Abstract
The immune system is replenished by self-renewing hematopoietic stem cells (HSCs) that produce multipotent progenitors (MPPs) with little renewal capacity. E-proteins, the widely expressed basic helix-loop-helix transcription factors, contribute to HSC and MPP activity, but their specific functions remain undefined. Using quantitative in vivo and in vitro approaches, we show that E47 is dispensable for the short-term myeloid differentiation of HSCs but regulates their long-term capabilities. E47-deficient progenitors show competent myeloid production in short-term assays in vitro and in vivo. However, long-term myeloid and lymphoid differentiation is compromised because of a progressive loss of HSC self-renewal that is associated with diminished p21 expression and hyperproliferation. The activity of E47 is shown to be cell-intrinsic. Moreover, E47-deficient HSCs and MPPs have altered expression of genes associated with cellular energy metabolism, and the size of the MPP pool but not downstream lymphoid precursors in bone marrow or thymus is rescued in vivo by antioxidant. Together, these observations suggest a role for E47 in the tight control of HSC proliferation and energy metabolism, and demonstrate that E47 is not required for short-term myeloid differentiation.
Introduction
Despite progress in deciphering the cellular and environmental cues involved in hematopoietic stem cell (HSC) self-renewal and repopulation, the specific transcriptional regulators that control the functional integrity of HSCs are still being defined.1,2 Recent studies implicate E proteins, a family of basic helix-loop-helix transcription factors, in controlling the maintenance and lineage repopulation activities of HSCs.3,4 The E protein inhibitor Id1 has been shown to modulate long-term HSC self-renewal and differentiation.3 E2A, an essential basic helix-loop-helix transcription factor in immune system development, contributes to HSC maintenance and early lineage commitment.4-6 However, the precise roles and mechanisms of E proteins in regulating HSC dynamics, including the size of functional HSC pool, long-term HSC persistence, and short-term HSC myeloid differentiation, remain unclear. In addition, recent studies conflict on whether E47 promotes myeloid development4 or prevents myeloid development7 of uncommitted hematopoietic progenitors.
Little is known about the transcription regulatory pathways that control the size of the long-term HSC pool. Although multiple groups uniformly suggest that E47 is required for the development of early hematopoietic progenitors, including the nonrenewing multipotent progenitors (MPPs) and the downstream lymphoid-myeloid primed MPPs (LMPPs),4-6 results from the self-renewing HSC pool are discordant. Two groups found normal numbers of HSCs in E47 knockout (KO) mice, using 3 independent phenotypic schemes, including the flk2− LSK (lineage− Sca-1+ c-kit+), CD27− LSK, and CD150+CD48− LSK definitions.5,6 Another group, however, showed a reduction of HSCs in mice lacking E47 using the definition CD150+flk2− LSK.4 Because none of these schemes defines an entirely pure population of long-term HSCs, the results from these studies might reflect the discrepancy between phenotypic HSCs and functional HSCs.8-10 Indeed, only 1 in 3 CD150+CD48− LSKs has functional long-term HSC properties, and this ratio is even lower in aged mice.10,11 The precise role of E47 in regulating the size of functional HSC pool remains to be definitively established and may be best assessed by quantitative in vivo limit dilution adoptive transfer assays rather than by simple resolution of phenotypic HSC subsets.
Numerous advances define a requirement for E proteins in hematopoiesis.12 E proteins are an essential transcription factor in lymphoid lineage differentiation13-15 but are not required for megakaryocyte/erythroid potential.5 However, the role of E proteins in myeloid lineage development is controversial. A previous report showed that myeloid progenitors are reduced in E2A-deficient mice, suggesting that E2A promotes myelopoiesis.4 In contrast, another recent study found that a variant of E47 prevents myeloid lineage differentiation of LMPPs in in vitro culture assays.7 A third study showing that mice lacking the Id inhibitor of E47 activity have normal myeloid differentiation3 suggests that E proteins may be dispensable at least for short-term myeloid activity. These conflicting observations might reflect the different roles of E proteins within hematopoietic progenitors (cell-autonomous) versus within the hematopoietic progenitor cell niche (cell nonautonomous). Indeed, both cell-autonomous and cell nonautonomous roles for the Id inhibitors of E47 activity are being defined.7,16 A careful separation of the cell-intrinsic versus extrinsic effects of E47, with specific attention to quantitative differences, is critical for a precise understanding of the specific functions of E proteins.
Here, we performed quantitative in vivo and in vitro assays to examine the cell-intrinsic role of E47 in regulating the functional potential of HSCs, including short-term activation and myeloid differentiation, and the size of the functional HSC pool. We found that E47-deficient bone marrow progenitors showed functional niche engraftment. Unexpected relative to recent findings, E47 null HSCs demonstrated competent short-term myeloid differentiation potential in response to transplantation stress in vivo and lipopolysaccharide (LPS) stimulation in vitro. However, the long-term repopulation and self-renewal activities of HSCs were compromised. Self-renewal defects of E47 null HSCs are cell-intrinsic and are associated with hyperproliferation and premature exhaustion under transplantation stress. Quantification of this defect revealed a 3-fold reduction in the frequency of functional HSCs in E47-deficient mice by limit dilution adoptive transfer assays in vivo. E47-deficient HSCs and MPPs had altered expression of genes associated with metabolism, suggesting that E47 might regulate early hematopoiesis, in part, through maintaining cellular energetics. In support of this hypothesis, in vivo exposure to antioxidants appreciably restored MPP cellularity in E47-deficient mice. Together, these observations quantify a cell-intrinsic role of E47 in regulating long-term HSC self-renewal, but not the short-term activation or myeloid differentiation capabilities.
Methods
Mice
E47 KO mice generated on an FVB/N background13 were backcrossed to C57BL/6 mice for 11 generations. N-acetyl cysteine was administered orally for 14 days (1 mg/mL in sterile deionized water). Mice were bred in accordance with Institutional Animal Care and Use Committee policies at the University of Pittsburgh School of Medicine, and all mice experiments were approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
Flow cytometry
Hematopoietic progenitors were stained as we have reported15 ; detailed antibody panel is available on request. For apoptosis analysis, surface-stained cells were incubated with annexin V (BD Biosciences) and 4,6-diamidino-2-phenylindole (DAPI) at room temperature for 15 minutes according to the manufacturer's instruction. Flow cytometry was performed on a 4-laser, 12-detector LSR II (BD Biosciences) and data analyzed with FlowJo software Version 8.8.6 (TreeStar).
Microarray and quantitative PCR validation
Two independent sorts were performed for each sample. Cells were sorted directly into SuperAmp tubes containing lysis buffer (Miltenyi Biotec) and sent to Miltenyi Biotec for global cDNA amplification followed by one-color hybridization using Agilent Whole Mouse Genome. Data were evaluated with the Gene Expression Data Analyzer statistical package (www.bioinformatics.upmc.edu/GE2/GEDA.html) using the J5 test. The J5 statistic is designed to be robust for analyses with a low number of replicates, thereby minimizing the chances of false positives because of outliers.17 Genes with a J5 score greater than 2 and a fold change more than 2 were considered significant. Supplemental Tables 1 and 2 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article) list both the J5 value and fold change for the relevant genes; complete array data are available on request. For target validation, cDNA was prepared from sorted cells using the Cells-to-Ct kit (Applied Biosystems) per the manufacturer's instructions. Quantitative polymerase chain reaction (PCR) analysis was performed in triplicate reactions using TaqMan probes (Applied Biosystems), and the data are normalized to actin. The results are expressed as the fold difference of knockout relative to wild-type (2-ddCt). All microarray data have been deposited into the Gene Expression Omnibus (National Center for Biotechnology Information) under accession number GSE26788.
BrdU incorporation and cell-cycle analysis
Bromodeoxyuridine (BrdU) incorporation and cell-cycle analysis were performed as we have described.6,15,18 Mice were injected intraperitoneally with 200 μg BrdU in phosphate-buffered saline, or phosphate-buffered saline alone as a control, at 12-hour intervals. Twenty-four hours after the first injection, bone marrow cells were stained for surface markers using the anti-BrdU FITC flow kit (BD Biosciences) according to the manufacturer's instructions. Ki-67 intracellular staining and cell-cycle analyses were performed as described.6
Cell culture
Lineage-marker (CD3, CD19, CD11b, Gr-1, Ter119, NK1.1, and B220) negative bone marrow cells were depleted by magnetic automated cell separation, and stained with surface antibodies to flk2, Sca1, and c-kit.6 The indicated populations were double sorted on a 3 laser, 11 detector FACSAria (BD Biosciences).
For the long-term culture-initiating cell assay, flk2− LSKs were double sorted directly onto S17 stromal cells in 96-well plates.19 After 35 days, cultures overlaid with MethoCult M3434 (StemCell Technologies), and colony-forming potential was assessed according to the manufacturer's protocol. For serial replating assays, primary cultures were trypsinized after 10 days and hematopoietic progenitors replated by sorting on fresh S17 stromal cells for another 25 days before colony formation was assessed in MethoCult.
For in vitro colony-forming assay, flk2− LSKs cells were double sorted directly onto MethoCult M3434 (StemCell Technologies) at a limit dilution dose (1, 2, and 5 cells per well) in 96-well plates. Colony forming potential was assessed as in the preceding paragraph.
For serum-free medium culture assays, double-sorted flk2− LSKs were cultured with X-VIVO 15 (Biowhittaker) supplemented with 10% bovine serum albumin (StemCell Technologies) and the recombinant murine cytokines (PeproTech): 20 ng/mL stem cell factor, 50 ng/mL flk2/flt3 ligand, 10 ng/mL interleukin-3, and 50 ng/mL thrombopoietin, in the presence or absence of 10 μg/mL LPS (Sigma-Aldrich).
Adoptive transfer assays
For limiting-dilution competitive repopulation assays, serial dilutions of bone marrow cells from wild-type (WT) or E47 knockout littermates (CD45.2) were mixed with 2 × 105 CD45.1 WT competitor cells and injected into lethally irradiated (10 Gy) CD45.1 recipient mice via the tail vein. Repopulation of the peripheral blood of the recipient mice was measured at 16 weeks after transplantation, and the competitive reconstitution unit was calculated according to Poisson statistics by L-Calc software Version 1.1.1 (StemCell Technologies).
For serial transplantation, 2 × 106 WT or E47-deficient CD45.2 bone marrow cells were injected into lethally irradiated CD45.1 recipients. Sixteen weeks after transplantation, 2 × 106 bone marrow cells from primary recipients were serially transferred into lethally irradiated CD45.1 secondary recipients. Multilineage reconstitution was examined monthly in the peripheral blood and at 16 weeks after transplantation in the spleen of both primary and secondary recipients.
For niche engraftment analysis, 2 × 106 WT or E47 knockout CD45.2 bone marrow cells were injected into lethally irradiated CD45.1 recipients. Recipient mice were killed at 2 weeks after transplantation, and CD45.2+ flk2− LSKs were counted.
Statistics
The statistical significance of differences between group means (P < .05) was established using Student t test. Progenitor frequencies in limiting dilution assays were calculated according to Poisson statistics by L-Cal software.
Results
E47 KO HSCs have efficient in vitro myeloid differentiation under pathogen-free conditions and after LPS stimulation
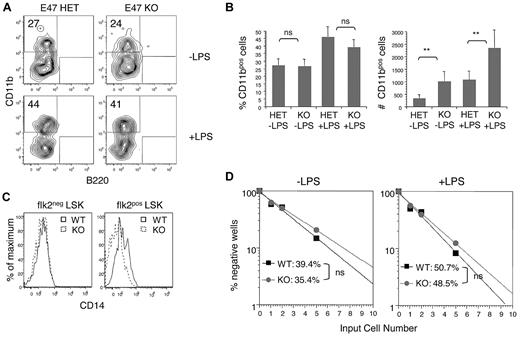
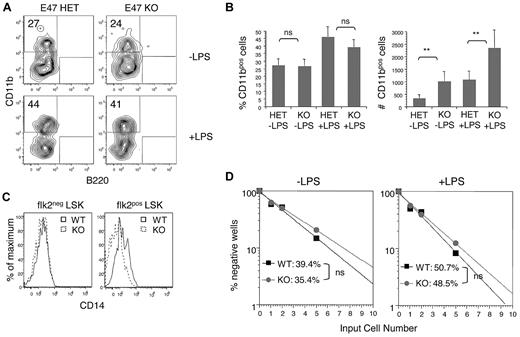
The precise role of E47 in regulating the myeloid differentiation potential of HSCs remains unclear, with one study suggesting that E proteins promote myeloid development4 and other studies suggesting differently.3,7 Although E47-deficient mice have reduced numbers of flk2brightest LMPPs,5,6 E47 does not appear to directly regulate flk2 mRNA expression, rendering this cytokine receptor one useful marker of HSC discrimination.5 Focusing on the HSC-enriched flk2− LSK subset, we examined the requirement for E47 in myeloid differentiation under defined conditions in bulk culture as well as at the single-cell level. We found that 27.4% ± 4.13% versus 26.4% ± 4.49% of CD11b+ cells emerged from E47 KO versus heterozygous (HET) flk2− LSKs after culture in defined serum-free medium, suggesting that E47 KO HSCs have efficient myeloid differentiation (Figure 1A, n = 9 wells, P > .05). However, although the frequency of myeloid generation was comparable between E47 KO and HET flk2− LSKs, the absolute number of CD11b cells emerging from E47 KO progenitors was increased more than 2-fold (Figure 1B). Recent work demonstrates that Toll-like receptor ligation induces rapid myeloid differentiation of HSCs, possibly to promote innate immune responses during infection.20 We confirmed normal expression of the LPS coreceptor CD14 on E47 KO flk2− LSKs (Figure 1C) and found that LPS increased the percentage of CD11b+ cells from both E47 KO and E47 HET progenitors to a similar degree (Figure 1B left panel). Again, although the frequency of CD11b+ cells was similar between KO and HET flk2− LSKs, the absolute number of myeloid cells was significantly increased in the absence of E47 (Figure 1B right panel).
E47-deficient HSCs show efficient myeloid differentiation under pathogen-free conditions and after stimulation with LPS. (A-B) Flk2− LSKs from E47 HET or KO mice were cultured at 300 cells per well in 96-well plates for 72 hours in the presence or absence of 10 μg/mL LPS. Cells were harvested, counted, and stained with lineage specific antibodies. The number and frequency of CD11b+ myeloid cells were measured (n = 9 wells). **P < .05. ns indicates not significant. The data are representative of 2 independent experiments. (C) The expression of CD14 on flk2− LSKs and flk2+ LSKs from WT and KO littermates was examined by flow cytometric analysis. The data are representative of 2 independent experiments. (D) flk2− LSKs from E47 WT or KO mice were cultured in MethoCult at 1, 2, and 5 cells per well in 96-well plates for 7 days in the presence or absence of 10 μg/mL LPS. Wells with colony-forming unit-granulocyte, macrophage colonies were scored positive. The frequency of colony-forming cells was calculated according to Poisson statistics (n = 48 wells per group). The data are representative of 2 independent experiments.
E47-deficient HSCs show efficient myeloid differentiation under pathogen-free conditions and after stimulation with LPS. (A-B) Flk2− LSKs from E47 HET or KO mice were cultured at 300 cells per well in 96-well plates for 72 hours in the presence or absence of 10 μg/mL LPS. Cells were harvested, counted, and stained with lineage specific antibodies. The number and frequency of CD11b+ myeloid cells were measured (n = 9 wells). **P < .05. ns indicates not significant. The data are representative of 2 independent experiments. (C) The expression of CD14 on flk2− LSKs and flk2+ LSKs from WT and KO littermates was examined by flow cytometric analysis. The data are representative of 2 independent experiments. (D) flk2− LSKs from E47 WT or KO mice were cultured in MethoCult at 1, 2, and 5 cells per well in 96-well plates for 7 days in the presence or absence of 10 μg/mL LPS. Wells with colony-forming unit-granulocyte, macrophage colonies were scored positive. The frequency of colony-forming cells was calculated according to Poisson statistics (n = 48 wells per group). The data are representative of 2 independent experiments.
To carefully evaluate myeloid developmental potential at the single-cell level in the absence of confounding proliferative effects, we performed in vitro limit dilution colony-forming unit assays. We found that E47 KO and WT flk2− LSKs have comparable colony-forming activity (without LPS, 35.4% vs 39.4% positive wells; with LPS, 48.5% vs 50.7% positive wells, respectively, Figure 1D). Together, these observations demonstrate that E47 KO HSCs can be efficiently activated for myeloid differentiation at a per-cell level and that E47 does not appear to restrain or promote myeloid potential in this compartment. As such, a finding of reduced myeloid progenitors in E2A-deficient mice4 may reflect a failure of HSC persistence rather than perturbations in myeloid lineage potential per se.
E47 null HSCs have competitive short-term myeloid repopulation but defective long-term repopulation activities
Within the flk2− LSK subset, long-term HSCs can be more specifically identified using the CD150 and CD48 SLAM markers.11 We found that the frequencies of phenotypic long-term HSCs (CD150+CD48− LSKs) are relatively similar: 0.0050% ± 0.0038% cells versus 0.0049% ± 0.0034% cells in the bone marrow of WT versus E47 KO mice (n = 6 mice). However, whether these HSCs retain competent short-term and long-term myeloid repopulation activity remains unclear. This is a particularly important point because even the SLAM LSK phenotypic definition does not resolve a pure HSC subset.11
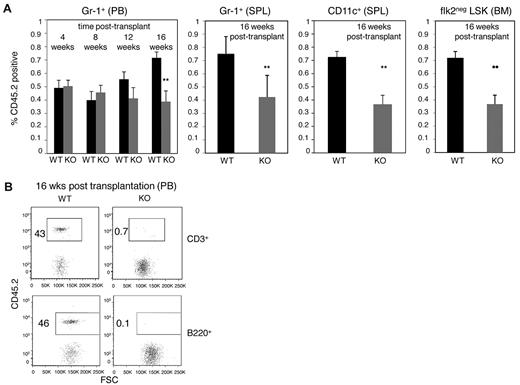
To definitively assess the cell-intrinsic requirement for E47 in myeloid repopulation in vivo, we performed competitive adoptive transfer assays in which the developmental potential of E47-deficient progenitors is compared relative to WT progenitors. For these analyses, we focused on the short-lived Gr-1+ subset as a sensitive indicator of ongoing hematopoiesis.19 We found comparable short-term reconstitution of Gr-1+ myeloid cells by E47 null or WT donor bone marrow up to 12 weeks after transplantation (Figure 2A). Specifically, 48% ± 7% versus 50% ± 6% of Gr-1+ cells were CD45.2+ in the peripheral blood of the mice reconstituted by WT versus KO donor cells at 4 weeks after transplantation, and 40% ± 9% versus 45% ± 7% of Gr-1+ cells were CD45.2+ from WT versus KO donor cells at 8 weeks after transplantation, respectively (n = 5 mice, P > .05). These data suggest that cell-intrinsic E47 is dispensable for short-term myeloid differentiation of E47 null HSCs in the context of a WT developmental environment, supporting the in vitro findings in Figure 1.
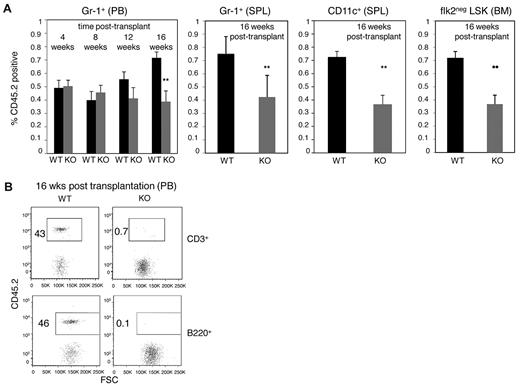
E47-deficient bone marrow cells display compromised long-term competitive repopulation activity in vivo. (A) CD45.2 WT and E47 KO bone marrow cells (2 × 105) mixed with an equal number of CD45.1 competitor cells were adoptively transferred into lethally irradiated CD45.1 recipient mice. The proportion of test donor-derived (CD45.2+) Gr-1+cells was monitored every 4 weeks in the peripheral blood of the recipient mice. Then, the recipients were killed at 16 weeks after transplantation, and lineage repopulation in the spleen (Gr-1+, CD11c+) or bone marrow (flk2− LSK) was measured (n = 5 mice). P < .05. The data are representative of 2 independent experiments. (B) Competitive lymphoid repopulation activity of WT and E47 KO bone marrow cells was measured at 1:1 test donor/competitor ratio. The percentage of test donor-derived (CD45.2+) lymphoid cells in the peripheral blood of the recipient mice at 16 weeks after transplantation is shown (n = 8 mice). PB indicates peripheral blood; SPL, spleen; and BM, bone marrow.
E47-deficient bone marrow cells display compromised long-term competitive repopulation activity in vivo. (A) CD45.2 WT and E47 KO bone marrow cells (2 × 105) mixed with an equal number of CD45.1 competitor cells were adoptively transferred into lethally irradiated CD45.1 recipient mice. The proportion of test donor-derived (CD45.2+) Gr-1+cells was monitored every 4 weeks in the peripheral blood of the recipient mice. Then, the recipients were killed at 16 weeks after transplantation, and lineage repopulation in the spleen (Gr-1+, CD11c+) or bone marrow (flk2− LSK) was measured (n = 5 mice). P < .05. The data are representative of 2 independent experiments. (B) Competitive lymphoid repopulation activity of WT and E47 KO bone marrow cells was measured at 1:1 test donor/competitor ratio. The percentage of test donor-derived (CD45.2+) lymphoid cells in the peripheral blood of the recipient mice at 16 weeks after transplantation is shown (n = 8 mice). PB indicates peripheral blood; SPL, spleen; and BM, bone marrow.
However, significantly reduced myeloid reconstitution by E47 null donor cells was observed long-term (Figure 2A). Hematopoiesis at 16 weeks is thought to reflect the contribution of long-term HSCs as short-lived subsets cannot durably sustain reconstitution at this point.19 At 16 weeks after transplantation, 72% ± 10% versus only 39% ± 18% of Gr-1+ cells were CD45.2+ in the peripheral blood of the mice reconstituted by WT versus KO donor cells, respectively (Figure 2A, n = 5 recipients per group, P < .05). E47 KO donor cells also gave rise to significantly fewer splenic Gr-1+ granulocytes or CD11c+ dendritic cells, as well as bone marrow flk2− LSKs (Figure 2A, n = 5 recipients per group, P < .05). Not surprisingly given known defects in lymphoid potential, E47-deficient donor bone marrow cells fail to reconstitute detectable T cells or B cells in the presence of an equal number of competitor cells (Figure 2B, n = 8 recipients, P < .05). Together, these observations suggest that E47 KO HSCs can efficiently mediate rapid short-term myeloid lineage repopulation under transplantation stress but that long-term repopulation activities are compromised.
E47 null HSCs display poor self-renewal efficiency in vivo
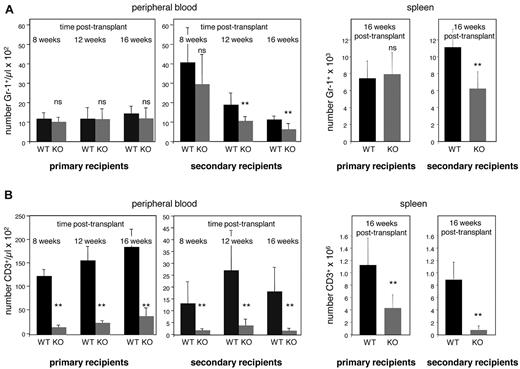
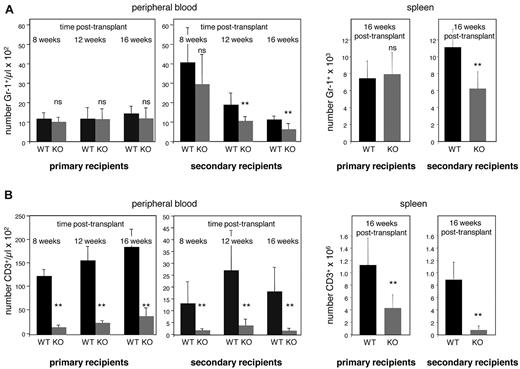
To examine the cell-autonomous role of E47 in maintaining the self-renewal potential of long-term HSCs, we performed serial transplantation under noncompetitive conditions. Consistent with our in vitro findings, E47 KO bone marrow showed competent myeloid reconstitution in primary WT recipients. Comparable numbers of donor-derived Gr-1+ cells were detected in both the peripheral blood and spleen in mice transplanted with WT or E47 KO donor bone marrow (Figure 3A). However, significant repopulation defects were observed in the secondary recipients of E47 null bone marrow. Reconstitution of Gr-1+ cells derived from CD45.2 E47-deficient donor bone marrow was decreased as early as 12 weeks, and at 16 weeks after transplantation E47 null donor bone marrow showed a 2-fold reduction in both peripheral blood and spleen. The number of WT versus KO donor-derived CD45.2+Gr-1+ cells was 1114 ± 196/μL versus 619 ± 293/μL in the peripheral blood, and 10 858 ± 2087/μL versus 6108 ± 1922/μL per spleen (n = 6-8 mice, P < .05). The observation that myeloid repopulation is grossly normal in primary recipients but significantly reduced in the secondary recipients suggests progressive depletion of HSC repopulation activity caused by diminished self-renewal. Had the E47 defect been restricted to short-lived HSCs or MPPs, then the magnitude of reconstitution would be similar across primary and secondary recipients, which is not the case.
E47-deficient HSCs display poor self-renewal efficiency in vivo. Serial transplantation was performed to examine the long-term self-renewal efficiency of HSCs from WT and E47 KO mice. The bone marrow cells from WT and E47 KO littermates were first adoptively transferred into primary recipients for 16 weeks and then serially transferred into secondary recipients. Blood reconstitution was measured monthly. Recipients were killed at 16 weeks after transplantation, and lineage reconstitution in the spleen was examined. Myeloid or lymphoid reconstitution efficiency was indicated by the number of donor-derived CD45.2+ Gr-1+ cells (A) or CD3+ cells (B), respectively (n = 6 to 8 mice per group). **P < .05. ns indicates not significant.
E47-deficient HSCs display poor self-renewal efficiency in vivo. Serial transplantation was performed to examine the long-term self-renewal efficiency of HSCs from WT and E47 KO mice. The bone marrow cells from WT and E47 KO littermates were first adoptively transferred into primary recipients for 16 weeks and then serially transferred into secondary recipients. Blood reconstitution was measured monthly. Recipients were killed at 16 weeks after transplantation, and lineage reconstitution in the spleen was examined. Myeloid or lymphoid reconstitution efficiency was indicated by the number of donor-derived CD45.2+ Gr-1+ cells (A) or CD3+ cells (B), respectively (n = 6 to 8 mice per group). **P < .05. ns indicates not significant.
Progressive loss of repopulation activity was also observed for T lymphoid reconstitution (Figure 3B). T-cell reconstitution by E47-deficient bone marrow was reduced 5-fold in the peripheral blood and 3-fold in the spleen of primary recipients, and a more pronounced defect was observed in secondary recipients (a 9-fold decrease in peripheral blood and 10-fold decrease in the spleen; n = 6-8 mice, P < .05). That E47-deficient bone marrow showed progressive loss in both myeloid and T lymphoid reconstitution in secondary recipients indicated a defect in the long-term in vivo self-renewal of HSCs. This finding contrasts with the apparent integrity of myeloid production in the short term.
Mechanisms underlying HSC exhaustion
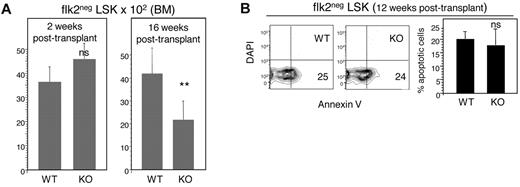
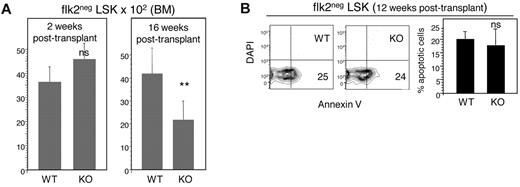
We performed a series of in vivo assays to examine the mechanisms underlying the functional defects of E47 KO HSCs. First, we examined whether E47 KO HSCs can mediate functional niche engraftment, the critical step subsequent to bone marrow homing. In this assay, we measured the number of donor-derived flk2− LSKs engrafted 2 weeks after adoptive transfer of CD45.2 WT or E47 KO bone marrow cells to CD45.1 hosts. Comparable numbers of CD45.2+ flk2− LSKs (3616 ± 623 vs 4604 ± 639, n = 3 mice, P > .05) were detected in the bone marrow of mice reconstituted by WT or E47 KO bone marrow cells (Figure 4A left panel). Therefore, E47 KO HSCs do not have detectable defects in niche engraftment. In contrast to short time points, at 16 weeks after transplantation, the number of CD45.2+ flk2− LSKs decreased 50% from 4184 ± 1189 in mice reconstituted by WT donor cells to 2174 ± 818 in those reconstituted by E47 KO donor cells (Figure 4A right panel, n = 8 mice, P < .05). The reduction of E47 KO HSCs at later time points suggests premature exhaustion under transplantation stress. HSC exhaustion can be associated with poor survival21-23 ; however, we found similar frequencies of apoptosis for E47 KO or WT-derived flk2− LSKs isolated either directly ex vivo from donor animals6 or at 12 weeks after transplantation (Figure 4B, n = 3 mice, P > .05), a time point at which E47 KO HSCs begin to show defects in competitive reconstitution. Therefore, gross apoptosis is unlikely to be a major mechanism responsible for depletion of E47-deficient HSCs.
E47 null HSCs show normal homing, niche engraftment, and apoptosis. (A) CD45.2 WT or E47 KO bone marrow cells (2 × 106) were injected into lethally irradiated CD45.1 recipient mice. The number of donor-derived flk2− LSKs was examined at 2 weeks (n = 3 mice) and at 16 weeks (n = 8 mice) after transplantation. (B) Lethally irradiated CD45.1 recipient mice reconstituted with E47 KO or WT CD45.2 donor bone marrow cells were killed at 12 weeks after transplantation. The bone marrow cells from these recipients were stained with antibodies to resolve donor-derived CD45.2 flk2− LSKs and then labeled with annexin V and DAPI for apoptosis analysis. (Left panels) Representative flow cytometry profiles used to generate the bar graph on the right (n = 3 mice). **P < .05. ns indicates not significant.
E47 null HSCs show normal homing, niche engraftment, and apoptosis. (A) CD45.2 WT or E47 KO bone marrow cells (2 × 106) were injected into lethally irradiated CD45.1 recipient mice. The number of donor-derived flk2− LSKs was examined at 2 weeks (n = 3 mice) and at 16 weeks (n = 8 mice) after transplantation. (B) Lethally irradiated CD45.1 recipient mice reconstituted with E47 KO or WT CD45.2 donor bone marrow cells were killed at 12 weeks after transplantation. The bone marrow cells from these recipients were stained with antibodies to resolve donor-derived CD45.2 flk2− LSKs and then labeled with annexin V and DAPI for apoptosis analysis. (Left panels) Representative flow cytometry profiles used to generate the bar graph on the right (n = 3 mice). **P < .05. ns indicates not significant.
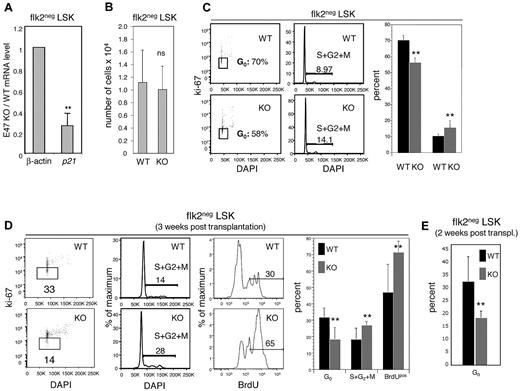
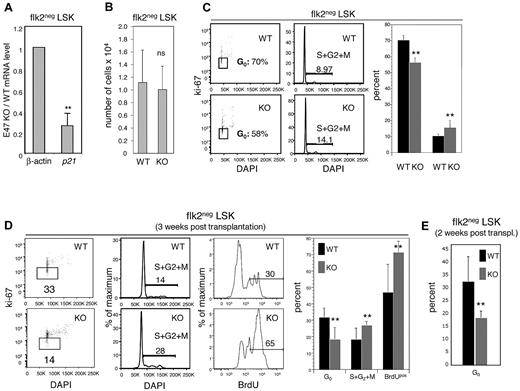
We have previously shown that E47-deficient total LSKs exhibit a 50% reduction in transcripts of the cell-cycle inhibitor p21.6 Consistent with our previous findings, p21 was reduced 3-fold in E47-deficient flk2− LSKs compared with WT flk2− LSKs (Figure 5A, n = 4 independent sorts). Thus, p21 might play an important role in linking abnormalities of cell-cycle kinetics with functional defects in E47 null HSCs. Indeed, the HSCs in p21 KO mice display long-term self-renewal defects that are strikingly similar to the defects in E47 null HSCs,24 and E47 has been shown to directly bind to the p21 promoter and induce transcriptional expression.6,25 We then assessed the cell-cycle status of E47 null versus WT HSC-enriched flk2− LSKs at steady-state homeostasis. Whereas flk2− LSKs were numerically comparable in WT versus E47 null mice (Figure 5B), E47-deficient flk2− LSKs showed hyperproliferation as indicated by a loss of G0 quiescent cells and increased percentage of the actively cycling cells (Figure 5C). Specifically, the proportion of Ki67-negative quiescent cells was 70.3% ± 3.0% versus 56.0% ± 3.2% in WT and E47 KO flk2− LSKs, respectively (n = 3 mice, P < .05). Conversely, the proportion of cells in the S + G2 + M phases increased from 10.1% ± 1.1% to 15.1% ± 4.3% in these WT versus KO flk2− LSKs (n = 3 mice, P < .05). Thus, E47 restricts the proliferation of HSCs at steady-state homeostasis, a result consistent with our previous findings.
E47 null HSCs displayed hyperproliferation under steady state and after transplantation stress. (A) Flk2− LSKs from WT and E47 KO littermates were sorted by flow cytometry, and the expression of p21 and β-actin was examined by quantitative reverse-transcription PCR. The data are presented as KO/WT ratios for each transcript (n = 4 independent sorts). **P < .05. (B) Bone marrow cells from WT and E47 KO littermates were stained with cell surface antibodies to resolve HSC-enriched flk2− LSKs, and the number of flk2− LSKs was counted (n = 11 mice). (C) Surface-stained flk2− LSKs from E47 WT or KO mice were fixed and then stained with antibodies to the Ki67 proliferation antigen and DAPI for cell-cycle analysis. (Left panels) Representative flow cytometric profiles used to generate the bar graph on the right (n = 3 or 4 mice). **P < .05. (D) Lethally irradiated mice reconstituted with E47 KO or WT CD45.2 donor bone marrow cells were killed at 3 weeks after transplantation. A total of 100 μg BrdU was injected into recipient mice at a 12-hour interval for 24 hours before death. The donor-derived CD45.2 flk2− LSKs were fixed and stained with antibodies to BrdU, Ki67, or DAPI for proliferation and cell-cycle analysis (n = 4 mice). **P < .05. ns indicates not significant. (E) Bone marrow from E47 KO (CD45.2) and WT (CD45.1/2) mice was cotransferred into lethally irradiated CD45.1 hosts, and cell-cycle status was examined as in panel D at 2 weeks after transplantation. (n = 6 mice). P < .05.
E47 null HSCs displayed hyperproliferation under steady state and after transplantation stress. (A) Flk2− LSKs from WT and E47 KO littermates were sorted by flow cytometry, and the expression of p21 and β-actin was examined by quantitative reverse-transcription PCR. The data are presented as KO/WT ratios for each transcript (n = 4 independent sorts). **P < .05. (B) Bone marrow cells from WT and E47 KO littermates were stained with cell surface antibodies to resolve HSC-enriched flk2− LSKs, and the number of flk2− LSKs was counted (n = 11 mice). (C) Surface-stained flk2− LSKs from E47 WT or KO mice were fixed and then stained with antibodies to the Ki67 proliferation antigen and DAPI for cell-cycle analysis. (Left panels) Representative flow cytometric profiles used to generate the bar graph on the right (n = 3 or 4 mice). **P < .05. (D) Lethally irradiated mice reconstituted with E47 KO or WT CD45.2 donor bone marrow cells were killed at 3 weeks after transplantation. A total of 100 μg BrdU was injected into recipient mice at a 12-hour interval for 24 hours before death. The donor-derived CD45.2 flk2− LSKs were fixed and stained with antibodies to BrdU, Ki67, or DAPI for proliferation and cell-cycle analysis (n = 4 mice). **P < .05. ns indicates not significant. (E) Bone marrow from E47 KO (CD45.2) and WT (CD45.1/2) mice was cotransferred into lethally irradiated CD45.1 hosts, and cell-cycle status was examined as in panel D at 2 weeks after transplantation. (n = 6 mice). P < .05.
The magnitude of proliferative defects was even more pronounced after adoptive transfer stress. Three weeks after adoptive transfer of E47 KO and WT donor bone marrow cells into WT recipients, E47 null flk2− LSKs exhibited greater proliferation relative to WT (Figure 5D). Cells residing in G0 were decreased from 31.7% ± 5.8% for WT donor-derived flk2− LSKs to 18.2% ± 7.2% for E47 KO donor-derived flk2− LSKs (summarized in far right panel, n = 3 mice, P < .05). Reciprocally, the proportion of cells in S + G2 + M phases increased from 18.3% ± 6.8% for donor-derived WT flk2− LSKs to 27% ± 2.0% for donor-derived E47 KO flk2− LSKs in these mice. Furthermore, BrdU incorporation increased from 47% ± 17% for WT flk2− LSKs to 71.6% ± 6.5% for donor-derived E47 KO flk2− LSKs. These observations suggest that cell-intrinsic E47 regulates the proper cell-cycle activation of HSCs under transplantation stress. Thus, not only do E47 null progenitors exhibit hyperproliferation in the context of an E47 KO background (Figure 5C) but also in the context of a WT background (Figure 5D), suggesting cell-autonomous requirements.
One concern is that the failure of normal lymphoid production in mice reconstituted by E47-deficient HSCs contributes to a lymphopenia that may alter the developmental microenvironment. In this case, hyperproliferation of E47-deficient HSCs might be exacerbated by perturbed cytokine secretion or related effects, but not necessarily cell-intrinsic defects within HSCs per se. To directly test this possibility, we cotransferred E47 WT (CD45.1/2) and E47 KO (CD45.2) bone marrow cells into the same recipients (CD45.1). We found that E47-deficient HSCs displayed a loss of G0 quiescence and reciprocal hyperproliferation of the same magnitude as that observed in noncompetitive adoptive transfer assays (compare Figure 5D with Figure 5E). This observation confirms that the defects in E47-deficient HSCs are cell-intrinsic.
Quantitative analysis of long-term HSC defects in E47-deficient mice
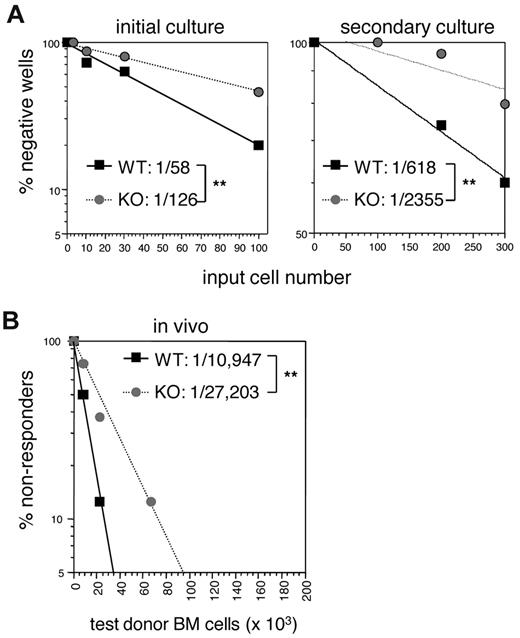
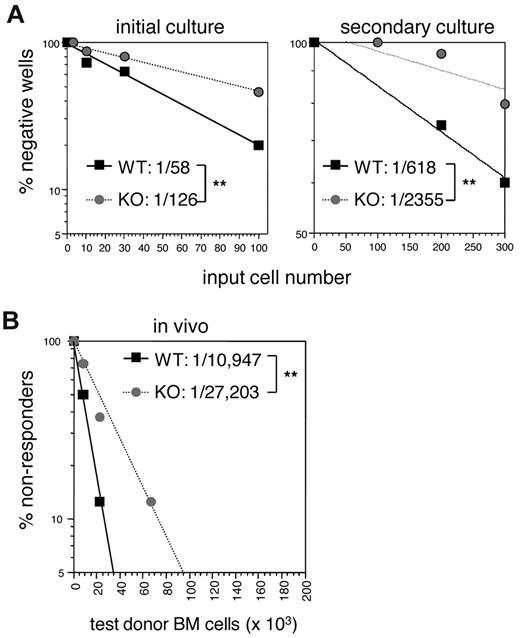
A quantitative study of the defects in E47-deficient LT HSCs in the context of a WT developmental environment is essential for a clear understanding of cell-autonomous requirement for E47. We first examined the role of E47 in regulating the long-term colony-initiating potential of HSCs in vitro. Double-sorted flk2− LSKs were cultured at limiting dilution doses for 35 days, a time frame that selects for cells with long-term hematopoietic reconstitution potential, after which functional differentiation was assessed using MethoCult.19 We found a 2-fold reduction in colony-forming cells in the absence of E47. As shown in Figure 6A (left panel), the frequency of colony-forming cells decreased from 1 in 58 of WT flk2− LSKs to 1 in 126 of E47 KO flk2− LSKs (P < .05). Serial long-term culture assays were then performed to measure the frequency of progenitors capable of serial repopulation activity. The frequency of colony-forming cells that survived serial replating was reduced 3-fold from 1 in 618 versus 1 in 2355 of WT versus E47 KO flk2− LSK, respectively (Figure 6A right panel, P < .05). Thus, E47-deficient HSCs showed severe cell-autonomous defects in colony-forming activities, and the magnitude of these defects is progressive during serial passage.
Quantitative analysis of long-term HSC defects in E47-deficient mice. (A) Long-term culture-initiating cell assay was performed with double-sorted flk2− LSKs from WT and E47 KO littermates to determine the frequency of long-term colony-forming cells. Plotted is the percentage of wells that did not give rise to colonies after a single plating (left panel) or serial replating (right panel) at the indicated input cell numbers. The frequency of long-term colony-forming cells was calculated according to Poisson statistics. (B) Limit dilution doses (0.67 × 105, 0.22 × 105, and 0.073 × 105) of CD45.2 WT and E47 KO bone marrow cells mixed with a constant number (2 × 105) of CD45.1 competitor cells were adoptively transferred into lethally irradiated CD45.1+ recipient mice. The graph depicts the percentage of CD45.1 recipient mice that had less than 1% of donor CD45.2+ Gr-1+ cells at 16 weeks after adoptive transfer of the indicated donor cell numbers. The frequency of functional HSCs was calculated using Poisson statistics. Eight recipient mice were used at each cell dose per genotype. **P < .05.
Quantitative analysis of long-term HSC defects in E47-deficient mice. (A) Long-term culture-initiating cell assay was performed with double-sorted flk2− LSKs from WT and E47 KO littermates to determine the frequency of long-term colony-forming cells. Plotted is the percentage of wells that did not give rise to colonies after a single plating (left panel) or serial replating (right panel) at the indicated input cell numbers. The frequency of long-term colony-forming cells was calculated according to Poisson statistics. (B) Limit dilution doses (0.67 × 105, 0.22 × 105, and 0.073 × 105) of CD45.2 WT and E47 KO bone marrow cells mixed with a constant number (2 × 105) of CD45.1 competitor cells were adoptively transferred into lethally irradiated CD45.1+ recipient mice. The graph depicts the percentage of CD45.1 recipient mice that had less than 1% of donor CD45.2+ Gr-1+ cells at 16 weeks after adoptive transfer of the indicated donor cell numbers. The frequency of functional HSCs was calculated using Poisson statistics. Eight recipient mice were used at each cell dose per genotype. **P < .05.
To directly quantify the cell-intrinsic role of E47 in regulating the pool size of functional long-term HSCs in vivo, we performed limiting dilution adoptive transfer assays in which serial dilutions of test donor bone marrow are competed against a constant number of competitor bone marrow cells. Blood reconstitution analyses at 16 weeks after transplantation, a time point reflecting the long-term repopulation activity of HSCs, revealed a 3-fold reduction in the frequency of long-term repopulating cells derived from the bone marrow of E47-deficient mice compared with WT mice (Figure 6B). Specifically, the frequency of functional long-term HSCs from WT and KO mice was 1 of 10 947 and 1 of 27 203, re-spectively (Table 1, n = 8 recipient mice/group, P < .05). That the donor cells were functioning in the context of a WT environ-ment demonstrates specific cell-intrinsic defects of E47 null donor HSCs.
Restoration of MPPs in E47-deficient mice
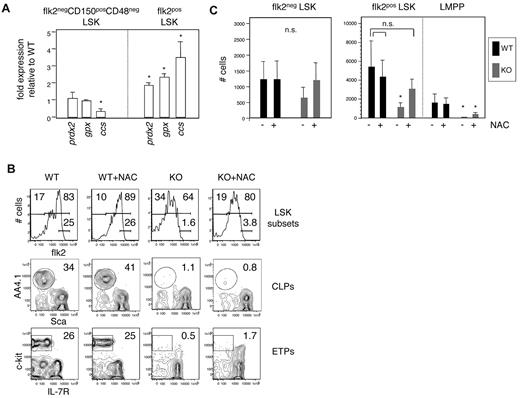
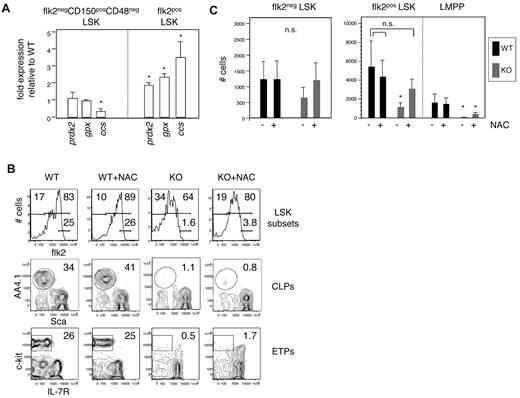
Our results defining a 3-fold reduction in the number of functional HSCs in E47-deficient mice do not fully explain the striking 10-fold reduction in downstream MPPs in these animals. Thus, the mechanisms underlying the failure of HSCs to produce or maintain MPPs remain unclear. Examination of transcription factors important in early hematopoietic differentiation (hoxA9, runx1, ikaros, gata2, and gata3) did not reveal major differences (data not shown). Therefore, we performed microarray analyses, in duplicate, on highly purified E47 WT and KO CD150+CD48−flk2− LSKs that been subject to the stress of each primary and secondary adoptive transfer. Notable among the differently expressed genes were several transcripts associated with cellular energy metabolism, including copper chaperone for superoxide dismutase (ccs), glutathione peroxidase (gpx), peroxiredoxin (prdx2), glutathione-S-transferase (gst4a), and NADH dehydrogenase (ndufa5; supplemental Tables 1-2). For validation, we focused on a subset of these genes in HSCs and MPPs isolated directly from E47 WT and KO mice under homeostatic circumstances (ie, not subject to adoptive transfer stress). Although ccs, gpx, and prdx2 were only modestly elevated in E47 KO HSCs isolated under homeostasis, these genes were significantly overexpressed in MPPs from these same animals (Figure 7A; P < .05).
Antioxidant restoration of E47-deficient MPPs. (A) Quantitative PCR analysis of redox-associated genes in purified HSCs (flk2−CD150+CD48− LSKs) or MPPs (flk2+ LSKs) from E47 KO mice compared with E47 WT mice. Data are mean ± SD of triplicate wells, representative of 2 or 3 independent sorts. *P < .05. (B) Mice treated with 1 mg/mL N-acetyl cysteine in the drinking water were killed after 14 days, and lymphoid organs stained to detect bone marrow multipotent progenitors (flk2− and flk2+ LSKs), common lymphoid progenitors (CLPs: lin−IL7R+AA4.1+Sca-llo), and early thymic progenitors precursors (ETPs: lin−CD44+CD25+c-kit+IL-7R−). (C) Quantitation of hematopoietic subsets in B (n = 5 mice/group). *P < .05. ns indicates not significant.
Antioxidant restoration of E47-deficient MPPs. (A) Quantitative PCR analysis of redox-associated genes in purified HSCs (flk2−CD150+CD48− LSKs) or MPPs (flk2+ LSKs) from E47 KO mice compared with E47 WT mice. Data are mean ± SD of triplicate wells, representative of 2 or 3 independent sorts. *P < .05. (B) Mice treated with 1 mg/mL N-acetyl cysteine in the drinking water were killed after 14 days, and lymphoid organs stained to detect bone marrow multipotent progenitors (flk2− and flk2+ LSKs), common lymphoid progenitors (CLPs: lin−IL7R+AA4.1+Sca-llo), and early thymic progenitors precursors (ETPs: lin−CD44+CD25+c-kit+IL-7R−). (C) Quantitation of hematopoietic subsets in B (n = 5 mice/group). *P < .05. ns indicates not significant.
Alterations in cellular metabolism associated with hyperproliferation or environmental stresses can be alleviated by therapeutic administration of antioxidants, including N-acetyl cysteine. After 2 weeks of in vivo exposure to N-acetyl cysteine in the drinking water, absolute numbers of MPPs (flk2+ LSKs) in E47 KO mice were appreciably restored relative to WT levels (Figure 7B top row, C; n = 5 mice per group; P < .05). Downstream LMPPs (flk2bright LSKs) were only slightly increased after treatment (Figure 7C; P > .05), and there was no reconstitution of common lymphoid progenitors in the bone marrow or early thymic progenitors in the thymus (Figure 7B middle and bottom rows). Thus, antioxidant treatment alleviates the MPP deficiency in E47 KO animals but does not correct the paucity of downstream subsets, which presumably depends on lymphoid-lineage gene expression.12 Together, our findings indicate a role for E47 in the proliferative integrity and persistence of HSCs and MPPs independent of effects on lineage-specific differentiation.
Discussion
In this study, we quantified the cell-intrinsic requirement for the transcription factor E47 in HSC differentiation and persistence. E47 null HSCs showed competent short-term myeloid differentiation but compromised long-term multilineage repopulation activity in both in vivo quantitative and qualitative adoptive transfer assays as well as in defined cultures in vitro. We found that defects in the long-term self-renewal capabilities of E47 KO HSCs were associated with cell-autonomous hyperproliferation under replication stress and loss of p21. In vivo treatment with an antioxidant appreciably restored numbers of MPPs in E47 KO mice. That myeloid differentiation remains intact despite disruption in each lymphoid potential, self-renewal, and proliferative activity of HSCs helps to pinpoint the specific biologic activities of cell-intrinsic E47 in this pivotal subset.
Previous studies established that E47 transcriptional activity, cell-intrinsic or otherwise, is dispensable for restriction to the megakaryocyte/erythroid lineages and is required for lymphoid lineage potential.5,12,26 Absent from this developmental cascade is a clear understanding of myeloid lineage potential. Although the germline loss of E47 reduces myelopoiesis,4 suggesting that E47 is required for myeloid potential, mice deficient in the E protein inhibitor Id1 exhibit normal short-term myeloid activity.3 Moreover, forced expression of a variant form of E47 restricts myeloid development of LSKs,7 rendering the role of E47 unclear. Here, we carefully show that E47-deficient progenitors have competent short-term myeloid potential in vivo and also under in vitro conditions of LPS stimulation. These results indicate that E47 is dispensable for efficient myeloid restriction of flk2− LSKs and that previously observed defects in myeloid output4 can be attributed to a basic loss of HSC integrity over time rather than a specific defect in myeloid potential per se. Even though E47 is not absolutely required for myeloid differentiation, it may still be involved in other aspects of myeloid progenitor dynamics. Indeed, E2A-deficient LMPPs give rise to granulocytes of atypical morphology in clonal assays, and the biologic significance remains to be investigated.5 Our studies are broadly consistent with findings in Id1 knockout mice that short-term myelopoiesis is unaffected by perturbed E protein activities, whereas long-term myelopoiesis is compromised because of HSC failure.3
We identified a striking hyperproliferation of donor-derived E47-deficient HSCs under transplantation stress. Cell-cycle regulation has been established as an important mechanism governing the long-term self-renewal potential of HSCs.2 For instance, in mice lacking the key cell-cycle inhibitors p21 or p16, hyperproliferation increased the number of phenotypic HSCs under steady-state circumstances, but the repopulation activities of HSCs were gradually exhausted during serial transplantation.8,24 In E47-deficient mice, we and others found normal numbers of phenotypic HSCs under steady-state homeostasis5,6 but a 3-fold reduction in the frequency of functional HSCs by competitive adoptive transfer assays (Figures 5B, 6B; Table 1). As E47-deficient progenitors show efficient engraftment 2 weeks after adoptive transfer (Figure 4A), these findings cannot be explained by a failure to productively lodge in the bone marrow. Rather, this divergence is likely the result of exhaustion of E47 KO HSCs under replication stress. Indeed, the number of flk2− LSKs derived from E47 KO donor cells was reduced by 2-fold at 16 weeks after transplantation compared with WT donor-derived flk2− LSKs (Figure 4A), indicating HSC exhaustion in the context of a WT environment. Similar exhaustion of HSCs was observed after administration of mitotoxic drug 5-fluorouracil.6 Together, these observations suggest that E47 may prevent the premature exhaustion of HSCs under replication stress, possibly by acting on cell cycling.
The results from this study link E47 deficiency with long-term repopulation defects of HSCs in part through regulation of cell proliferation and oxidative stress. What remains unknown is the mechanism through which E47 activity is regulated in HSCs and other early progenitor subsets. JNK-mediated inactivation of E47 via Mixed Lineage Kinase 2 has been reported to be involved in neuronal cell death.27 Mixed Lineage Kinase 2 regulates the activity of E47 through phosphorylation of the conserved transcriptional activation domain AD2.27 Understanding the phosphorylation status of E47 in multipotent hematopoietic progenitors may help decipher the E47 transcriptional pathways in regulating cell-cycle progression in early hematopoiesis.
Together, our results quantify the cell-intrinsic role of the transcription factor E47 in the functional integrity of bone marrow long-term HSCs. We show that E47 maintains the size of a functionally robust HSC pool and prevents the premature exhaustion of long-term self-renewal of HSCs under replication stress. We also show that E47 is not required for short-term rapid lineage differentiation under transplantation stress or after LPS challenge. Not only does premature exhaustion of HSCs significantly reduce the efficiency of long-term hematopoiesis in response to hematopoietic injury, microbial challenge, aging, and other replication stresses, but hyperproliferation and constant activation of HSCs under these persistent stresses might foster mutation accumulation and provide the molecular basis for tumorigenesis. Indeed, disruption of E protein activity is associated with the cancers of both lymphoid and myeloid lineages in humans and mouse.28-31 Thus, further investigations on the role of E47 in HSC replication and transformation may provide new insights into tissue damage repair, aging, and cancer development.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dewayne Falkner for excellent cell sorting; Christine Milcarek and Kathee Martincic for helpful advice; Micke Sigvardsson, Lisa Denzin, and Kim Payne for critical comments on the manuscript; and Xiao-hong Sun, whose criticisms elevate this work.
This work is supported by the National Institutes of Health (AI079047 and AR054529; L.B.).
National Institutes of Health
Authorship
Contribution: Q.Y. performed experiments in all figures except Figures 6A and 7, which were performed by L.B.; Q.Y. and L.B. designed experiments, analyzed the results, and wrote the manuscript; B.E. contributed key ideas to the development of this work; and all authors read and approved the final paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Lisa Borghesi, Department of Immunology, University of Pittsburgh School of Medicine, 200 Lothrop St, Pittsburgh, PA 15261; e-mail: borghesi@pitt.edu.