Abstract
After allogeneic stem cell transplantation (SCT), T lymphocyte function is reestablished from the donor's postthymic T cells and through thymic T-cell neogenesis. The immune repertoire and its relation to that of the donor have not been characterized in detail in long-term adult SCT survivors. We studied 21 healthy patients in their second decade after a myeloablative SCT for hematologic malignancy (median follow-up, 12 years). Immune profiles were compared with donor samples cryopreserved at transplant and beyond 10 years from SCT. Only one recipient was on continuing immunosuppression. Compared with the donor at transplant, there was no significant difference in CD4, CD8, natural killer, and B-cell blood counts. However, compared with donors, recipients had significantly fewer naive T cells, lower T-cell receptor excision circle levels, fewer CD4 central memory cells, more effector CD8+ cells, and more regulatory T cells. TCR repertoire analysis showed no significant difference in complexity of TCRVβ spectratype between recipients and donors, although spectratype profiles had diverged with both gain and loss of donor repertoire peaks in the recipient. In conclusion, long-term allogeneic SCT survivors have subtle defects in their immune profile consistent with defective thymic function but compatible with normal health. This study is registered at http://www.clinicaltrials.gov as NCT00106925.
Introduction
Ninety percent of allogeneic stem cell transplantation (SCT) recipients who are still living 2 years after SCT will become long-term survivors,1 and as survivors age, more attention must be paid to their long-term status and potential complications.2
After SCT, prolonged immune deficiency and delayed T-cell reconstitution results in significant morbidity and mortality. Full immune recovery after SCT implies a normal distribution and number of lymphocyte subsets and antibody production, in addition to immune competence against infectious agents, immunesurveillance of malignant cells, and absence of active GVHD. Although many studies show that most immune parameters return to a normal range within a few years of SCT, recipient age and occurrence of chronic GVHD (cGVHD) govern the pace and completeness of immune reconstitution.3 In adult SCT recipients, failure of thymic T-cell maturation limits the contribution of new donor-derived naive T cells to the repertoire, which are supplied from long-lived postthymic T cells transfused with the stem cell graft.4 GVHD further impairs full immune recovery, in part through an associated immune imbalance, and in part because GVHD damages the thymus. In addition, immunosuppressive treatment for GVHD impairs immune function.
Despite these barriers to complete normalization of the immune system, many adult recipients become healthy long-term posttransplant survivors. However, there are no comprehensive data on the immune profile of recipients surviving into their second decade after SCT. In particular it is not known how much of the immune repertoire is derived from postthymic T cells and how much it is complemented by new thymic emigrants. Here we describe the immune characteristics of 21 healthy patients surviving into their second decade after myeloablative T-cell–depleted SCT for leukemia. The availability of cryopreserved donor samples from the time of transplant permitted paired comparisons between recipients and donors and revealed greater evolution of the donor T-cell repertoire in the recipient than within the donor during the same time period
Methods
Study design
These data were drawn from an National Heart, Lung, and Blood Institute (NHLBI)/National Institutes of Health Institutional Review Board–approved long-term follow-up study of 121 recipients who received a T-cell–depleted SCT from an HLA-identical sibling donor between 1993 and 2004 (NHLBI 2005-H-0130, registration number: NCT00106925). Consent was obtained in accordance with the Declaration of Helsinki. Forty-two of 121 recipients survived 10 years or longer. Four of these patients died (2 from chronic GVHD, and 2 from cardiovascular causes). Twenty-one recipients and 15 of their donors consented to participate in this study, which required the collection of blood samples for immunologic studies. A cross-sectional analysis of clinical outcomes and T-cell reconstitution was completed in a sample of recipients surviving into their second decade after allogeneic SCT.
Transplantation protocols
All recipients received cyclophosphamide 120 mg/kg and 12- to 13.6-Gy total body irradiation conditioning. Recipients received T-cell–depleted BM (n = 15) or G-CSF mobilized peripheral blood (PBSC) SCT (n = 6) with cyclosporine for GVHD prophylaxis and delayed add-back of donor lymphocytes 30-90 days after transplantation. Acute and chronic GVHD were graded on the basis of published criteria.5,6
T-cell depletion
All transplants were T-cell depleted ex vivo. In the first protocol (93-H-0212), the bone marrow harvest was depleted of T cells by elutriation.7 In protocol 97-H-0099, T cells were depleted by CD34+ selection on the Ceprate SC column (CellPro), followed by CD2 selection on a second column (CellPro). In subsequent protocols, T cells were depleted from the graft by the use of the Isolex 300i immunomagnetic cell selection system (Baxter Healthcare) for positive selection of CD34+ cells, followed by negative selection of T cells by the use of an antibody cocktail of anti-CD2, anti-CD6, and anti-CD7, as previously described.8
Donor lymphocyte infusion
To prevent relapse and facilitate immune reconstitution, 1 or 2 lymphocyte infusions (total dose 1-10 × 106 CD3 cells/kg) were given between days +30 and +90, provided the recipient was not receiving steroid treatment for acute GVHD grade ≥ II.
Reagents for flow cytometry
The following reagents were used for immunophenotypic analyses of leukocyte subsets: (1) αCD14 Pacific Blue, αCD19 Pacific Blue, αCD8-APC Alexa 750, αCD45RO-PE, and ViViD (Live/Dead Fixable Violet Dead cell staining kit; Invitrogen); (2) αCD56-FITC or PECy5.5, αCD57 FITC, α-TCR γδ-PE, αCD19-PECy5, αCD3-PECy7, αCD16-APCCy7, αCD4-allophycocyanin, and αFOXP3 Alexa 647 plus the buffer set (Becton Dickinson Biosciences); and (3) αNKG2A-PE (CD159a), αNKG2D-allophycocyanin (CD314), and αCD27-PECy5 were from Beckman Coulter.
T-cell surface and intracellular staining for flow cytometry
Flow cytometric analysis was performed as described previously.9,10 In brief, PBMCs were thawed, rested overnight at 37°C in RPMI 1640 supplemented with 10% heat-inactivated human AB serum (Gemini Bio-Product), 2mM l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin (Invitrogen) with 50 units DNase I (Roche)/mL and stained with ViViD (Live/Dead Fixable Violet Dead cell staining kit, Invitrogen L34955) for 15 minutes at room temperature. After washing, the cells were stained with the appropriate mAbs for 15 minutes at room temperature, washed again, and acquired on a Canto II flow cytometer (Becton Dickinson Biosciences). For the staining of intracellular markers, the cells were first stained with ViViD and antisurface antigen mAb, fixed, permeabilized, and stained with anti-FoxP3 mAb.11,12 Data were acquired on a Canto II flow cytometer. The data were analyzed with DiVa (BD Biosciences, version 5.0.1). Using this panel of mAb, we defined naive, central memory, central effector, and effector T cells as follows: Regulatory T cells (Tregs), CD4+CD25+FOXP3+; naive T cells, CD27+CD45RO−CD57−; central memory T cells, CD27+CD45RO+CD57−; effector memory T cells, CD27−CD45RO+CD57+/−; effector T cells, CD27−CD45RO−CD57+.
Flow cytometric cell sorting
PBMCs were thawed and rested overnight at 37°C, then stained with ViViD for 15 minutes at room temperature. After a wash step with FACS buffer (PBS with 2% FCS and 0.05% sodium azide), the cells were stained with mAb to CD14, CD19, CD3, CD4, and CD8, and sorted on a Vantage flow cytometer (BD Biosciences). A minimum of 300 000 CD4+ or CD8+ T cells were sorted as dry pellets and into RNA isolation reagent Trizol (Invitrogen) for T-cell receptor excision circle (TREC) and TCR spectratype analysis, respectively.
TREC analysis
TRECs were quantified in sorted CD4+ and CD8+ T cells as described.13 In brief, cells were lysed in 100 μg/mL proteinase K for 1 hour at 56°C, after which the enzyme was heat-inactivated for 10 minutes at 95°C. Real-time quantitative PCR was performed on 5 μL of cell lysate with an ABI7700 system (Applied Biosystems) as previously described.13 A standard curve was generated from pZero Blunt (pCR Blunt) plasmid (Invitrogen), and TREC values for samples were calculated by the ABI7700 software. Samples were analyzed in duplicate.13,14
TCR spectratyping
Total RNA was extracted from the sorted CD4+ and CD8+ T cells, reverse-transcribed, and examined for spectratypes as described.4,15 In brief, cDNA aliquots were amplified to saturation with 1 of the 23 TCRVβ oligonucleotide primers in combination with a fluorescently labeled constant region primer. Amplicons were denatured in gel loading buffer and loaded onto a denaturing polyacrylamide sequencing gel and analyzed in an automated DNA sequencer for size fluorescence intensity determination (spectratyping profile). Applied Biosystems GeneMapper software was used for quantitative repertoire analysis of Vβ subfamilies. Overall complexity within a Vβ subfamily was determined by counting the number of discrete peaks for a Vβ subfamily, with each subfamily graded on a score of 0 to 5.16 Spectratypes containing more than 5 peaks were given a score of 5; spectratypes with 0, 1, 2, 3, or 4 peaks were given a score of 0, 1, 2, 3, or 4, respectively. The maximum complexity score for a sample is 115 (23 Vβ × 5).17 A diversity score was created to describe differences in spectratype between recipient and donor reference samples. A score of 1 was given either for peak heights ≥ 2 × than that of the reference sample or ≤ 1/2 the height of the reference sample. The sum of these scores for CD4 or CD8 T cells is the diver-sity score.
Statistics
Descriptive statistics were used to summarize the demographic and clinical characteristics. The risk factors associated with long-term outcomes in the recipients who survived the first 10 years after SCT were determined by analyses of clinical and transplant variable characteristics of recipients. Two-sided Fisher exact tests or χ2 tests were used in univariate analyses for categorical variables. The paired t test and Mann-Whitney U test were used for continuous variables. Statistical significance was accepted at P < .05. The data analyses were performed with the use of the Prism 4 for Windows (GraphPad Software, Inc).
Results
Patient characteristics
Of 121 recipients enrolled in a long-term evaluation protocol, 21 of 42 patients who survived a median of 12 years (range, 10-15 years) after SCT were studied. The indication for transplant was chronic myelogenous leukemia (n = 17), acute myelogenous leukemia and myelodysplastic syndrome (n = 3), and multiple myeloma (n = 1). All recipients received donor lymphocyte infusion at 30-90 days after transplantation. Recipient characteristics are summarized in Table 1. Median patient age at SCT was 36 years (range, 13-56 years) for recipients and 36 years (range, 16-58 years) for donors. Previously, 6 (29%) had developed acute GVHD (grade 2-IV) and 18 (86%) chronic GVHD (13 limited, 5 extensive; Table 1). Six (29%) recipients were on prolonged immunosuppressive therapy for cGVHD, 3 for up to 5 years, and 2 from 6 to 11 years. One recipient with extensive cGVHD remained on treatment at the time of study. At the time of study all recipients were healthy, with no intercurrent infection or disease relapse.
Quantitative immunoglobulins
Quantitative immunoglobulins were measured in 21 recipients in their second decade after transplant. Median concentrations (±95% confidence interval) for IgG, IgA, and IgM were 1030 mg/dL (952-1209 mg/dL); 198 mg/dL (177-264 mg/dL); and 106 mg/dL (101-157 mg/dL), respectively. All values were well within the normal ranges (IgG, 642-1730 mg/dL; IgA, 91-499 mg/dL; and IgM, 34-342 mg/dL; Table 2).
Lymphocyte counts
Absolute CD3+, CD3+/CD4+, CD3+/CD8+, and NK (CD3−/CD56+) counts were measured in 18 recipients and were within the normal range. CD19+ counts (median, 453/μL) were elevated compared with the normal population (range, 61-321/μL; Table 2).
Comparisons with donor at time of SCT
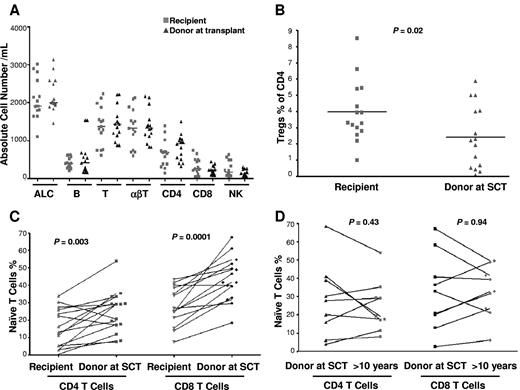
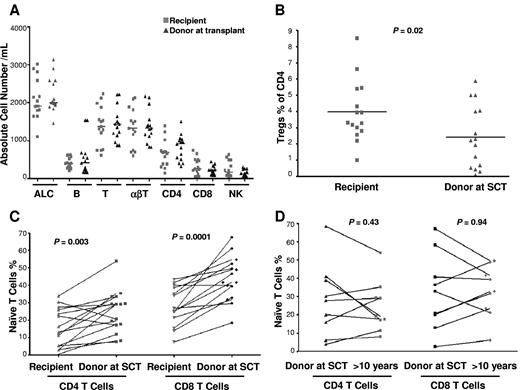
Leukocyte subsets were compared in donor-recipient pairs with the use of donor cells obtained at transplant. There was no significant difference in the total lymphocyte, neutrophil, monocyte, natural killer (NK) cell, CD4+ and CD8+ T cell, and B-cell absolute counts compared with the donor pretransplant (Figure 1A). However, SCT survivors had significant differences in CD4+ and CD8+ T-cell and NK-cell subset composition compared with the donor sample taken at transplant: In the CD4 compartment, recipients had significantly more Tregs (as a percentage of CD4 cells, almost double in the recipient compared with the donor, P = .02; Figure 1B) and significantly fewer naive (P = .003; Figure 1C) and central memory cells (P = .03) compared with their donors. In the CD8 compartment, recipients had more effector (P = .02) and fewer naive cells (P = .0001; Figure 1C). In the NK compartment, there were fewer CD56[int]CD16-NKG2A+NKG2D+ NK cells (P = .02). Recipients with a history of cGVHD had a significant decrease in naive CD8+ T cells (P = .02 and a trend to decreased naive CD4+ T cells (P = .31) compared with those with no cGVHD. The recipient (UPN no. 76) who had extensive cGVHD and who was receiving immunosuppression had the lowest number of naive CD4+ and CD8+ T cells. In 9 instances, donor blood samples contemporaneous with those from transplant survivors in this study were also obtained. In paired comparisons of donor samples taken at transplant and a median of 12 years later there was no significant difference of T-cell phenotypes, including absolute lymphocyte count, naive CD4 and CD8 T cells, NK cells, T-cell subsets, B cells, and Tregs lymphocytes (Figure 1D).
Lymphocytes (T, B, NK, Treg, and naive T cells) in recipients and donors at transplant (15 recipient-donor pairs). (A) Absolute total lymphocyte (ALC), T-, B-, and NK-cell counts. (B) CD4+CD25+FOXP3+ regulatory T cells as a percentage of CD4+ T cells. (C) Paired analysis of naive (CD27+CD45RO−CD57−) CD4+ and CD8+ T cells in recipients and donors at SCT. (D) Paired analysis of naive CD4+ and CD8+ T cells in donors at SCT and donors in the second decade after SCT (9 pairs).
Lymphocytes (T, B, NK, Treg, and naive T cells) in recipients and donors at transplant (15 recipient-donor pairs). (A) Absolute total lymphocyte (ALC), T-, B-, and NK-cell counts. (B) CD4+CD25+FOXP3+ regulatory T cells as a percentage of CD4+ T cells. (C) Paired analysis of naive (CD27+CD45RO−CD57−) CD4+ and CD8+ T cells in recipients and donors at SCT. (D) Paired analysis of naive CD4+ and CD8+ T cells in donors at SCT and donors in the second decade after SCT (9 pairs).
TREC analysis
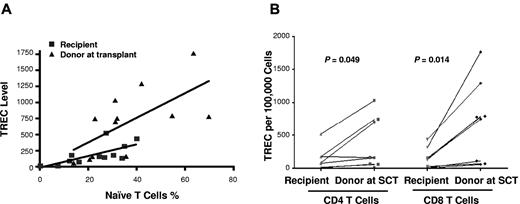
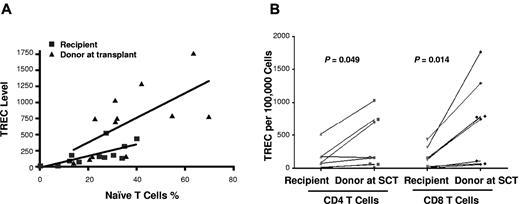
To measure thymic output after SCT, the TREC levels were examined separately for sorted CD4+ and CD8+ T cells in donor-recipient pairs. Samples from 8 donors were sufficient for sorting CD4 and CD8 T cells to compare TREC levels with their individual recipients. TREC levels correlated with numbers of naive CD4+ and CD8+ T cells, with lower TREC levels in the recipients (r = 0.69, P = .01) than that in the donors (r = 0.65, P = .02; Figure 2A), suggesting persisting thymic deficiency. Compared with donor samples taken at SCT, TREC levels were lower in recipient CD4+ (P = .05) and CD8+ (P = .01) T cells (Figure 2B). However, in 3 cases in whom comparisons could be made, there were no significant differences in CD4+ and CD8+ T-cell TREC levels between the contemporaneous donor samples and those taken at SCT (data not shown). However, TREC levels were significantly lower in the CD4+ T cells of recipients with history of GVHD than that of the cohort of recipients without history of GVHD (P = .02). The recipient (UPN no. 76) with extensive cGVHD receiving immunosuppression, had the lowest TREC levels in CD4+ or CD8+ T cells. Although there was a trend to lower TREC levels in older patients, differences in TREC levels in either recipients or donors age > 36 years at SCT compared with younger patients were not significant (data not shown).
TREC analysis for thymic function. (A) Correlation between the naive CD4+ and CD8+ T-cell numbers and TREC levels of recipients (■) and donors (▴) at transplant, with lower TREC levels in the recipients (r = 0.69, P = .01) than that in the donors (r = 0.65, P = .02). (B) TREC levels of CD4+ and CD8+ T cells of recipients and donors at transplant (8 pairs).
TREC analysis for thymic function. (A) Correlation between the naive CD4+ and CD8+ T-cell numbers and TREC levels of recipients (■) and donors (▴) at transplant, with lower TREC levels in the recipients (r = 0.69, P = .01) than that in the donors (r = 0.65, P = .02). (B) TREC levels of CD4+ and CD8+ T cells of recipients and donors at transplant (8 pairs).
TCR repertoire spectratyping
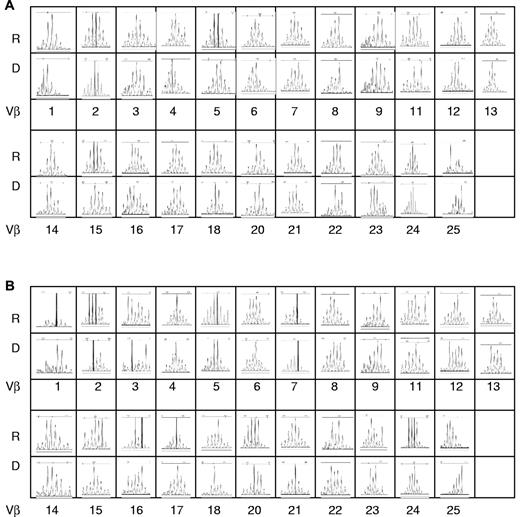
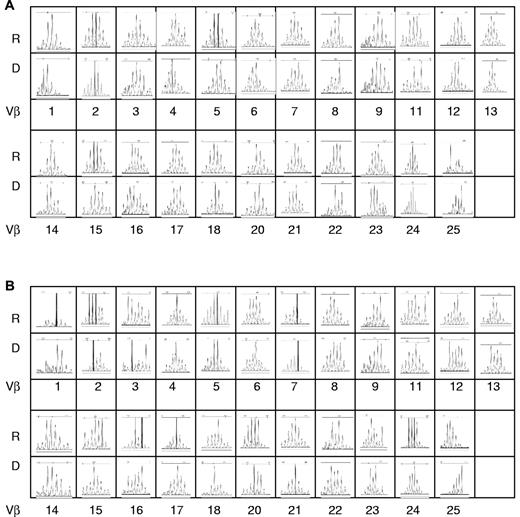
TCR repertoire analysis was used to evaluate thymic function. Total RNA was extracted from sorted CD4+ or CD8+ cells and spectratype profiles of the 23 Vβ subfamilies were analyzed in recipient and their donors at SCT. The samples from 6 donors had sufficient sorted CD4 and CD8 T cells for RNA extraction to compare TCR repertoire with their individual recipients. A representative TCR Vβ spectratype from one donor-recipient pair is shown in Figure 3. The recipient shows a full normal-looking repertoire in the second decade after transplant. The TCRVβ diversity (total complexity score) describes the polyclonality of the T-cell repertoire. There was no significant difference in total complexity scores of T cells between donors at SCT (CD4+, mean ± SD, 101 ± 3; CD8+, mean ± SD, 100 ± 3) and recipients (CD4+, mean ± SD, 104 ± 2; CD8+, mean ± SD: 101 ± 2; Figure 4B).
Representative TCRVβ spectratype from a pair of recipient and donor pair at SCT. The spectratype profiles of the 23 Vβ subfamilies were analyzed. Shown are the CDR3 lengths on the x-axis versus the fluorescence intensity in the y-axis. R indicates recipient; D, donor. (A) CD4+ cells. (B) CD8+ cells.
Representative TCRVβ spectratype from a pair of recipient and donor pair at SCT. The spectratype profiles of the 23 Vβ subfamilies were analyzed. Shown are the CDR3 lengths on the x-axis versus the fluorescence intensity in the y-axis. R indicates recipient; D, donor. (A) CD4+ cells. (B) CD8+ cells.
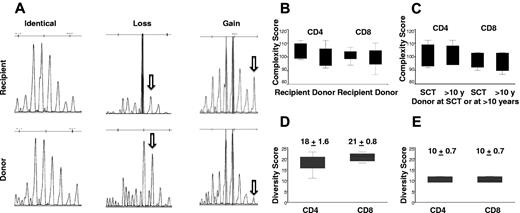
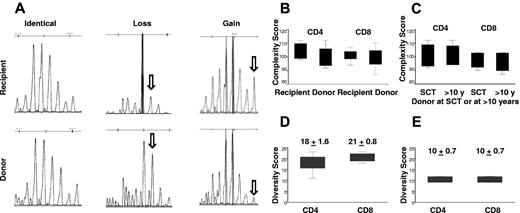
TCRVβ repertoire complexity and diversity scores in recipients and donors. (A) An example of pattern diversity between recipient and donor with both gains and losses of repertoire peaks in the recipient. (B) The TCRVβ diversity (total complexity score) as a measure of the polyclonality of the CD4+ or CD8+ T-cell repertoire. Overall complexity within a Vβ subfamily was determined by counting the number of discrete peaks for a Vβ subfamily, with each subfamily graded on a score of 0 to 5. There was no significant difference in total complexity score of CD4+ (P = .30) or CD8+ (P = .64) T cells between recipients and donors at SCT (6 pairs). (C) There was no significant difference in total complexity score of CD4+ (P = .54) or CD8+ (P = .25) T cells between donors at SCT and donors in the second decade after SCT (4 pairs). (D) There are significant different diversity patterns in TCR Vβ subfamilies between recipients and donors at SCT (6 pairs). The median diversity score for CD4+ and CD8+ T cells was 18 ± 1.6 and 21 ± 0.8, respectively. (E) The median diversity score for CD4+ and CD8+ T cells was 10 ± 0.7 for both CD4+ and CD8+ T cells, between donors at SCT and donors in the second decade after SCT (4 pairs).
TCRVβ repertoire complexity and diversity scores in recipients and donors. (A) An example of pattern diversity between recipient and donor with both gains and losses of repertoire peaks in the recipient. (B) The TCRVβ diversity (total complexity score) as a measure of the polyclonality of the CD4+ or CD8+ T-cell repertoire. Overall complexity within a Vβ subfamily was determined by counting the number of discrete peaks for a Vβ subfamily, with each subfamily graded on a score of 0 to 5. There was no significant difference in total complexity score of CD4+ (P = .30) or CD8+ (P = .64) T cells between recipients and donors at SCT (6 pairs). (C) There was no significant difference in total complexity score of CD4+ (P = .54) or CD8+ (P = .25) T cells between donors at SCT and donors in the second decade after SCT (4 pairs). (D) There are significant different diversity patterns in TCR Vβ subfamilies between recipients and donors at SCT (6 pairs). The median diversity score for CD4+ and CD8+ T cells was 18 ± 1.6 and 21 ± 0.8, respectively. (E) The median diversity score for CD4+ and CD8+ T cells was 10 ± 0.7 for both CD4+ and CD8+ T cells, between donors at SCT and donors in the second decade after SCT (4 pairs).
However, spectratyping profile diversity showed significant differences between recipients and donors. Figure 4A shows an example of pattern diversity between donor and patient with both gains and losses of repertoire peaks in the recipient. The median diversity score for CD4+ and CD8+ T cells was 18 ± 1.6 and 21 ± 0.8, respectively (Figure 4D). The altered but diverse TCRVβ spectratyping profiles indicate considerable TCR repertoire plasticity in the recipient. We also compared changes in the TCRVβ repertoire in 4 donors at transplant and in the second decade after SCT. During the period between sample collections there was no significant difference in total complexity scores of CD4+ cells or CD8+ T cells (CD4+, mean ± SD, 99 ± 4.3 vs 100 ± 4.3; CD8+, mean ± SD, 97.5 ± 3.6 and 99 ± 2.9; Figure 4C). There was evidence of TCR repertoire plasticity with a diversity score of 10.2 ± 0.7 for both CD4+ and CD8+ T cells. However, this finding represented less diversity than was seen between recipient and donor (Figure 4E). A representative TCRVβ spectratype from a donor sample at SCT and more than a decade later is shown in Figure 3.
Discussion
It is well established that immune recovery after SCT is prolonged and incomplete and that T-cell immunity may remain impaired for years after SCT.18 Naive T cells of donor origin appear around 6 months after SCT,19 but restoration of the naive T-cell pool may require 1 to 2 years and may remain incomplete especially in recipients > 45 years.4 Naive T-cell recovery after SCT is much slower in adults than in children, reflecting an age-associated post-SCT thymic rebound.14
Here we describe the first detailed immune profiling of adult transplant recipients in the second decade after SCT. Although absolute values of T cells, NK cells, and immunoglobulins were in the normal range, we found more subtle differences in T-cell and NK-cell subsets when recipients were compared with their donors. Notably, recipients still showed reduced frequencies of naive T cells and memory CD4 T cells. Together with the reduced TREC levels, these findings point to a persisting defect in thymic output in these long-term SCT survivors. These data from adult SCT recipients contrast with the report from Cavazzana-Calvo et al20 in primary T-cell–immunodeficient children who had undergone SCT > 10 years previously. All recipients had persistent thymopoiesis despite myeloablative conditioning with presence of naive T cells carrying TRECs.
Because many adult recipients have a history of GVHD, it was not possible to determine whether age alone inversely correlated with TREC levels.14 Although there was a trend for older recipients to have lower TRECs, there was no significant decrease in TRECs in the donors during the 10-year interval. Both SCT conditioning and GVHD contribute to reduced thymic function. We found that patients with a history of chronic GVHD had decreased numbers of naive T cells and levels of TRECs compared with those with no chronic GVHD, and the single recipient studied with active cGVHD had the lowest TREC levels. These data are consistent with previous reports indicating that thymopoiesis is inhibited by GVHD.14 An interesting observation was that recipients had significantly greater numbers of CD4+CD25+FOXP3+ regulatory T cells. The reason for this is unclear. We did not study the activity of the regulatory T cells detected, and it is unclear whether these Tregs contribute to the relative tolerance observed in these patients. It is possible that Tregs increase in response to donor alloimmune responses and remain high even after cGVHD has become quiescent. It is also possible that the regulatory cells detected caused excess turnover of mature T cells, thereby inducing more rapid dilution of TRECs in these patients. Alternatively, the thymic precursor pool may have been more limited. These Tregs would reduce TRECs irrespective of the status of the thymic microenvironment. However, in one study from Ritz's group,21 they found that the regulatory T-cell frequencies are reduced in patients with active cGVHD, but the function of theses Tregs remains normal.
In the first year after transplantation, TCR repertoires remain abnormal and TREC values low,22 implying that the recovery of the full T-cell receptor repertoire requires restoration of thymic function. Thymopoiesis after SCT has been shown to be critical for the restoration of peripheral CD4 T-cell populations with broad TCR diversity,4 and de novo T-cell generation by the thymus is important in long-term immune reconstitution in recipients after SCT.15 The fully diverse CD4+ and CD8+ T-cell spectratypes in our survivors, in the face of a defective thymic output, raises the question how much the peripheral compartment was diversified by a slow trickle of thymic emigrants during the course of a decade and how much the peripheral T-cell repertoire evolved to meet novel or persisting antigenic challenges (both allogeneic and infectious) in the recipient. A comparison of spectratypes between recipient and donor revealed a further evolution of the repertoire in the recipient, that is, acquisition of new clonotypes and loss of other clonotypes. Consistent with the importance of the recipient allogeneic milieu in molding a new repertoire is the fact that the diversity in the recipient compared with the donor sample taken at SCT was twice as great as the change occurring within the donor during the same time period. This finding would be consistent with both alloantigen,23 and viral antigenic challenge from persisting viruses.24
These observations were made in a group of healthy transplant survivors who, with one exception, were off all immunosuppressive treatment. Nevertheless, although only one patient had active cGVHD, immune alterations related to a history of GVHD appear to persist long term. Such relatively minor abnormalities in immune profile were compatible with good health. However, it remains to be seen whether these defects presage worsening infectious complications when these long-term survivors become older and experience age-related attrition of their immune function. In conclusion, despite the evidence of poor thymic function in long-term SCT recipients the T-cell repertoire appears to have evolved further in the recipients and diversified sufficiently to render these patients immune competent.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the National Institutes of Health intramural research programs of the NHLBI.
National Institutes of Health
Authorship
Contribution: R.Q.L. designed the research, provided clinical care, collected data, performed experiments, analyzed data, and drafted the manuscript; J.J.M. designed and performed experiments, analyzed data, and drafted the manuscript; M.B. provided clinical care, collected data, analyzed data, and drafted the manuscript; B.H. and S.M. designed and performed experiments, analyzed data and commented on the manuscript; B.N.S. and A.S. provided clinical care, collected patient data, and commented on the manuscript; E.K.K. provided clinical care, collected patient data, and commented on the manuscript; N.F.H. and K.K. performed experiments and commented on the manuscript; F.T.H and D.C.D. designed experiments, analyzed data, and commented on the manuscript; and A.J.B. designed and supervised the research, provided clinical care, collected and analyzed data, and drafted the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: A. John Barrett, MD, Stem Cell Allogeneic Transplantation Section Hematology Branch, NHLBI, National Institutes of Health Bldg 10, Hatfield CRC, Rm 3-5330 10 Center Dr MSC 1202, Bethesda, MD 20892-1202; e-mail: barrettj@nhlbi.nih.gov.