Abstract
To integrate available clinical and molecular information for cytogenetically normal acute myeloid leukemia (CN-AML) patients into one risk score, 275 CN-AML patients from multicenter treatment trials AML SHG Hannover 0199 and 0295 and 131 patients from HOVON/SAKK protocols as external controls were evaluated for mutations/polymorphisms in NPM1, FLT3, CEBPA, MLL, NRAS, IDH1/2, and WT1. Transcript levels were quantified for BAALC, ERG, EVI1, ID1, MN1, PRAME, and WT1. Integrative prognostic risk score (IPRS) was modeled in 181 patients based on age, white blood cell count, mutation status of NPM1, FLT3-ITD, CEBPA, single nucleotide polymorphism rs16754, and expression levels of BAALC, ERG, MN1, and WT1 to represent low, intermediate, and high risk of death. Complete remission (P = .005), relapse-free survival (RFS, P < .001), and overall survival (OS, P < .001) were significantly different for the 3 risk groups. In 2 independent validation cohorts of 94 and 131 patients, the IPRS predicted different OS (P < .001) and RFS (P < .001). High-risk patients with related donors had longer OS (P = .016) and RFS (P = .026) compared with patients without related donors. In contrast, intermediate-risk group patients with related donors had shorter OS (P = .003) and RFS (P = .05). Donor availability had no impact on outcome of patients in the low-risk group. Thus, the IPRS may improve consolidation treatment stratification in CN-AML patients. Study registered at www.clinicaltrials.gov as #NCT00209833.
Introduction
Acute myeloid leukemia (AML) is characterized by uncontrolled proliferation of hematopoietic progenitor cells, blocked maturation resulting from interruption of normal differentiation pathways, and activation of antiapoptotic pathways. In a large proportion of AML patients, standard cytogenetic analyses can identify specific recurrent chromosomal aberrations that are important for disease pathogenesis, response to therapy, and patient survival.1,2 However, almost half of all AML patients are classified as cytogenetically normal (CN-AML).2 Many efforts have been made to identify genetic mutations (eg, FLT3,3,4 NPM1,5,6 CEBPA,7-10 MLL,11,12 NRAS,13 IDH1/2,14-20 and WT121-23 ) that allow further subclassification and possibly risk-directed therapeutic intervention. In addition to mutations, modulated expression of genes important for proliferation, survival, and differentiation have also been shown to be predictive for CN-AML patient outcome (eg, MN1,24,25 BAALC,26 ERG,27 ID1,28 and WT129,30 ). This molecular heterogeneity of CN-AML is not fully reflected in current classification systems.31,32
Recently, 2 or 3 prognostic markers were combined to define patient subgroups with distinct prognosis. Most importantly, patients with mutated NPM1 and wild-type FLT3 have a favorable prognosis.5 Patients with low ERG, low EVI1, and high PRAME levels were also shown to have a good prognosis.33 In addition, WT1 single nucleotide polymorphism (SNP) rs16754, a recently described prognostic marker in CN-AML patients, identified a favorable subgroup in the NPM1/FLT3 high-risk group.34 The availability of sequence information from whole AML genomes is expected to lead to identification of even more prognostic markers in AML patients. Thus, new approaches are needed whereby the multitude of prognostic information is integrated, allowing individualized outcome prediction for specific treatments. In the present study, our goal was to optimize clinical outcome prediction by integrating a larger number of known biomarkers, as opposed to using individual or small sets of biomarkers, similar to integrative approaches in other malignant diseases.35
We here propose an integrative prognostic risk score (IPRS) for CN-AML patients based on clinical and molecular markers. This integrative approach may become a useful tool to deal with the ever-growing number of prognostic parameters.
Methods
Patients
Diagnostic bone marrow or peripheral blood samples were analyzed from 275 adult patients (age, 17-60 years) with de novo or secondary AML (French-American-British classification, M0-M2, M4-M7) and normal cytogenetics who were entered into the multicenter treatment trials AML SHG 0199 (www.clinicaltrials.gov #NCT00209833, June 1999 to September 2004, n = 181) or AML SHG 0295 (February 1995 to May 1999, n = 94), and for whom diagnostic material was available. Details of the treatment protocols have been previously reported.13,34,36 All patients received intensive, response-adapted double induction and consolidation therapy. Patients with a human leukocyte antigen-matched family donor were to receive an allogeneic transplant in first complete remission (CR) after the first consolidation cycle. Written informed consent was obtained according to the Declaration of Helsinki, and the study was approved by the Institutional Review Board of Hannover Medical School.
As an external validation cohort, diagnostic bone marrow or peripheral blood samples from 293 younger adults (age, 16-60 years) with de novo AML were analyzed. Patients were enrolled on Dutch-Belgian Haemato-Oncology Co-operative Group and Swiss Group for Clinical Cancer Research (HOVON/SAKK) protocols -04, -04A, -29, and -42 (www.hovon.nl).37-40 All trials were approved by the Institutional Review Board of Erasmus University Medical Center. Of the 293 patients, 131 patients (45%) were classified as CN-AML. Of the remaining 162 AML patients, 48 had a core-binding factor leukemia (CBF), 9 had a t(15;17), 13 had a monosomy 7, 13 had a complex karyotype, 11 had aberrations of chromosome band 11q23, 9 had a trisomy 8, 7 had a del(9q), 3 had a t(6;9), 38 had various other aberrations, and in 11 cases the cytogenetic information from diagnosis was not available.
Cytogenetic and molecular analysis
Pretreatment samples from all patients were studied centrally by G- and R-banding analysis. Chromosomal abnormalities were described according to the International System for Human Cytogenetic Nomenclature.41 Mutation analysis for CEBPA,7 FLT3-ITD,3,42 IDH1,14 IDH2,15 MLL-PTD,11 NPM1,5 NRAS,13 and WT134 (exons 7 and 9) were performed as previously described. Molecular analyses of patients enrolled in the HOVON/SAKK protocols were performed as described previously.17
Expression analysis of BAALC, ERG, EVI1, ID1, MN1, PRAME, and WT1 transcript levels
Real-time reverse-transcriptase polymerase chain reactions using patient-derived cDNA were carried out on a StepOne Plus real-time polymerase chain reaction system (Applied Biosystems) using the ABL FusionQuant Standard Kit as an endogenous control (Ipsogen). ERG and PRAME expression levels were quantified using the TaqMan Gene Expression Assays according to the manufacturer's instructions (Applied Biosystems, Assay ID: ERG, Hs01554635_m1; ID1, Hs00357821_g1; PRAME, Hs01022301_m1). Primers and polymerase chain reaction conditions were used for detection of BAALC,26 EVI1,43,44 MN1,25 and WT1.34 mRNA transcript levels were previously described. All reactions were quantified in duplicate. The data were analyzed using the StepOne Plus Software Version 2.0 (Applied Biosystems).
For the 293 patients from HOVON/SAKK protocols, gene expression levels were analyzed from gene expression profiling studies. All samples were analyzed using Affymetrix Human Genome U133Plus2.0 GeneChips (Affymetrix). Labeling, hybridization, scanning, and data normalization were performed as previously described.45
Statistical analysis
The definition of CR, relapse-free survival (RFS), and overall survival (OS) followed recommended criteria.46 The median follow-up time for survival was calculated according to the method of Korn.47 OS endpoints, measured from the date of entry into one of the prospective studies, were death (failure) and alive at last follow-up (censored). RFS endpoints, measured from the date of documented CR, were relapse (failure), death in CR (failure), and alive in CR at last follow-up (censored). Primary analysis was performed on OS. Sensitivity analyses were performed on CR and RFS, and the results are displayed for exploratory purposes.
For MN1, BAALC, ERG, ID1, and WT1 expression analysis, AML samples were dichotomized at the median expression value of patients in the learning set (median normalized copy number). PRAME expression analysis was evaluated using quartiles as cut-off points, as reported previously.33 To determine the expression levels of EVI1, a relative quantification was calculated using the equation 2−ΔΔCt, as previously described.44 Descriptive pairwise comparisons were performed by 2-sided χ2 tests for categorical variables and by 2-sided Mann-Whitney U tests for comparison of 2, or 2-sided Kruskal-Wallis tests for comparison of 3 continuous variables, and are provided for exploratory purposes. Kaplan-Meier curves and 2-sided log-rank tests were used to estimate the distribution of RFS, relapse risk, and OS and to compare differences between survival curves, respectively. For multivariate analysis, a Cox proportional hazards model was constructed for OS, adjusting for the 9 IPRS parameters and donor availability. A conditional backwards-elimination procedure was used to exclude redundant or unnecessary variables. Hazard ratios (HRs) and corresponding 95% confidence intervals (CIs) are presented for descriptive purpose. Two-sided P values less than .05 were considered as significant in the primary analysis and as indicators for a trend in all additional analyses. Statistical analyses were performed with the statistical software package SPSS Version 17.0 (SPSS Science).
Assignment of weights to prognostic markers
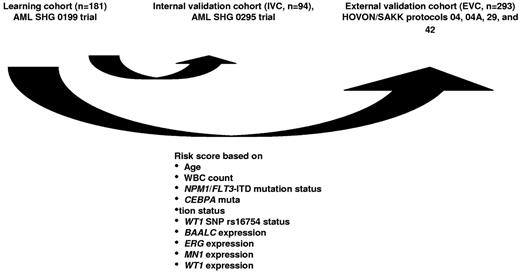
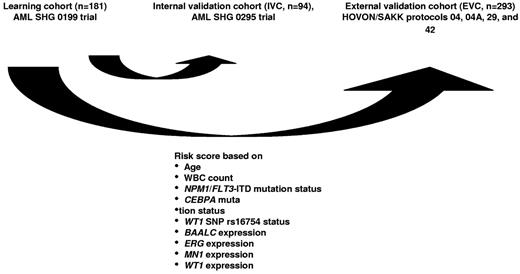
Integer weights for the risk score were derived from Cox proportional hazards modeling applied to the learning set, with OS as the outcome parameter. HRs for OS were calculated for each variable separately. Variables considered for model inclusion were age (dichotomized at < 41 years of age vs 41 years of age or older), white blood cell count, hemoglobin, platelet count, sex, Eastern Cooperative Oncology Group performance status (0 vs 1 or 2), de novo versus secondary AML, CEBPA, FLT3-ITD/NPM1, IDH1, IDH2, MLL-PTD, NRAS, and WT1 mutation status, WT1 SNP rs16754 status, BAALC, ERG, EVI-1, ID1, MN1, PRAME, and WT1 expression status. Variables with P values ≤ .05 in univariate analyses for OS were included in the model. The HRs were converted to integer weights according to the following: variables with HR of 1.2 or less were excluded from consideration, variables with HR ≥ 1.2 to ≤ 1.7 were assigned a weight of 1, variables with HR of > 1.7 and < 2.7 were assigned a weight of 2, and variables with HR ≥ 2.7 were assigned a weight of 3. The IPRS was the sum of these integer weights. Maximally selected log-rank statistics were applied48 to determine optimal cut-offs for risk group assignment. Using these cut-offs, the scores were collapsed into 3 risk groups: 0 to 7 (low-risk), 8 to 10 (intermediate-risk), and more than 10 (high-risk). The scores were then applied to patients in the internal and external validation sets to test their ability to predict HR and survival (Figure 1).
Results
Development of an IPRS in CN-AML patients
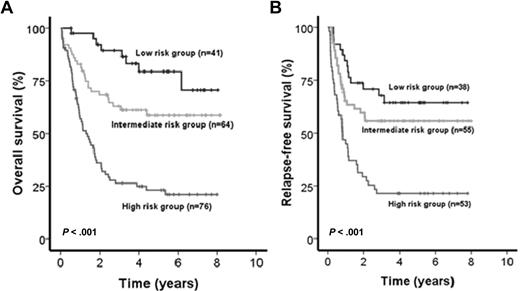
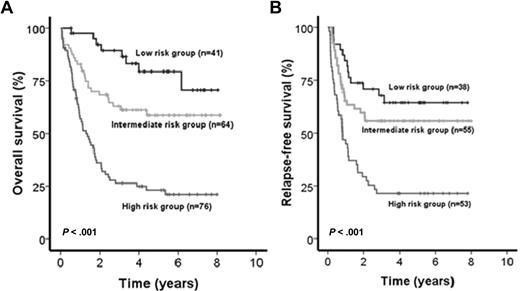
Patients included in the AML SHG 0199 trial (n = 181) were used as a learning set to establish prognostically relevant groups of patients based on different risk scores. Univariate Cox proportional hazards models were computed for OS using candidate prognostic markers (all possible parameters are listed in supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Parameters with P < .05 in univariate analysis for OS were included in the risk score. The IPRS that was derived involved 9 clinical, hematologic, and molecular factors, including age, white blood cell count, mutation status of NPM1, FLT3-ITD, CEBPA, and SNP rs16754, and expression levels of BAALC, ERG, MN1, and WT1 (Figure 1; Table 1). Other markers, such as NRAS, MLL-PTD, WT1, IDH1, or IDH2 mutations, were not significant and thus not included in the IPRS. Patients in the learning set had a median score of 10 (range, 2-15). Patients with 0 to 7, 8 to 10, or 11 to 15 points were assigned to the low-, intermediate-, or high-risk groups, respectively. The median follow-up of patients in the learning cohort was 5.2 years. CR was achieved in the low-risk (n = 41), intermediate-risk (n = 64), and high-risk (n = 76) group in 93%, 86%, and 70% of patients, respectively (P = .005). OS was different in the 3 risk groups (P < .001, Figure 2A), with shorter OS for intermediate-risk patients compared with low-risk patients (P = .029), and shorter OS for high-risk patients compared with intermediate-risk (P < .001) or low-risk patients (P < .001). Using the same cut-off for risk group assignment as for OS, RFS was different for the 3 risk groups (P < .001, Figure 2B).
Impact of the IPRS on survival of patients in the learning cohort. (A) OS and (B) RFS of CN-AML patients according to assignment to the low-, intermediate-, or high-risk groups (2-sided log-rank test).
Impact of the IPRS on survival of patients in the learning cohort. (A) OS and (B) RFS of CN-AML patients according to assignment to the low-, intermediate-, or high-risk groups (2-sided log-rank test).
Validation of the IPRS in 2 independent test populations
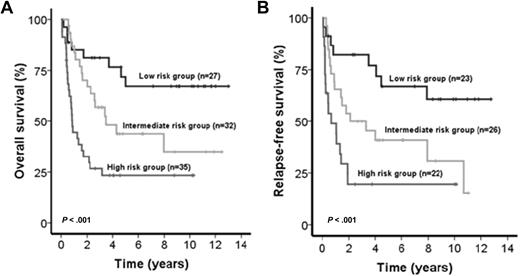
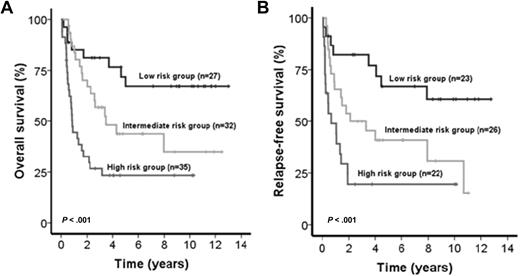
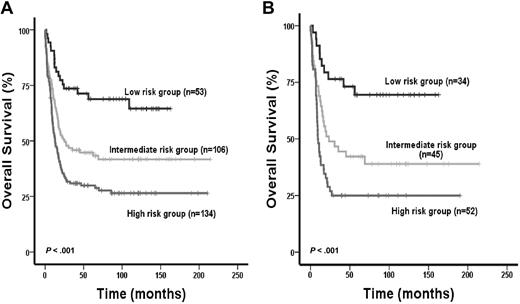
The IPRS was validated in 94 patients treated in the AML SHG 0295 trial (internal validation cohort [IVC]) and 131 patients from the HOVON/SAKK study group (external validation cohort [EVC]). Inclusion criteria and treatment regimens were similar in trials AML SHG 0295 and 0199.13 Baseline characteristics were similar for patients of the learning compared with patients of the 2 validation cohorts (data not shown). The median follow-up of patients in the IVC and EVC was 8.6 and 8.0 years, respectively. Patients from IVC assigned to the low-risk (n = 27), intermediate-risk (n = 32), and high-risk (n = 35) groups achieved CR in 85%, 81%, and 63%, respectively (P = .08). Patients in the 3 risk groups had significantly different OS (P < .001, Figure 3A) and RFS (P < .001, Figure 3B). Intermediate-risk patients showed shorter OS and RFS compared with low-risk patients (OS, HR = 2.4; 95% CI, 1.1-5.5; P = .04; RFS, HR = 2.4; 95% CI, 1.1-5.1; P = .02), and high-risk patients showed shorter OS and RFS compared with intermediate-risk patients (OS, HR = 2.3; 95% CI, 1.2-4.2; P = .001; RFS, HR = 2.2; 95% CI, 1.2-3.9; P = .02) and compared with low-risk patients (OS, HR = 2.1; 95% CI, 1.4-3.7; P < .001; RFS, HR = 2.1; 95% CI, 1.4-3.3; P = .001). The risk of relapse was 17%, 42%, and 59% in the low-, intermediate-, and high-risk groups (P < .001, supplemental Table 1). Cumulative incidence of relapse was higher in intermediate-risk compared with low-risk patients (P = .026) and in high-risk patients compared with intermediate-risk patients (P = .025, supplemental Figure 1). Baseline characteristics of parameters not included in the score and consolidation treatments were similar among the 3 risk groups (supplemental Table 1). As expected, all risk markers included in the score showed a different distribution among the 3 risk groups. However, within one risk group, all 9 risk markers were present with a similar proportion (supplemental Figure 2). Patients from EVC assigned to the low-risk (n = 53), intermediate-risk (n = 106), and high-risk (n = 134) groups achieved CR in 94%, 83%, and 76%, respectively (P = .01). Patients in the 3 risk groups had significantly different OS (P < .001, Figure 4A) and RFS (P < .001). Intermediate-risk patients had shorter OS and a trend for shorter RFS compared with low-risk patients (OS, HR = 2.3; 95% CI, 1.3-3.9; P = .003; RFS, HR = 1.6; 95% CI, 0.96-2.6; P = .07), and high-risk patients had shorter OS and a trend for shorter RFS compared with intermediate-risk patients (OS, HR = 1.5; 95% CI, 1.1-2.1; P = .012; RFS, HR = 1.4; 95% CI, 0.97-2.0; P = .07) and had shorter OS and RFS compared with low-risk patients (OS, HR = 1.9; 95% CI, 1.4-2.4; P < .001; RFS, HR = 1.5; 95% CI, 1.2-1.9; P = .001). When restricting the analysis to CN-AML patients of the EVC (n = 131), 34 patients were assigned to the low-risk group, 45 to the intermediate-risk, and 52 to the high-risk group. CR rates were 97% versus 87 versus 81% in the different risk groups (P = .09). Patients in the 3 risk groups had significantly different RFS (P < .001) and OS (P < .001, Figure 4B).
Impact of the IPRS on survival of patients in the internal validation cohort. (A) OS and (B) RFS of CN-AML patients according to assignment to the low-, intermediate-, or high-risk groups (2-sided log-rank test).
Impact of the IPRS on survival of patients in the internal validation cohort. (A) OS and (B) RFS of CN-AML patients according to assignment to the low-, intermediate-, or high-risk groups (2-sided log-rank test).
Impact of the IPRS on survival of patients in the external validation cohort. OS of all 293 AML patients (A) and of the 131 CN-AML patients (B) according to assignment to the low-, intermediate-, or high-risk groups (2-sided log-rank test).
Impact of the IPRS on survival of patients in the external validation cohort. OS of all 293 AML patients (A) and of the 131 CN-AML patients (B) according to assignment to the low-, intermediate-, or high-risk groups (2-sided log-rank test).
For further validation of the IPRS, the requirement of individual markers for OS prediction was then investigated by removing one marker from the score at a time in the learning cohort. Cut-off values for risk group assignment were adjusted depending on the weight of the removed risk marker. OS and RFS were compared between the 3 risk groups of the IVC. Exclusion of any single risk marker always resulted in inferior separation of the 3 risk groups compared with the model, including all 9 risk markers (data not shown).
Impact of allogeneic transplantation on treatment outcome in CN-AML patients based on the IPRS system
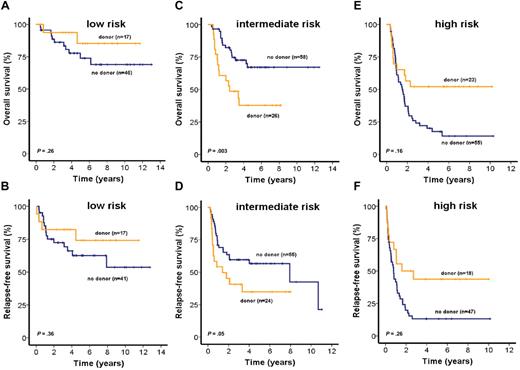
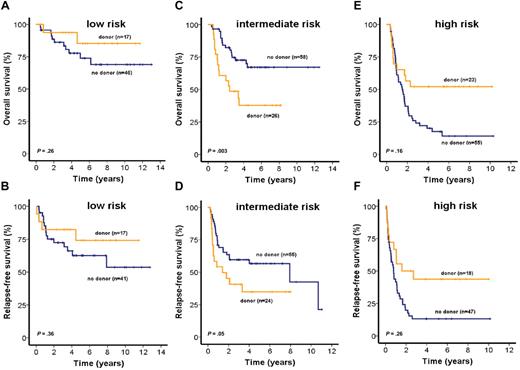
The role of allogeneic stem cell transplantation (alloSCT) for patients in each of the 3 risk groups was investigated by intent-to-treat analysis of all patients for whom information about the availability of a related donor was available (n = 225). To achieve a sufficient number of patients for this analysis, patients from both trials (AML SHG 0199 and 0295) were included. OS and RFS were similar in low-risk patients with donor (n = 17) compared with patients without donor (n = 46; OS, P = .26, RFS, P = .36, Figure 5A-B). Most baseline characteristics were similar between patients with or without a donor. However, the median PRAME expression was higher in patients with a related donor, but PRAME expression had no prognostic impact in this risk group (supplemental Table 2; and data not shown). Sixteen of 17 patients with a related donor actually received an alloSCT in first CR; 9 patients (20%) without a related donor and one (6%) with a related donor received an alloSCT from a human leukocyte antigen-matched unrelated donor in relapse (P = .16, supplemental Table 2).
Impact of matched related donor transplantation on survival of patients in the low-, intermediate-, or high-risk groups (intent-to-treat analysis). OS and RFS of CN-AML patients according to related donor availability for patients assigned to low-risk (A-B), intermediate-risk (C-D), or high-risk (E-F) groups (2-sided log-rank test).
Impact of matched related donor transplantation on survival of patients in the low-, intermediate-, or high-risk groups (intent-to-treat analysis). OS and RFS of CN-AML patients according to related donor availability for patients assigned to low-risk (A-B), intermediate-risk (C-D), or high-risk (E-F) groups (2-sided log-rank test).
Intermediate-risk patients with a related donor (n = 26) had shorter OS and RFS than patients without a related donor (n = 58; OS, P = .003, Figure 5C; RFS, P = .05, Figure 5D). Baseline characteristics in the intermediate-risk group were similar between patients with or without a related donor, except higher number of bone marrow blasts, higher frequency of NRAS mutations, and a higher median expression level of MN1 in patients without a related donor. However, neither MN1 expression nor NRAS mutations had a prognostic impact in this risk group (supplemental Table 2; and data not shown). Twenty intermediate-risk patients (77%) with a related donor actually received an alloSCT, whereas 3 patients (5%) without a related donor received an alloSCT from an unrelated donor in first CR. Nineteen patients (33%) without related donors and 3 (12%) with related donors received an alloSCT from an unrelated donor in relapse (P = .03, supplemental Table 2).
Patients in the high-risk group with a related donor (n = 23) had improved OS (P = .02, Figure 5E) and RFS (P = .03, Figure 5F) compared with patients without related donors (n = 55). Most baseline characteristics for high-risk group patients were similar regardless of donor availability; however, patients with donors were younger than patients without donors (supplemental Table 2). Fourteen patients (61%) with related donors and 3 (5%) without related donors actually received an alloSCT in first CR; 14 patients (25%) without a related donor and 5 patients (22%) with a related donor received an alloSCT from an unrelated donor in relapse (P = .86, supplemental Table 2). To evaluate whether donor availability was an independent predictor of OS, we performed multivariate analysis in each of the 3 risk groups, including donor availability and all 9 risk markers used for the IPRS. In the low-risk group, none of the parameters was independently associated with outcome. In the intermediate-risk group, only donor availability was independently associated with OS (supplemental Table 3). In the high-risk group, OS was independently associated with donor availability, NPM1/FLT3 mutation status, CEBPA mutation status, and WT1 expression (supplemental Table 4).
The impact of alloSCT in the 3 risk groups was also evaluated in an “as-treated” analysis considering alloSCT in first CR. This analysis yielded similar results as the intent-to-treat analysis with a negative impact of alloSCT on OS in intermediate-risk patients and a favorable impact of alloSCT on OS in high-risk patients (supplemental Figure 3). Finally, the impact of alloSCT after relapse was assessed in the 3 IPRS risk groups. OS was not significantly different in any of the 3 risk groups whether patients received an alloSCT after relapse or not (supplemental Figure 4). In intermediate-risk patients, Kaplan-Meier curves suggest that alloSCT after relapse may improve outcome; however, this should be assessed in a larger patient cohort in the future.
Discussion
During the last years, a large number of molecular prognostic markers have been identified in CN-AML. In addition to acquired somatic mutations, modified gene expression patterns have also been shown to impact prognosis of these patients. Availability of gene expression profiling studies and next-generation sequencing of whole genomes will lead to the detection of even more genetic changes of prognostic significance in AML patients. Despite abundant information about the prognostic impact of single markers, only little is known about the interaction of these risk factors and their cumulative effect on patient outcome. The aim of our study was therefore: (1) to generate an IPRS in CN-AML patients 60 years of age or younger based on currently available molecular and clinical markers and (2) to use this score for outcome prediction in the context of current consolidation treatment strategies, especially alloSCT in first CR. We hypothesized that prognostic markers, which are significant in univariate analysis, contain prognostic information that is not represented by any other marker. In our dataset based on 275 intensively treated patients with CN-AML and an additional validation cohort of 293 AML patients, including 131 CN-AML patients, 2 clinical (age, white blood cell count) and 7 molecular markers (mutation/polymorphism status of FLT3/NPM1, CEBPA, WT1 SNP rs16754, expression levels of MN1, BAALC, ERG, and WT1) fulfilled these requirements. In contrast to other reports, mutations of the WT1 gene21-23,34 and expression of PRAME33 had no prognostic impact in our patients and were therefore not included. High expression levels of EVI1, which mainly have prognostic value in AML with cytogenetic aberrations,49 were measurable only in a small proportion of CN-AML patients (8 of 227, 3.5%; data not shown) and were not included in the IPRS. Although CN-AML patients with low ID1 expression (dichotomized at the median expression value) showed a significantly longer OS than patients with high ID1 expression (P = .029) in the AML SHG trials, the ID1 expression status was not included in the IPRS as a strong association between ID1 expression and the CEBPA mutation status was observed (P < .001). This association has already been reported in earlier studies.50,51 In CEBPAwild-type patients, ID1 expression had no influence on patient outcome (RFS, P = .42; OS, P = .25). Based on the additive scores of the 9 markers, 3 risk groups were defined with different OS rates. The IPRS was validated in 2 independent patient cohorts for OS and RFS, indicating that it allows a robust estimation of the patient prognosis. Recently, other risk scores based on molecular markers have been proposed for CN-AML patients.33,52 In contrast to these studies, we analyzed a larger number of more homogeneously treated patients up to the age of 60 years. Moreover, we weighted the different risk factors according to their HR in univariate analysis. Using this score, we defined 3 clearly different risk groups and demonstrated that these factors retain their relative importance in the interaction with other risk factors.
Our second aim was an exploratory analysis of the differential effects of an alloSCT in the defined risk groups. Currently, the prognostic significance of an alloSCT in first CR from matched sibling donors in unselected patients with CN-AML is not clearly defined.53,54 It has been suggested that molecular risk factors, such as mutation of CEBPA, FLT3, and NPM1, can influence outcome of patients undergoing alloSCT.13 Therefore, we evaluated whether the IPRS could predict the efficacy of high-dose chemotherapy with alloSCT from a related donor in first CR compared with consolidation chemotherapy with high-dose cytarabine/daunorubicin or autologous transplantation. Because all patients with a matched sibling donor were scheduled for an alloSCT in first CR, we were able to perform this analysis on an intent-to-treat basis. This analysis revealed striking differences in the impact of donor availability on patient outcome. In the low-risk group, no differences in OS or RFS were detected between the donor and no-donor group. In the high-risk group, donor availability was independently associated with improved OS and RFS. In contrast, donor availability was an independent predictor for shorter OS in intermediate-risk patients, and by trend was associated with shorter RFS. OS and RFS are mainly determined by relapse and treatment-related mortality. Whereas the relapse rate was lower in the donor group, treatment-related mortality was higher than in the no-donor group (data not shown).
Gratwohl et al have developed a predictive score for CML patients to evaluate the risk of alloSCT for transplant-related mortality.55 This score was recently evaluated in other hematologic malignancies, including AML.56 Potential risk factors included in this score are disease stage, age, donor type, time interval from diagnosis to transplantation, and donor-recipient sex combination.56 However, biologic differences within one entity (eg, CN-AML) were not taken into account. Our data indicate that the IPRS may be an additive tool for outcome prediction after alloSCT that reflects the biologic heterogeneity of AML. Moreover, in the intermediate-risk group, survival after relapse in the no donor group was significantly longer, indicating that patients who relapse after chemotherapy have a higher probability for effective salvage strategies (data not shown). Therefore, it might be justified to postpone alloSCT in these patients until after relapse. This effect was not as prominent in the low-risk group, most probably because of the lower relapse rate. In the high-risk group, patients with a related donor had dramatically improved OS and RFS. This indicates that in these high-risk patients, the antileukemic activity of an alloSCT is superior to chemotherapy or autologous transplantation and outweighs the higher treatment-related mortality of this approach. Patients with related donor in the high risk group were younger than patients without related donor. Nevertheless, multivariate analysis for OS, including all 9 IPRS parameters and donor availability, showed that donor availability was an independent prognostic factor in this risk group. However, these observations need confirmation from larger, prospective studies. In the low-, intermediate-, and high-risk IPRS groups, 30%, 61%, and 92% of patients had NPM1/FLT3-ITD high-risk mutation status, respectively. Thus, most patients in the IPRS high-risk group are identified by NPM1/FLT3-ITD mutation status. However, a significant proportion of patients who appear not to benefit from alloSCT can be identified by IPRS but not by NPM1/FLT3-ITD mutation status.
In conclusion, we propose a weighted prognostic risk score of clinical and molecular prognostic markers in younger CN-AML patients who may become useful for outcome prediction of currently available consolidation treatment options. This score may be expanded when new markers are discovered, and it may be used to evaluate the efficacy of novel drug treatments in specific subsets of AML patients.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all patients and members of the HOVON-SAKK and the AML SHG Study Group that participated and our colleagues from the clinical genetics, stem cell transplantation, and molecular diagnostics laboratories of the Erasmus University Medical Center, Rotterdam, The Netherlands for storage of samples and cytogenetic and molecular analyses.
This work was supported by the Hannelore-Munke Fellowship and HiLF 2010 grant (F.D.), the Deutsche-José-Carreras Leukämie-Stiftung eV (grant DJCLS R 10/22 and H 06/04v), the Deutsche Krebshilfe eV (grant 109003), the H. W. & J. Hector Stiftung (grant M 47.1), Kompetenznetz “Akute und chronische Leukämien” (grant 01GI0378), and Bundesministerium für Bildung und Forschung (grants 01KG0605 and 01EO0802).
Authorship
Contribution: F.D., M.H., J.K., and A. Ganser conceived and designed the study; F.D., M.H., K.W., C.R., W.F., M.L., L.K., G.H., R.D., B.L., P.J.M.V., J.K., and A. Ganser provided study materials or patients; F.D., M.H., K.G., M.M., I.H., K.W., F.T., E.S., G.H., R.D., B.L., P.J.M.V., J.K., and A. Ganser collected and assembled data; F.D., M.H., A. Großhennig, P.J.M.V., J.K., and A. Ganser analyzed and interpreted data; F.D., M.H., M.M., A. Großhennig, J.K., and A. Ganser wrote the manuscript; and F.D., M.H., M.M., K.W., K.G., A. Großhennig, I.H., F.T., E.S., W.F., M.L., L.K., C.R., G.H., R.D., B.L., P.J.M.V., J.K., and A. Ganser gave final approval of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Frederik Damm, Department of Hematology, Hemostasis, Oncology, and Stem Cell Transplantation, Hannover Medical School, Carl-Neuberg Strasse 1, 30625 Hannover, Germany; e-mail damm.frederik@mh-hannover.de.
References
Author notes
F.D. and M.H. contributed equally to this study.
J.K. and A. Ganser contributed equally to this study.