Abstract
Myeloid-derived suppressor cells (MDSCs) inhibit adaptive and innate immunity and accumulate in the blood of persons with cancer, chronic inflammation, trauma, infection, and stress. Some of the factors inducing their accumulation are known; however, mechanisms regulating their turnover have not been identified. Mass spectrometry showed prominent expression of apoptosis pathway proteins, suggesting that MDSC turnover may be regulated by Fas-FasL–mediated apoptosis. This hypothesis was confirmed by showing that blood MDSCs induced by 3 mouse tumors were Fas+ and apoptosed in response to Fas agonist in vitro and to activated FasL+ T cells in vivo. FasL-deficient mice contained significantly more blood MDSCs than FasL+/+ mice, and after removal of primary tumors MDSCs regressed in STAT6−/− and CD1−/− mice but not in STAT6−/−FasL−/− or CD1−/−FasL−/− mice. Fas+ macrophages and dendritic cells did not apoptose in response to activated T cells, indicating that Fas-FasL regulation of myeloid cells was restricted to MDSCs. These results identify a new mechanism regulating MDSC levels in vivo and show a retaliatory relationship between T cells and MDSCs in that MDSCs suppress T-cell activation; however, once activated, T cells mediate MDSC apoptosis.
Introduction
Tumor progression in patients and mice is associated with increasing levels of a population of suppressor cells known as myeloid-derived suppressor cells (MDSCs). MDSCs suppress antitumor immunity by blocking the activation of CD4+ and CD8+ T cells,1-3 skewing cytokine production toward a type 2 phenotype,4 inhibiting natural killer–cell cytotoxicity,5,6 promoting the accumulation of immune suppressive regulatory T cells,7,8 and perturbing lymphocyte trafficking.9 As a result, MDSCs are a significant obstacle to cancer immunotherapies that require activation of the host's immune system.
A hallmark of tumor-driven MDSCs is their elevated presence in the BM, spleen, blood, lymph nodes, and primary and metastatic tumor sites.1,10 Their accumulation is attributed to multiple proinflammatory factors, including IL-1β,11,12 IL-6,13 prostaglandin E2 (PGE2),14,15 S100A8/A9 proteins,10,16 GM-CSF,17 and VEGF,18,19 driving their differentiation from hemopoietic progenitor cells. These inflammatory mediators are produced by tumor cells13,20-22 or host cells23,24 or both. In persons with cancer, tumor cells are the predominant inducers because removal of tumor causes a rapid decrease in MDSCs.25,26 In contrast to induction of MDSCs, the factors that regulate MDSC maintenance and turnover are not well understood.
Accumulation of MDSCs associated with tumor growth could be because of various mechanisms, including the presence of excessive growth factors, cytokines, or inflammatory molecules that provide continuous survival signals; absence of signals that normally induce cell death; or nonresponsiveness of MDSCs to death signals. We have conducted a mass spectrometry screen of MDSCs and observed expression of multiple proteins associated with the Fas-FasL (Fas ligand) apoptosis pathway. Therefore, we have explored the role of this pathway in the survival and accumulation of MDSCs. We now report that MDSCs express the death receptor Fas and are killed when Fas is engaged by FasL. Activated T cells expressing FasL mediate apoptosis of MDSCs in vivo, suggesting that Fas-FasL interactions may regulate MDSC homeostasis and that Fas-FasL interactions could be exploited as a strategy to reduce MDSC levels in vivo.
Methods
Mice
BALB/c, transgenic BALB/c DO11.10 (I-Ad-restricted, OVA peptide 323-339–specific), transgenic BALB/c Clone4 (H-2Kd-restricted, influenza hemagglutinin [HA] peptide 518-526–specific), transgenic BALB/c TS1 (I-Ed-restricted, HA peptide 110-119–specific), transgenic C57BL/6 OT-1 (H-2Kb-restricted, OVA peptide 257-264 (SIINFEKL)–specific), gld BALB/c (hereafter called FasL−/−), perforin-deficient BALB/c (Pfp−/−), CD1-deficient BALB/c (CD1−/−), and STAT6-deficient BALB/c mice were bred in the University of Maryland Baltimore County animal facility. BALB/c, C57BL/6, and D011.10 breeding stock and C57BL/6 Fas−/− mice were from The Jackson Laboratory. Other strains were provided by colleagues (see “Acknowledgments”). All animal procedures were approved by the Institutional Animal Care and Use Committee at the University of Maryland Baltimore County.
Mass spectrometry
MDSCs were obtained from the peripheral blood of tumor-bearing mice14 (> 90% Gr1+CD11b+ cells; 5 × 106-107 cells/mouse) and lysed at a final concentration of 0.1% Rapigest acid–cleavable detergent (Waters) in 100mM NH4HCO3, pH 8.4. Cell lysates were digested with sequencing grade–modified trypsin (1:50 trypsin-to-protein ratio; Promega) for 1 hour at 37°C, after which trifluoroacetic acid was added to a final pH of ∼ 3. Lysates were incubated for 1 hour at 37°C, freeze-thawed at −80°C, and microfuged at 13 200 rpm for 5 minutes (Eppendorf 5415 D), and the trifluoroacetic acid–precipitated material was discarded.27 Supernatant fluid containing tryptic peptides was collected and brought to pH 7, and peptide concentration was measured by OD 280. Peptides (30 μg) were desalted (C18 spin cartridges; Pierce) and analyzed with a LTQ-FT Ultra mass spectrometer (ThermoFischer) interfaced with an Agilent 1100 nanoLC system. Tandem mass spectra were searched against the National Center for Biotechnology Information (NCBI) mouse protein database28 with the use of MASCOT data analysis software (v2.1; Matrix Science) with the following conditions: peptide mass tolerance at 10 ppm; fragment mass tolerance at 1.5 Da; a maximum of 2 allowed missed cleavages; and methionine oxidation and disulfide as the variable modifications. Proteins identified by ≥ 2 peptides with a MASCOT MOWSE (molecular weight search) score > 30 were considered reliable identifications.29
STAT6−/−FasL−/−, STAT6−/−Pfp−/−, and CD1−/−FasL−/− mice
STAT6−/− and CD1−/− mice were crossed to BALB/c FasL−/− or Pfp−/− mice, and the F1 generations were intercrossed to generate STAT6−/−FasL−/−, STAT6−/−Pfp−/−, or CD1−/−FasL−/− mice. Offspring were screened with PCR for homozygous deletion of STAT6, CD1, or perforin and the gld mutation.
Antibodies, flow cytometry, confocal microscopy
Fluorescently coupled mAbs to Gr1, CD11b, Fas, FasL, CD69, CD3, CD4, DO11.10 TCR (clone KJ1-26), caspase-3, and isotype control mAbs were from BD PharMingen; 4′,6-diamidino-2-phenylindole (DAPI) was from Invitrogen. Staining for flow cytometry was performed as described.25 Samples were run on a Beckman/Coulter XL or CyAn ADP flow cytometer and analyzed with FCS-Express or Summit software. For microscopy, live MDSCs were adhered to coverslips coated with Cell Tak (BD PharMingen) for 20 minutes at room temperature, washed twice with excess PBS, and fixed for 10 minutes with 2% formaldehyde in PBS at 37°C. For cell surface markers, fixed cells were washed with excess PBS containing 10% FCS (10% PBS) for 20 minutes, then stained in the dark for 1 hour at 4°C, followed by washing with excess 10% PBS. For intracellular staining, cells were incubated for 1 hour at 4°C in the dark with antibodies diluted in 10% PBS containing 0.2% saponin, followed by a 10-minute incubation with DAPI (5μg/mL), and a final wash in 10% PBS. Coverslips were mounted on slides with 10 μL of SlowFade (Invitrogen), visualized at room temperature with a Leica TCS SP5 Broadband Tandem scanning confocal microscope fitted with an HCX PL APO 63×/1.4 NA objective, and analyzed with Leica Image Browser software. Approximately 16 optical slices of 0.4 μm through the z-plane were sequentially collected per field. A minimum of 25 cells/samples was analyzed.
Tumor cells, tumor cell inoculation, surgery, and MDSC collection
AT3, TS/A, and 4T1 mouse mammary carcinoma cells were maintained as described.30-32 Mice were inoculated in the abdominal mammary gland with 7000 4T1 cells. For surgery experiments, primary tumors were excised when they were 5-6 mm in diameter (usually ∼ day 25).32 After surgery mice were killed when they became moribund. Mean survival times were calculated on the basis of the day of killing. Ten to 12 days after surgery some mice were tail bled into 500 μL of a 0.008% heparin solution, RBCs were removed by lysis, and the remaining leukocytes were characterized by flow cytometry.25 Absolute numbers of cells were determined by multiplying the total number of leukocytes in a fixed volume of blood by the percentage of Gr1+CD11b+ cells as assessed by flow cytometry.
Isolation of MDSCs from primary tumor and metastatic lungs
Primary tumors and lungs were cut into small pieces and incubated with shaking at 37°C for 20 minutes in 5 mL of RPMI containing 150 μL of collagenase IV (10 000 U/mL; Worthington) and 2 μL of DNAse (2 mg/mL; Sigma). Digested tissue was dissociated with the use of a GentleMACS (Miltenyi Biotec; “m imp tumor 01.01” program, C tube), followed by an incubation at 37°C with gentle shaking with a controlled environment incubator shaker (New Brunswick Scientific) for 20 minutes, followed by a second GentleMACS treatment. Resulting cells were passed through a 70-μM filter, RBCs were lysed using Gey Solution,25 and centrifuged on Ficoll-Paque Plus (GE Healthcare Biosciences; for 20 minutes at 20°C). Live cells were collected from the interface and washed with PBS. MDSCs were isolated from the mixture by Miltenyi magnetic bead purification with the use of Gr1 antibody (RB6-8C5) and LS MACS columns.25
T-cell suppression
Transgenic DO11.10 or Clone4 splenocytes were cocultured with cognate peptide and irradiated MDSCs (> 90% Gr1+CD11b+ cells) from tumor-bearing mice as described.25
Antibody-induced apoptosis
MDSCs were cultured in tumor cell–conditioned medium for 24 hours with Fas agonistic Jo-2 or isotype mAb (1 μg/mL). Tumor cell–conditioned medium was the supernatant fluids from confluent 4T1 cultures maintained in T-75 flasks with 20 mL of culture medium for 3 days.
Western blots
Western blots were performed per the manufacturer's protocol with the use of mAbs to activated caspase3 or β-actin (eBiosciences). MDSCs were stimulated for 16-20 hours with Jo-2 or isotype control mAb before lysis. Blots were stripped with stripping buffer (Thermo Scientific).
In vitro T-cell activation and MDSC apoptosis
T-25 flasks were coated with 4 mL of anti-CD3 antibody (clone 17A2; 15 μg/mL PBS), incubated overnight at 4°C, and washed with complete RPMI medium (10% FCS, 1% Glutamax, 1% penicillin-streptomycin, and 0.05% gentamycin). Splenic T cells were purified by negative selection with the use of an R&D Systems T-cell enrichment kit according to the manufacturer's directions (> 90% CD3+ as measured by flow cytometry). Purified T cells (2 × 107 in 4 mL of complete RPMI) were added to the coated T flasks and incubated for 6 hours at 37°C or 4°C for activated and resting T cells, respectively. MDSCs (2 × 107 cells/4 mL of complete RPMI) were then added to the T flasks. After a 16-hour incubation at 37°C, cells were stained with trypan blue and counted by hemocytometer, and MDSC apoptosis was determined with a caspase3 apoptosis kit as per the manufacturer's protocol (BD PharMingen).
In vivo T-cell activation and MDSC apoptosis
Transgenic DO11.10 or TS1 mice were injected subcutaneously at the base of the tail with 50 μg of OVA323-339 or HA110-119 peptide (1 mg/mL PBS) emulsified with complete Freund adjuvant (1:1 vol:vol). Spleens were removed 72 hours later, RBCs were removed by lysis, the Gr1+CD11b+ cells were analyzed for activated caspase3, and the CD3+DO11.10+ cells were analyzed for CD69.
In vivo adoptive transfer
Fas−/− or Fas+/+ C57BL/6 mice were injected in the tail vein with 5 × 106 purified CD3+ T cells from OT1 transgenic mice and within 24 hours challenged with OVA peptide (100 μg). Splenocytes were harvested 72 hours later, depleted of RBC, and analyzed for activated caspase3 and CD69.
Statistical analysis
The Student 2-tailed t test was performed with Microsoft Excel 2003. Mann-Whitney and log-rank tests were performed with the Web links http://faculty.vassar.edu/lowry/VassarStats.html and http://bioinf.wehi.edu.au/software/russell/logrank/. P values < .05 were considered significant.
Results
Apoptosis pathway proteins are prominently expressed by MDSCs
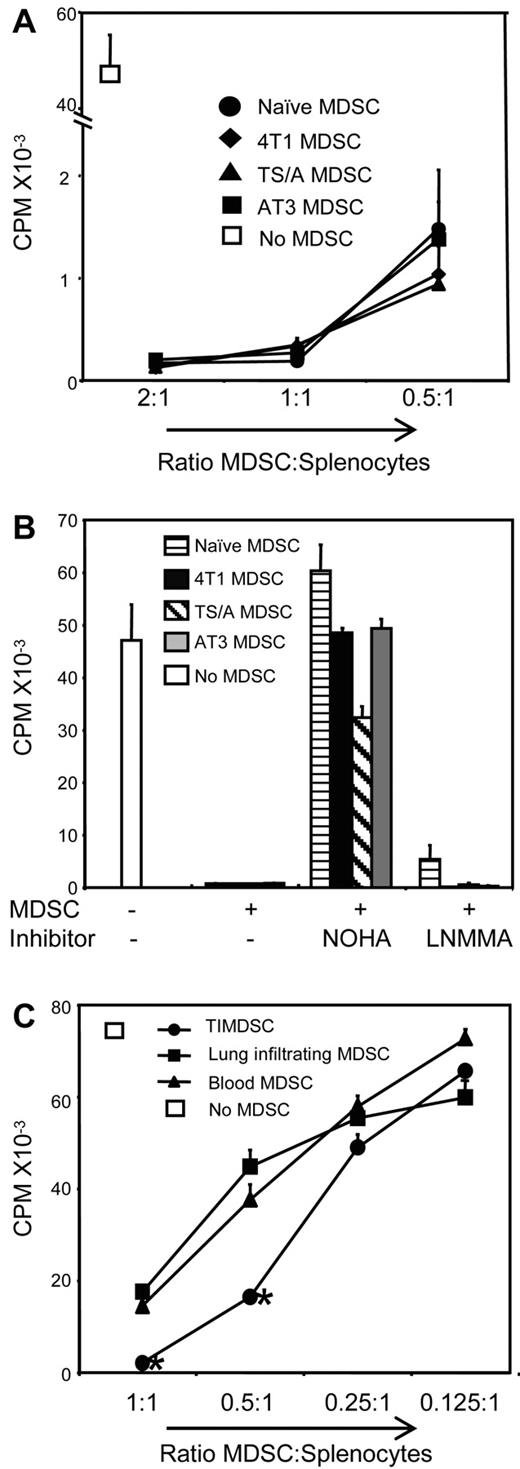
BALB/c mice were inoculated with the 4T1 mammary carcinoma, and Gr1+CD11b+ cells were harvested from the blood 35-41 days later. Phenotypically, the Gr1+CD11b+ cells were polymorphonuclear, CD11b+ Gr1+ Ly6Ghi Ly6Cneg−low IL-4Rαlow Arginase+ iNOS− CD115low F4/80low PDL1low CD80+ CD86+ ROS+ (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). To ascertain that these cells are MDSCs, they were tested for suppressive activity by coculturing with DO11.10 transgenic T cells in the presence of OVA323-339 peptide (Figure 1A). Suppressive activity was also tested in the presence of the inhibitors nor-NOHA (Nω-hydroxy-nor-l-arginine) and L-NMMA (L-NG-monomethyl-l-arginine) (Figure 1B), which inhibit arginase and inducible NO synthase (iNOS), respectively. In the absence of inhibitors, T-cell activation was significantly suppressed, whereas nor-NOHA, but not L-NMMA, partially restored T-cell activation. MDSCs purified from primary 4T1 tumors (tumor-infiltrating MDSCs, TIMDSCs), from lungs with metastatic 4T1 nodules, and from blood were significantly suppressive (Figure 1C), with TIMDSCs being the most suppressive. 4T1-induced blood-derived MDSCs, which, according to their phenotypic and suppressive profile, are granulocytic MDSCs, were used in the subsequent experiments.
4T1, TS/A, and AT3 mammary tumors induce Gr1+CD11b+ immune-suppressive MDSCs in BALB/c and C57BL/6 mice, respectively. (A) Gr1+CD11b+ cells were obtained from the blood of tumor-free BALB/c mice or mice with 4T1, TS/A, or AT3 tumors and incubated at the indicated ratios with splenocytes from DO11.10 transgenic mice and OVA323-339 peptide. T-cell activation was measured by incorporation of 3H-thymidine. MDSCs were pooled from 3-4 mice/group and were > 90% Gr1+CD11b+ cells. (B) MDSCs and splenocytes from panel A were incubated at a 2:1 ratio in the presence of the arginase inhibitor nor-NOHA or the iNOS inhibitor L-NMMA. Average +SD of triplicates. One of 2 independent experiments is shown. (C) MDSCs were purified and pooled from primary tumors (TIMDSCs) of 12 mice or peripheral blood of 3 mice and were incubated at the indicated ratios with splenocytes from Clone4 transgenic mice and HA518-526 peptide.
4T1, TS/A, and AT3 mammary tumors induce Gr1+CD11b+ immune-suppressive MDSCs in BALB/c and C57BL/6 mice, respectively. (A) Gr1+CD11b+ cells were obtained from the blood of tumor-free BALB/c mice or mice with 4T1, TS/A, or AT3 tumors and incubated at the indicated ratios with splenocytes from DO11.10 transgenic mice and OVA323-339 peptide. T-cell activation was measured by incorporation of 3H-thymidine. MDSCs were pooled from 3-4 mice/group and were > 90% Gr1+CD11b+ cells. (B) MDSCs and splenocytes from panel A were incubated at a 2:1 ratio in the presence of the arginase inhibitor nor-NOHA or the iNOS inhibitor L-NMMA. Average +SD of triplicates. One of 2 independent experiments is shown. (C) MDSCs were purified and pooled from primary tumors (TIMDSCs) of 12 mice or peripheral blood of 3 mice and were incubated at the indicated ratios with splenocytes from Clone4 transgenic mice and HA518-526 peptide.
Similar suppression assays were performed for Gr1+CD11b+ cells obtained from the blood of BALB/c mice with TS/A mammary tumors and C57BL/6 mice with AT3 mammary tumors and from tumor-free BALB/c mice (Figure 1A-B). Gr1+CD11b+ cells from these mice were also suppressive, and suppression was partially reversed by nor-NOHA.
As a first step in defining signaling pathways that regulate MDSC activity, we used mass spectrometry to identify proteins that are highly expressed by MDSCs. MDSCs from 4T1 tumor-bearing mice (> 90% Gr1+CD11b+ cells) were lysed, and the resulting lysate was digested with trypsin. After desalting the tryptic peptides were analyzed on a LTQ-FT Ultra mass spectrometer interfaced with an Agilent 1100 nano-liquid chromatograph system. Tandem mass spectra were searched against the NCBI mouse protein database28 with the use of the MASCOT search engine. Seven hundred seventy-seven proteins were identified with ≥ 2 peptides per protein and with MASCOT MOWSE scores of ≥ 30. Four of the 777 proteins belonged to the apoptosis pathway activated by Fas-FasL signaling (Table 1), leading us to hypothesize that the Fas-FasL pathway may regulate MDSC survival.
MDSCs express the death receptor Fas (CD95/APO-1)
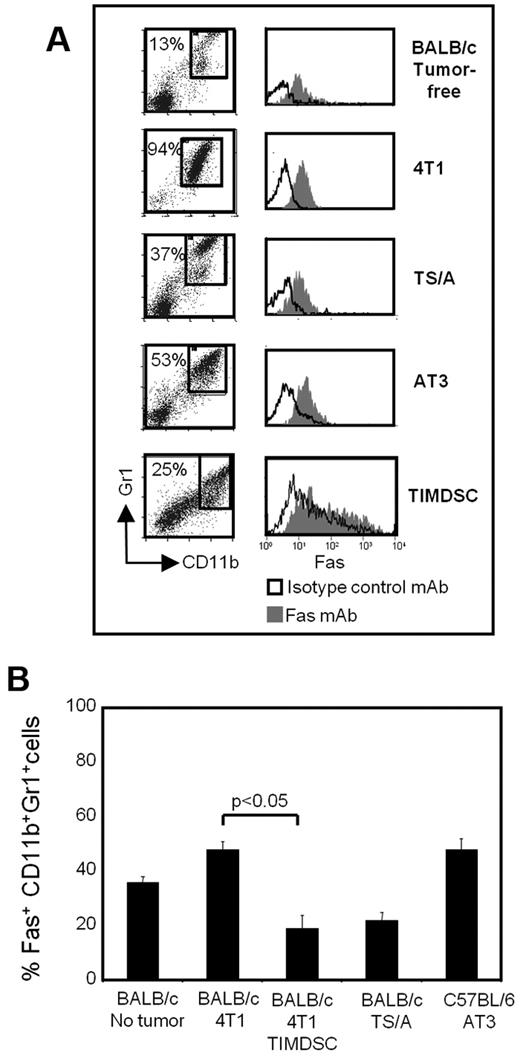
If Fas/FasL-mediated apoptosis contributes to MDSC turnover, then MDSCs will express the death receptor Fas. To test this hypothesis, BALB/c mice were inoculated with 4T1 or TS/A cells, and C57BL/6 mice were injected with AT3 cells on day 0 and bled 28-61 days later when they had large primary tumors (8.24 ± 0.65 mm, 6.66 ± 1.65 mm, and 17.40 ± 3.56 mm in diameter, respectively). Blood was also collected from tumor-free BALB/c mice, and MDSCs were isolated from 4T1 primary tumors. Leukocytes were stained for Gr1, CD11b, and Fas, and the gated Gr1+CD11b+ cells (Figure 2A) were analyzed for Fas expression by flow cytometry (Figure 2B). Gated Gr1+CD11b+ cells from both tumor-bearing and tumor-free mice express Fas.
MDSCs express the death receptor Fas. Leukocytes from the blood of tumor-free BALB/c mice, BALB/c mice with 4T1 or TS/A mammary tumors, C57BL/6 mice with AT3 mammary tumors, and TIMDSCs from BALB/c mice with 4T1 tumors were labeled for Gr1, CD11b, and Fas, and the Gr1+CD11b+ cells were gated and analyzed by flow cytometry for expression of Fas. (A) Flow cytometry of cells from individual mice showing Fas expression on the gated CD11b+Gr1+ cells. (B) Percentage +SD of Fas+CD11b+Gr1+ cells from 2 experiments (n = 6 mice/group).
MDSCs express the death receptor Fas. Leukocytes from the blood of tumor-free BALB/c mice, BALB/c mice with 4T1 or TS/A mammary tumors, C57BL/6 mice with AT3 mammary tumors, and TIMDSCs from BALB/c mice with 4T1 tumors were labeled for Gr1, CD11b, and Fas, and the Gr1+CD11b+ cells were gated and analyzed by flow cytometry for expression of Fas. (A) Flow cytometry of cells from individual mice showing Fas expression on the gated CD11b+Gr1+ cells. (B) Percentage +SD of Fas+CD11b+Gr1+ cells from 2 experiments (n = 6 mice/group).
MDSCs are susceptible to Fas/FasL-mediated apoptosis
The caspase family of cysteine proteases plays an essential role in apoptosis. Caspase3, like other members of the caspase family, is synthesized as an inactive proenzyme. In cells undergoing apoptosis, caspase3 is activated by self-proteolysis or cleavage by another protease or both actions, making activated/cleaved caspase3 a marker for cells undergoing apoptosis.33 To determine whether MDSCs are susceptible to FasL-mediated apoptosis, leukocytes from the blood of BALB/c mice with 4T1 or TS/A tumors, C57BL/6 mice with AT3 tumors, or tumor-free BALB/c mice were incubated with the Fas agonist Jo2 mAb or with an irrelevant control mAb.
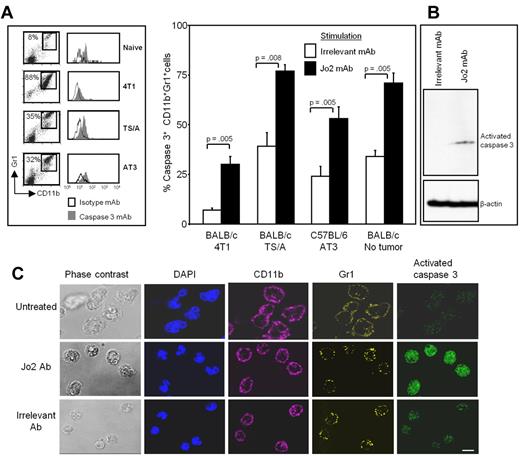
After an incubation of 16-20 hours, the cells were stained for CD11b, Gr1, and activated caspase3, and the gated Gr1+CD11b+ cells were analyzed for activated caspase3. Figure 3A shows the caspase3 staining of Gr1+CD11b+ cells from a representative individual mouse from each group and the pooled results for 6 mice/group. Although control antibody-treated MDSCs from tumor-free and tumor-bearing mice had some background-cleaved caspase3 expression, Jo2 mAb-treated cells from both tumor-bearing and tumor-free mice had significantly higher levels of cleaved caspase3. Jo2-treated MDSCs also contained activated caspase3 as detected by Western blot analysis (Figure 3B). Therefore, MDSCs are susceptible to FasL-induced apoptosis.
MDSCs undergo apoptosis and express activated caspase3 when treated in vitro with the Fas agonist antibody Jo2. Leukocytes from the blood of tumor-free or tumor-bearing mice were cultured for 24 hours with Fas agonist Jo2 antibody or irrelevant control antibody, harvested, and labeled for Gr1, CD11b, and cleaved caspase3, and the Gr1+CD11b+ cells were gated and analyzed for cleaved caspase3. (A) Flow cytometric staining of labeled cells from representative individual mice (left) and percentage of CD11b+Gr1+ cells expressing cleaved caspase3 from 6 mice/group (right). Average +SD of 2 experiments; P values were calculated with the Mann-Whitney test. (B) Jo2 or irrelevant control mAb-treated MDSCs were lysed, and the lysates were electrophoresed on 12% SDS-PAGE gels, transferred to nitrocellulose, and probed for activated caspase3. The blots were stripped and then reprobed for β-actin. (C) Leukocytes from 4T1 tumor-bearing BALB/c mice were treated with Jo2 or irrelevant mAb; stained with DAPI and with mAbs to Gr1 (Alexa700), cleaved caspase3 (FITC), and CD11b (Alexa-647); and analyzed by confocal microscopy. Bar = 10 μm.
MDSCs undergo apoptosis and express activated caspase3 when treated in vitro with the Fas agonist antibody Jo2. Leukocytes from the blood of tumor-free or tumor-bearing mice were cultured for 24 hours with Fas agonist Jo2 antibody or irrelevant control antibody, harvested, and labeled for Gr1, CD11b, and cleaved caspase3, and the Gr1+CD11b+ cells were gated and analyzed for cleaved caspase3. (A) Flow cytometric staining of labeled cells from representative individual mice (left) and percentage of CD11b+Gr1+ cells expressing cleaved caspase3 from 6 mice/group (right). Average +SD of 2 experiments; P values were calculated with the Mann-Whitney test. (B) Jo2 or irrelevant control mAb-treated MDSCs were lysed, and the lysates were electrophoresed on 12% SDS-PAGE gels, transferred to nitrocellulose, and probed for activated caspase3. The blots were stripped and then reprobed for β-actin. (C) Leukocytes from 4T1 tumor-bearing BALB/c mice were treated with Jo2 or irrelevant mAb; stained with DAPI and with mAbs to Gr1 (Alexa700), cleaved caspase3 (FITC), and CD11b (Alexa-647); and analyzed by confocal microscopy. Bar = 10 μm.
MDSCs were also analyzed for cleaved caspase3 by confocal microscopy (Figure 3C). 4T1-induced MDSCs were either untreated (top) or incubated with Jo2 (middle) or irrelevant mAb (bottom) and were externally stained for Gr1 and CD11b and internally stained with DAPI and mAb to cleaved caspase3. Ninety-two percent of Gr1+CD11b+ MDSCs treated with Jo2 mAb were strongly positive for cleaved caspase3, whereas only 5% of control mAb-treated cells were strongly positive. These data confirm that MDSCs apoptose in response to a Fas agonist.
MDSCs apoptose in response to FasL-expressing activated T cells
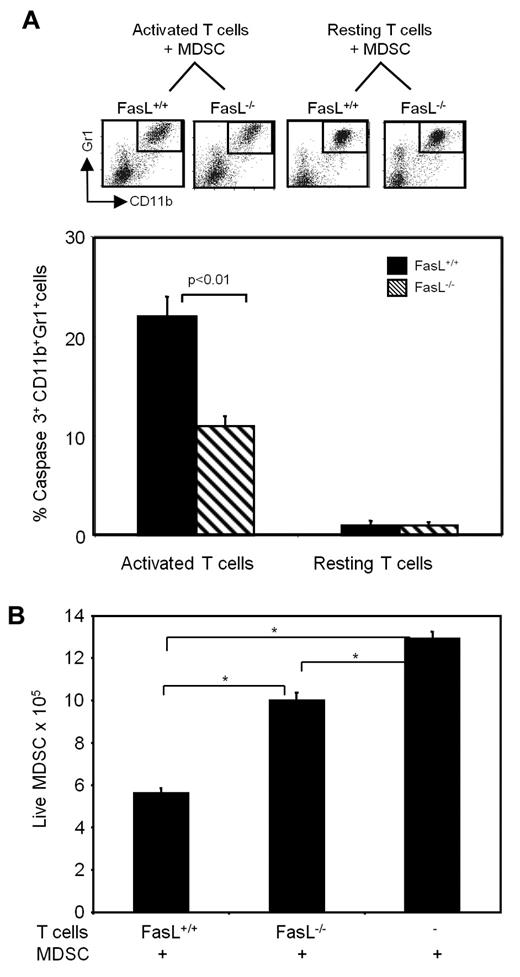
To determine whether MDSCs apoptose in response to a natural ligand by the caspase3 pathway, purified splenic T cells (> 90% CD3+) from BALB/c (FasL+/+) and FasL-deficient BALB/c mice were activated for 6 hours with plate-bound anti-CD3 antibody to induce expression of FasL, after which time MDSCs were added, and the cells were incubated for another 16-20 hours. MDSCs were also cocultured with non-CD3-activated (resting) wild-type BALB/c T cells or FasL−/− T cells. At the end of the culture period, the absolute number of viable cells was determined by counting and trypan blue exclusion, and the cells were then stained with antibodies to Gr1, CD11b and cleaved caspase3. Figure 4A shows the Gr1 and CD11b staining of cells from representative individual mice and the pooled results of cleaved caspase3+ Gr1+CD11b+ cells for the 6 mice/group. Twenty-one percent of MDSCs cocultured with CD3-activated FasL+/+ T cells contained cleaved caspase3, whereas only 12% of MDSCs cocultured with activated FasL−/− T cells contained cleaved caspase3. MDSCs cocultured with resting T cells from either wild-type BALB/c or FasL−/− mice were < 1% caspase3+. Assessment of the absolute number of live MDSCs similarly showed that coculture with FasL+/+ T cells resulted in a significant reduction of surviving MDSCs compared with coculture with FasL−/− T cells (Figure 4B). These results show that activated T cells induce apoptosis of MDSCs.
MDSCs undergo apoptosis by the caspase3 pathway when cocultured with activated FasL+ T cells. Purified splenic T cells from FasL+/+ or FasL−/− mice were incubated in anti-CD3–coated T flasks at 37°C (“activated”) or 4°C (“resting”). Blood MDSCs (> 90% Gr1+CD11b+ cells) were added 6 hours later, and the flasks were incubated for an additional 16 hours. (A) Cells were then stained with mAbs to CD11b, Gr1, and cleaved caspase3. (Top) Gating of Gr1+CD11b+ cells from individual mice. (Bottom) Average percentage of CD11b+Gr1+ cells expressing cleaved caspase3. Data are average + SD of 2 experiments; n = 6 mice/group. (B) At the end of the 16-hour culture, cells were stained with trypan blue, and the absolute number of live CD11b+Gr1+ cells was determined. Cells were pooled from 3 mice/group, data are average + SD of triplicates. *P < .01. Percentage of live MDSCs = (total trypan blue negative cells) × (percentage of Gr1+CD11b+ cells as assessed by flow cytometry).
MDSCs undergo apoptosis by the caspase3 pathway when cocultured with activated FasL+ T cells. Purified splenic T cells from FasL+/+ or FasL−/− mice were incubated in anti-CD3–coated T flasks at 37°C (“activated”) or 4°C (“resting”). Blood MDSCs (> 90% Gr1+CD11b+ cells) were added 6 hours later, and the flasks were incubated for an additional 16 hours. (A) Cells were then stained with mAbs to CD11b, Gr1, and cleaved caspase3. (Top) Gating of Gr1+CD11b+ cells from individual mice. (Bottom) Average percentage of CD11b+Gr1+ cells expressing cleaved caspase3. Data are average + SD of 2 experiments; n = 6 mice/group. (B) At the end of the 16-hour culture, cells were stained with trypan blue, and the absolute number of live CD11b+Gr1+ cells was determined. Cells were pooled from 3 mice/group, data are average + SD of triplicates. *P < .01. Percentage of live MDSCs = (total trypan blue negative cells) × (percentage of Gr1+CD11b+ cells as assessed by flow cytometry).
Resistance to metastatic disease and regression of MDSCs require FasL
We previously reported that BALB/c STAT6−/− and BALB/c CD1−/− mice reject established 4T1 metastatic disease if their primary 4T1 tumors are surgically removed.25,26 Resistance and extension of survival time required elimination of M2 macrophages and MDSCs and activation of tumor-specific T cells. Because activated T cells mediate MDSC apoptosis in vitro (Figure 4), the rapid and virtually complete regression of MDSCs in STAT6−/− and CD1−/− mice could be mediated by Fas-FasL interactions. To test this hypothesis we created STAT6−/−FasL−/− and CD1−/−FasL−/− mice by crossing STAT6−/− or CD1−/− mice with FasL−/− mice and then intercrossing the F1 heterozygotes. The homozygous knockout genotypes of the 2 strains were ascertained by PCR (supplemental Figure 2).
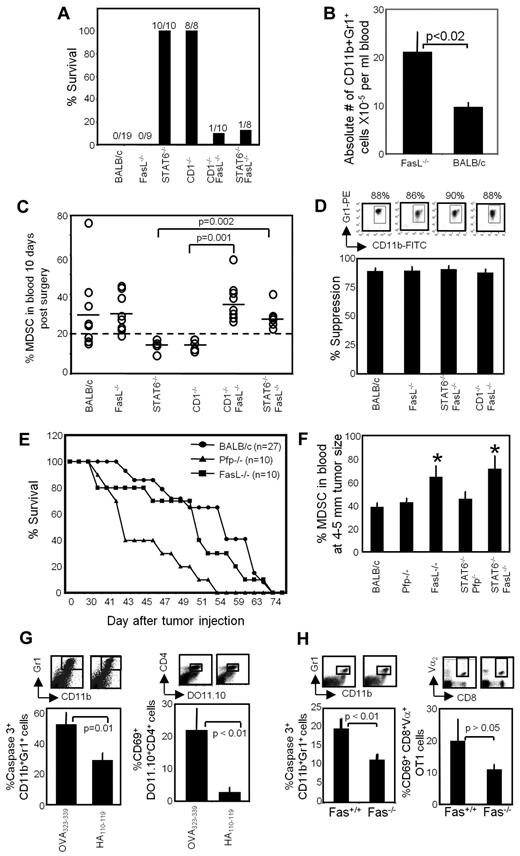
Groups of STAT6−/−FasL−/− and CD1−/−FasL−/− mice along with STAT6−/−, CD1−/−, FasL−/−, and syngeneic BALB/c mice were inoculated in the abdominal mammary gland with 4T1 tumor cells, and the tumors were allowed to progress for 3-4 weeks until they were 5.31 ± 1.61, 5.35 ± 1.23, 5.29 ± 1.35, 5.33 ± 1.24, 5.26 ± 1.47, and 5.42 ± 1.54 mm, respectively, in diameter and metastatic disease was established. The primary tumors were then surgically removed, and the mice were followed for > 247 days (Figure 5A). All STAT6−/− and CD1−/− mice after surgery were resistant to metastatic disease. In contrast, only 10% and 12.5% of STAT6−/−FasL−/− and CD1−/−FasL−/− mice, respectively, survived > 247 days. Mean survival times for the 90% of STAT6−/− FasL−/− and 87.5% of CD1−/−FasL−/− mice that died were 63 ± 10 and 56 ± 7 days, respectively. BALB/c and FasL−/− mice died with mean survival times of 54 ± 9 and 54 ± 10 days, respectively. Therefore, host cell expression of FasL is essential for survival and rejection of metastatic mammary carcinoma in a postsurgery setting.
Resistance to metastatic disease and MDSC regression require FasL. (A) Resistance to metastatic disease requires host cell expression of FasL. BALB/c, FasL−/−, STAT6−/−, CD1−/−, CD1−/−FasL−/−, and STAT6−/−FasL−/− mice were inoculated on day 0 in the mammary gland with 7000 4T1 tumor cells. Primary tumors were removed 3-4 weeks later when metastatic disease was established, and mice were followed after surgery for survival. Numbers indicate the number of mice surviving > 247 days/total number of mice/group. Data are the pooled results of 2 independent experiments. (B) FasL−/− mice contain elevated levels of MDSCs. Data are the average + SD of 5 mice/group. (C) MDSC regression requires host cell expression of FasL. The BALB/c, FasL−/−, STAT6−/−, CD1−/−, CD1−/−FasL−/−, and STAT6−/−FasL−/− mice of Figure 5A were bled 10-12 days after removal of their primary tumors, and the percentage of Gr1+CD11b+ MDSCs in the blood was determined by flow cytometry. Dotted line represents the average percentage of Gr1+CD11b+ cells in the blood of tumor-free mice; solid lines represent the average percentage of Gr1+CD11b+ cells in each group. Each ○ represents an individual mouse. Data are pooled from 2 independent experiments. (D) Gr1+CD11b+ cells from postsurgery BALB/c, FasL−/−, STAT6−/−FasL−/−, and CD1−/−FasL−/− mice are equally suppressive. Gr1+CD11b+ cells from the peripheral blood of 4T1 tumor–bearing mice were cocultured with DO11.10 splenocytes and cognate (OVA323-339) peptide. T-cell activation was assessed by 3H-thymidine incorporation. Average +SD of 4 independent experiments for BALB/c and 2 independent experiments for FasL−/−, CD1−/−FasL−/−, and STAT6−/−FasL−/−mice. (E) Postsurgery resistance to 4T1-induced metastatic disease is perforin dependent. BALB/c, FasL−/−, and Pfp−/− mice were inoculated on day 0 in the abdominal mammary gland with 7000 4T1 tumor cells. Primary tumors were removed 3-4 weeks later when metastatic disease was established, and mice were followed after surgery for survival. Data are pooled from 5 independent experiments for BALB/c (n = 27 mice) and from 3 independent experiments for Pfp−/− and FasL−/− mice (n = 10 mice for each group). Pfp−/− mice survived significantly shorter than wild-type and FasL−/− mice (P < .01 by log-rank test). (F) FasL is required for MDSC reduction. BALB/c, Pfp−/−, FasL−/−, STAT6−/−Pfp−/−, and STAT6−/−FasL−/− mice were inoculated in the abdominal mammary gland with 7000 4T1 tumor cells. When primary tumors were 4-5 mm in diameter, the mice were bled, and the percentage of Gr1+CD11b+ MDSCs in the peripheral blood was determined by flow cytometry. *Statistically significantly different groups (P < .05); Average +SD of 3 mice/group. (G) MDSCs apoptose in vivo in response to activated T cells. DO11.10 transgenic mice were injected subcutaneously on day 0 with cognate peptide (OVA323-339) or irrelevant peptide (HA110-119). Splenocytes were collected on day 3, depleted for RBCs, and stained for Gr1, CD11b, and cleaved caspase3 or for CD4, DO11.10 TcR, and CD69. (Top) Gated Gr1+CD11b+ and CD4+DO11.10+ cells from representative individual mice; (bottom) percentage of CD11b+Gr1+cleaved caspase3+ and percentage of activated (CD69+) CD4+DO11.10+ cells. Average +SD of 5 mice/group. Data are from 1 of 2 independent experiments. (H) Fas+/+ but not Fas−/− MDSCs apoptose in vivo in response to activated T cells. C57BL/6 Fas+/+ and Fas−/− mice were adoptively transferred with CD8+ OT-1 T cells and challenged subcutaneously with cognate peptide. Splenocytes were harvested on day 3 and analyzed according to Figure 5G for percentage of caspase3+ MDSCs and for percentage of activated (CD69+) OT-1 T cells. Average +SD of 4 mice/group.
Resistance to metastatic disease and MDSC regression require FasL. (A) Resistance to metastatic disease requires host cell expression of FasL. BALB/c, FasL−/−, STAT6−/−, CD1−/−, CD1−/−FasL−/−, and STAT6−/−FasL−/− mice were inoculated on day 0 in the mammary gland with 7000 4T1 tumor cells. Primary tumors were removed 3-4 weeks later when metastatic disease was established, and mice were followed after surgery for survival. Numbers indicate the number of mice surviving > 247 days/total number of mice/group. Data are the pooled results of 2 independent experiments. (B) FasL−/− mice contain elevated levels of MDSCs. Data are the average + SD of 5 mice/group. (C) MDSC regression requires host cell expression of FasL. The BALB/c, FasL−/−, STAT6−/−, CD1−/−, CD1−/−FasL−/−, and STAT6−/−FasL−/− mice of Figure 5A were bled 10-12 days after removal of their primary tumors, and the percentage of Gr1+CD11b+ MDSCs in the blood was determined by flow cytometry. Dotted line represents the average percentage of Gr1+CD11b+ cells in the blood of tumor-free mice; solid lines represent the average percentage of Gr1+CD11b+ cells in each group. Each ○ represents an individual mouse. Data are pooled from 2 independent experiments. (D) Gr1+CD11b+ cells from postsurgery BALB/c, FasL−/−, STAT6−/−FasL−/−, and CD1−/−FasL−/− mice are equally suppressive. Gr1+CD11b+ cells from the peripheral blood of 4T1 tumor–bearing mice were cocultured with DO11.10 splenocytes and cognate (OVA323-339) peptide. T-cell activation was assessed by 3H-thymidine incorporation. Average +SD of 4 independent experiments for BALB/c and 2 independent experiments for FasL−/−, CD1−/−FasL−/−, and STAT6−/−FasL−/−mice. (E) Postsurgery resistance to 4T1-induced metastatic disease is perforin dependent. BALB/c, FasL−/−, and Pfp−/− mice were inoculated on day 0 in the abdominal mammary gland with 7000 4T1 tumor cells. Primary tumors were removed 3-4 weeks later when metastatic disease was established, and mice were followed after surgery for survival. Data are pooled from 5 independent experiments for BALB/c (n = 27 mice) and from 3 independent experiments for Pfp−/− and FasL−/− mice (n = 10 mice for each group). Pfp−/− mice survived significantly shorter than wild-type and FasL−/− mice (P < .01 by log-rank test). (F) FasL is required for MDSC reduction. BALB/c, Pfp−/−, FasL−/−, STAT6−/−Pfp−/−, and STAT6−/−FasL−/− mice were inoculated in the abdominal mammary gland with 7000 4T1 tumor cells. When primary tumors were 4-5 mm in diameter, the mice were bled, and the percentage of Gr1+CD11b+ MDSCs in the peripheral blood was determined by flow cytometry. *Statistically significantly different groups (P < .05); Average +SD of 3 mice/group. (G) MDSCs apoptose in vivo in response to activated T cells. DO11.10 transgenic mice were injected subcutaneously on day 0 with cognate peptide (OVA323-339) or irrelevant peptide (HA110-119). Splenocytes were collected on day 3, depleted for RBCs, and stained for Gr1, CD11b, and cleaved caspase3 or for CD4, DO11.10 TcR, and CD69. (Top) Gated Gr1+CD11b+ and CD4+DO11.10+ cells from representative individual mice; (bottom) percentage of CD11b+Gr1+cleaved caspase3+ and percentage of activated (CD69+) CD4+DO11.10+ cells. Average +SD of 5 mice/group. Data are from 1 of 2 independent experiments. (H) Fas+/+ but not Fas−/− MDSCs apoptose in vivo in response to activated T cells. C57BL/6 Fas+/+ and Fas−/− mice were adoptively transferred with CD8+ OT-1 T cells and challenged subcutaneously with cognate peptide. Splenocytes were harvested on day 3 and analyzed according to Figure 5G for percentage of caspase3+ MDSCs and for percentage of activated (CD69+) OT-1 T cells. Average +SD of 4 mice/group.
If FasL regulates homeostatic levels of Gr1+CD11b+ cells, then FasL−/− mice should have more Gr1+CD11b+ cells than wild-type BALB/c mice. To test this hypothesis, the absolute number of Gr1+CD11b+ cells in the blood of tumor-free BALB/c and FasL−/− mice was determined (Figure 5B). FasL−/− mice have ∼ 2-fold more blood Gr1+CD11b+ cells than FasL+/+ mice, consistent with the concept that FasL regulates MDSC levels in vivo.
If regression of MDSCs in tumor-bearing mice is regulated by FasL, then postsurgery STAT6−/− and CD1−/− mice will have fewer MDSCs than postsurgery STAT6−/−FasL−/−, CD1−/−FasL−/−, and BALB/c mice. To test this possibility, postsurgery STAT6−/−FasL−/−, CD1−/−FasL−/−, STAT6−/−, CD1−/−, FasL−/−, and BALB/c mice were bled 10-12 days after removal of primary tumor, and the percentage of Gr1+CD11b+ cells in the blood was determined (Figure 5C). As previously reported,25 Gr1+CD11b+ cells in the blood regress to background levels (< 20% Gr1+CD11b+ cells) in STAT6−/− and CD1−/− mice but remain elevated in BALB/c mice.25,26 In contrast, Gr1+CD11b+ cells remain elevated in STAT6−/−FasL−/− and CD1−/−FasL−/− mice. Gr1+CD11b+ cells from all of the groups are equally suppressive in a suppression assay with the use of DO11.10 transgenic T cells and OVA323-339 peptide (Figure 5D), showing they are MDSCs. These data show that the susceptibility of the STAT6−/−FasL−/− and CD1−/−FasL−/− mice is because of the absence of FasL and suggest that FasL+ cells contribute to the in vivo regulation of MDSCs.
FasL expression is essential for reducing MDSCs
We previously demonstrated that rejection of 4T1 metastatic disease after surgical removal of primary tumor required CD8+ T cells and concurrent reduction of MDSCs.25,34 These observations raised the possibility that FasL is critical for tumor rejection because activated T cells eliminate 4T1 tumor cells through Fas-FasL interactions. If T cells kill tumor cells by a Fas-FasL mechanism, then tumors should progress more rapidly in FasL−/− mice than in wild-type BALB/c mice. To test this possibility, BALB/c and BALB/c FasL−/− mice were inoculated in the mammary fat pad with 4T1 cells, primary tumors were removed when metastatic disease was established, and the mice were followed for survival. Pfp−/− BALB/c mice were included to determine whether the required CD8+ T cells eliminated tumor through a perforin-dependent or a Fas-FasL–dependent mechanism (Figure 5E). Mean survival times of the wild-type BALB/c and FasL−/− mice were not significantly different (55 ± 9 and 53 ± 10 days, respectively), whereas the mean survival time of Pfp−/− mice was significantly shorter (45 ± 5 days). To determine whether perforin regulates MDSC levels, we generated STAT6−/−Pfp−/− mice (see supplemental Figure 2 for genotyping) and measured the percentage of MDSCs in BALB/c, FasL−/−, Pfp−/−, STAT6−/−Pfp−/−, and STAT6−/−FasL−/− mice (Figure 5F). MDSC levels in BALB/c, Pfp−/−, and STAT6−/−Pfp−/− mice were significantly reduced relative to MDSC levels in FasL−/− and STAT6−/−FasL−/− mice. Therefore, FasL controls MDSC levels, whereas perforin regulates T cell–mediated tumor cell elimination. To confirm that Fas-FasL interactions do not directly affect tumor cell proliferation, 4T1 cells were tested by flow cytometry for Fas and treated with the Fas agonist antibody Jo2 or an irrelevant mAb (supplemental Figure 3). Although 4T1 tumor cells express Fas, they do not apoptose when treated with Jo2. Therefore, perforin is required for tumor cell killing, whereas FasL is essential for depletion of MDSCs.
MDSCs apoptose in vivo in response to activated T cells
Activated T cells and tumor cells are the main expressors of FasL in vivo.35 To determine whether tumor cells regulate MDSC levels by FasL, we stained 4T1 primary and metastatic tumor cells and Gr1+CD11b+ cells from naive and 4T1 tumor–bearing mice for FasL (supplemental Figure 4). None of these cells expressed FasL. To determine whether T cells were the relevant population, we activated T cells in vivo in tumor-free mice and assessed the apoptotic levels of naturally occurring Fas+Gr1+CD11b+ cells (see Figure 1A). Tumor-free mice were used because of the difficulty of activating T cells in tumor-bearing mice with elevated levels of MDSCs. Transgenic DO11.10 mice were injected with their cognate peptide (OVA323-339) or an irrelevant peptide (HA110-119). Splenic leukocytes were harvested 3 days later and stained for CD11b, Gr1, and cleaved caspase3, and the gated Gr1+CD11b+ cells were analyzed for cleaved caspase3 (Figure 5G). To ascertain that the transgenic T cells were activated, splenocytes were also stained for CD4, DO11.10, and the activation marker CD69, and the gated CD4+DO11.10+ cells were analyzed for CD69 expression. Approximately 20% of CD4+DO11.10+ cells from OVA323-339-injected mice were specifically antigen activated, and ∼ 52% of the Gr1+CD11b+ cells expressed cleaved caspase3. In contrast, < 4% of CD4+DO11.10+ cells from HA110-119-injected mice were CD69+, and only 29% of the Gr1+CD11b+ cells expressed cleaved caspase3. These results indicate that MDSCs undergo apoptosis in vivo in the presence of activated T cells.
To further test if MDSCs apoptose by Fas-FasL interactions, we compared the in vivo apoptotic rate of Fas+/+ versus Fas−/− MDSCs. Wild-type C57BL/6 and Fas−/− C57BL/6 mice were adoptively transferred with OT-1 T cells, the mice were challenged with cognate peptide, and the percentage of activated caspase3+ Gr1+CD11b+ cells in the spleen was determined (Figure 5H). Significantly more MDSCs in adoptively transferred Fas+/+ recipients were caspase3+ than with Fas−/− recipients, confirming that MDSCs apoptose in vivo in response to FasL+-activated T cells.
To determine whether Fas-FasL interactions mediate apoptosis of other myeloid cells, splenocytes were stained with mAbs to Fas, F4/80, CD11c, CD3, and B220, and the gated F4/80+CD11c−CD3−B220− macrophages and F4/80−CD11c+CD3−B220− dendritic cells (DCs) were analyzed for Fas (supplemental Figure 5A). Macrophages and DCs both express Fas. To determine whether macrophages and DCs apoptose in vivo in response to activated FasL+ T cells, transgenic TS1 mice were inoculated with cognate (HA110-119) or nonspecific (OVA323-339) peptide as in Figure 5G, and splenic macrophages and DCs were analyzed for activated caspase3 (supplemental Figure 5B). Neither cell population had significant numbers of apoptotic cells. Therefore, Fas-FasL–mediated apoptosis regulates the level of MDSCs but does not affect the level of other myeloid cells in vivo.
Discussion
Gr1+CD11b+ MDSCs have the phenotype of immature myeloid cells and are progenitors for DCs, macrophages, and granulocytes. In healthy mice, Gr1+CD11b+ cells differentiate into these normal cell types. However, under chronic inflammatory conditions1 during periods of stress,36 in aged persons,37 and in persons with infections,38 cancer,39,40 or autoimmunity,41 MDSC levels are elevated. Proinflammatory mediators such as IL-1β,11,12 PGE2,14,15 IL-6,13,42 complement component C5a,43 VEGF,20 and GM-CSF17 are inducers of MDSCs. Our findings that MDSCs express the death receptor Fas and apoptose by activated FasL+ T cells identify a new mechanism regulating MDSC turnover. These results also lead to the surprising conclusion that MDSCs and T cells engage in a retaliatory relationship in that MDSCs suppress T-cell activation; however, once activated, T cells mediate MDSC apoptosis.
Fas is constitutively or inducibly expressed by many cells and tissues,44 and Fas-FasL interactions are well established as critical regulatory factors for lymphocyte homeostasis.45 However, a role for Fas-FasL interactions in the regulation of myeloid lineage cells has not previously been reported. The studies reported here suggest that Fas-FasL–mediated apoptosis may be involved in the homeostatic regulation of Gr1+CD11b+ myeloid cells in healthy persons. Because the immune system is routinely exposed to antigen, persons maintain a low level of FasL+-activated T cells. Through Fas-FasL interactions, these T cells could contribute to the homeostatic regulation of MDSCs. The finding that tumor-free FasL−/− mice have elevated levels of Gr1+CD11b+ cells compared with wild-type mice supports this concept and shows that FasL−/− mice not only have lymphoproliferative disease but also have elevated levels of myeloid cells. Myeloid cell elevation is obvious in FasL−/− mice if one analyzes the absolute number of MDSCs, but it may not be apparent if one determines the percentage of MDSCs because the absolute number of lymphoid cells is also elevated in FasL−/− mice.
Although the levels of Gr1+CD11b+ cells are regulated by Fas-FasL interactions in healthy mice, this regulation does not obviously occur in persons with cancer, chronic inflammation, or stress. Under these conditions, the production of molecules that induce MDSC accumulation drastically increases. The increasing quantities of MDSCs prevent T-cell activation, and, as a result, there is a deficit of FasL+ T cells capable of inducing MDSC apoptosis. Because MDSCs produce proinflammatory molecules, they may drive their own accumulation through an autocrine feedback loop. As a consequence, T-cell activation will be blocked, thereby producing a self-propagating cycle that expands MDSCs and decreases T-cell activation. Our results showing that MDSC levels regress in tumor-bearing postsurgery STAT6−/−FasL+/+ and CD1−/−FasL+/+ mice, but not in STAT6−/−FasL−/− and CD1−/− FasL−/− mice, further support a role for Fas-FasL interactions in MDSC turnover.
It is intriguing that other myeloid cells (eg, DCs and macrophages) express Fas but do not appear to be homeostatically regulated through Fas-FasL interactions. The difference in Fas-FasL susceptibility is not because of differences in Fas expression but is probably because of differences in downstream signaling molecules because DCs and macrophages do not apoptose in response to activated T cells. Additional studies are needed to clarify the differential effect of FasL on MDSCs versus other myeloid cells.
MDSCs are a significant obstacle to cancer immunotherapies that rely on natural killer cells or the in vivo activation of T cells. Therefore, the reduction or inactivation of MDSCs is viewed as critical if these immunotherapies are to be effective, and there has been considerable focus on strategies for eliminating MDSCs.1 Several drugs reduce or inactivate MDSCs in some persons. Retinoic acid induces the differentiation of monocytic MDSCs into mature myeloid cells such as DCs.46 Gemcitabine4,5 and amino-biphosphonate reduce MDSC levels.47 Inhibitors of phosphodiesterase-5 (eg, sildenafil)48 and the tyrosine kinase inhibitor Sutent (sunitinib) reduce MDSC suppressive activity.49 Fas-induced apoptosis may be another therapeutic approach. The studies of Loeffler et al50 support this concept. Those investigators demonstrated that treatment with Salmonella typhimurium engineered to express FasL reduced progression of breast cancer lesions. Antitumor immunity required Fas on host cells and not tumor cells, and the antitumor effect decreased if Gr1+ cells were deleted, suggesting that Gr1+CD11b+ MDSCs were the targeted cells. MDSC reduction may also occur after adoptive transfer of in vitro–activated tumor-specific T cells if these cells express FasL.
At least one factor that induces MDSC accumulation may also facilitate survival by blocking MDSC turnover. PGE2 and the EP2 receptor agonist butaprost induce MDSC differentiation from c-kit+ progenitor cells.14 However, PGE2 also inhibits FasL up-regulation on activated T cells.51 Therefore, PGE2 not only induces MDSCs but also enhances MDSC survival by inhibiting T cell–induced MDSC apoptosis.
Although originally identified in patients with cancer, MDSCs accumulate under a variety of conditions, and their role as immune modulators is becoming increasingly appreciated. Therefore, if Fas-FasL–mediated apoptosis could be exploited to lower MDSC levels, such an approach may affect multiple diseases and abnormal physiologic conditions.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Drs Robert Wiltrout, Luc Van Kaer, Michael Grusby, and Ephraim Fuchs for providing FasL−/−, CD1−/−, STAT6−/−, and TS1 and Clone4 mice, respectively; Dr Scott Abrams for the AT3 cell line; Ms Chere Petty for assistance with confocal microscopy; Ms Sandra Tickle for mouse husbandry; and Mss Tiffany Williams and Chineye Emeche for generating CD1−/−FasL−/− and STAT6−/−FasL−/− mice, respectively.
This work was supported by American Cancer Society (IRG-97-153-07) (P.S.), NIH (RO1CA84232 and RO1CA115880) (S.O.-R.). O.C. was supported by predoctoral fellowship W81XWH-10-10027 from the DOD CDMRP.
National Institutes of Health
Authorship
Contribution: P.S. designed research, performed experiments, collected, analyzed and interpreted data, performed statistical analyses, and wrote the paper; O.C. designed research, performed experiments, collected, analyzed and interpreted data; V.K.C. performed experiments and collected data; K.A.A. and R.A.Z. designed research and interpreted data; and S.O.-R. designed research, analyzed and interpreted data, and wrote the paper
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Suzanne Ostrand-Rosenberg, Department of Biological Sciences, University of Maryland Baltimore County, 1000 Hilltop Circle, Baltimore, MD 21250; e-mail: srosenbe@umbc.edu; or Pratima Sinha, Department of Biological Sciences, University of Maryland Baltimore County, 1000 Hilltop Circle, Baltimore, MD 21250; e-mail: pratima@umbc.edu.