Abstract
The development of immune cytopenias is a well-recognized side effect of many drugs. Quinine- and quinidine-dependent antibodies are classic examples of drug-induced effects that cause severe, life-threatening thrombocytopenia. Whereas the effects of drug-dependent antibodies on platelets have been well documented, their effects on megakaryocyte (Mk) biology are still unclear. We analyzed sera from several quinine-induced thrombocytopenia (QITP) patients on highly pure Mks (98% glycoprotein IIb-positive [GPIIb+]; 92% GPIX+) derived from human CD34+ cells cultured with human thrombopoietin. We demonstrate by flow cytometry and confocal microscopy that QITP IgGs bind Mks efficiently in the presence of quinine. Incubation of day-4 Mks with QITP sera or purified IgG resulted in induction of apoptosis, a significant decrease in cell viability, and an increase in cell death. Furthermore, QITP sera preferentially reduced the number of late GPIX+/GPIbα+ Mks and the number of receptors per cell in the surviving population. Ploidy distribution, lobularity, and average cell size of Mks remained unchanged after treatment. In addition, treated Mks showed a marked decrease in their proplatelet production capacity, suggesting that drug-dependent antibodies hinder platelet production. Therefore, QITP antibodies considerably reduce the proplatelet production capabilities of Mks despite undetectable effects on DNA content, morphology, and cell size.
Introduction
The development of immune cytopenias is a well-recognized side effect of many drugs, including heparin, penicillin and its derivatives, abciximab, quinine, and quinidine. Whereas several cell types, such as erythrocytes and leukocytes,1 are targeted by drug-dependent antibodies, it is the effect on platelets that appears to be more prevalent. Of several causative agents, quinine is a classic example of drugs that cause severe, life-threatening thrombocytopenia, and its mechanisms of action may be applicable to other drug-induced thrombocytopenias.
The antibodies responsible for quinine-induced thrombocytopenia (QITP) are immunoglobulins that usually recognize the VWF or the fibrinogen receptors glycoprotein (GP) Ib/IX or GPIIb/IIIa. Quinine-dependent antibodies are notable by the fact that they have no significant affinity for the platelet antigen unless the drug is present at therapeutic concentrations.2 Typically, patients with QITP present with severe thrombocytopenia (platelet count < 10 × 109/L) of abrupt onset after a variable period of taking quinine; they often have petechiae and mucosal bleeding. After cessation of the offending drug, the platelet counts usually increase to above baseline levels generally after a week but occasionally may require longer recovery periods.3
The platelet antigens (GPIb/IX and GPIIb/IIIa) are also expressed on megakaryocytes (Mks) during differentiation.4 Therefore, it is likely that quinine-dependent antibodies may also bind to cell-surface GPs on Mks. Indeed, the likelihood of antiplatelet factors present in primary immune thrombocytopenia (ITP) patients compromising the function of Mks was discussed in the early 1960s.5 It was subsequently shown that ITP serum inhibits megakaryopoiesis in vitro,6 and McMillan et al found that antibodies from chronic ITP patients do not only impair Mk production in vitro but also inhibit their maturation.7 More recently, it was reported that a patient treated with eptifibatide developed severe thrombocytopenia.8 The investigators then demonstrated that treatment of cultured Mks with IgG isolated from this patient resulted in a significant decrease in cell viability only when the drug was present in the medium.8 Therefore, we hypothesized that antiplatelet drug-dependent antibodies may also act on Mks as part of the pathogenesis of thrombocytopenia. To examine this hypothesis, in the present study, highly pure Mks derived from human CD34+ cells were used to analyze the effect on megakaryopoiesis of QITP sera from several patients in vitro. We show that QITP antibodies bind Mks efficiently, induce cell death via apoptosis, and reduce the expression of the GPIb/IX complex. Moreover, we demonstrate that cells treated with QITP sera drastically reduce their capacity to produce proplatelets despite undetectable effects on DNA content, morphology, and cell size. These observations suggest that the severity of the thrombocytopenia induced by drug-dependent antibodies may be in part due to both a reduction in the number of Mks and a decrease in their capacity to produce proplatelets and, consequently, platelets.
Methods
Patients
Samples were selected randomly from stored sera collected with informed consent from 5 patients with QITP (4 males/1 female, age 20-71 years, platelet counts 0-10 × 109/L). These patient serum samples were prepared from blood taken after the patients had stopped taking quinine. The sera of the patient WJ was collected at 2 different times (referred to as WJ1 and WJ2). Unless specified, WJ1 serum was used. The diagnosis of QITP was made according to the criteria outlined previously.9 Human mobilized peripheral blood samples enriched for CD34+ cells were obtained from the unused cell pool in the Bone Marrow Transplant Laboratory at St George Hospital (Sydney, New South Wales, Australia). The study was approved by the St George Hospital ethics committee.
CD34+ cell isolation and megakaryocytic differentiation culture
CD34+ cells were isolated using CD34-conjugated magnetic beads and separation columns (Miltenyi Biotec) according to the manufacturer's instructions. For megakaryocytic differentiation, the isolated CD34+ cells were cultured in StemPro-34 serum-free complete medium (Invitrogen) in the presence of human recombinant thrombopoietin (TPO; Genentech) at 50 ng/mL.
QITP antibody binding
Expired platelets from the blood bank at St. George Hospital were washed with PBS, pH 7.4 (Invitrogen), and resuspended at 20 × 106 in 100 μL of PBS or PBS containing 0.3mM quinine (Qn; Sigma-Aldrich). QITP serum/IgG or normal serum (NS)/IgG was added to the platelets (1:10) and incubated for 30 minutes at room temperature, washed with PBS or PBS/0.3mM Qn, and then incubated for 30 minutes at 4°C with goat F(ab′)2 anti–human IgG:RPE (1:10; AbD Serotec). Platelets were washed with PBS or PBS/0.3mM Qn, and analyzed by flow cytometry in a FACSCalibur flow cytometer (BD Biosciences). CHO cells expressing GPIb/IX or GPIIb/IIIa were stained and analyzed as described for platelets in the previous paragraph.
Day 10-12 Mks were washed and the Fc receptor blocked with the IV.3 antibody (1:5) at 4°C for 30 minutes. Mks were then treated as described in the above paragraph for platelets, stained with anti-human CD42a (GPIX)–Alexa Fluor 647 (1:10; AbD Serotec), and analyzed by flow cytometry. The GPIX-positive cells were gated for QITP antibody–binding detection.
Confocal microscopy
Platelets and Mks were stained with QITP sera in the presence or absence of Qn and with the anti-GPIX monoclonal antibody FMC25, followed by anti-human IgG–Alexa Fluor 488 and anti-mouse IgG (H+L)–Alexa Fluor 594 (Invitrogen). The cells were fixed in 2% paraformaldehyde for 15 minutes at room temperature, centrifuged onto slides using a Cyto-Tek cytocentrifuge (Miles), and analyzed under a 60× oil objective with a confocal laser-scanning microscope (Olympus) using Olympus Fluoview Software, Version 4.3, FV300.
Kinetic expression of megakaryocytic markers on CD34+ cells cultured with TPO
CD34+ cells were collected on different days during the differentiation culture and stained with anti-human CD41a(GPIIb)-FITC (1:5; BD Pharmingen) and anti-human CD42a-Alexa Fluor 647 (1:10). Mks were also stained with anti CD34-PE/CD15-FITC antibodies (1:5; BD Pharmingen). Both FITC- and PE-conjugated isotype antibody (1:5; BD Pharmingen) and Alexa Fluor 647–conjugated isotype antibody (1:10; AbD Serotec) were used as controls. After washing, the cells were analyzed by flow cytometry.
Counting of cultured Mks
CD34+ cells were cultured with TPO for 4 days and then collected, washed, counted, and reseeded in duplicate in 24-well plates at 1 × 105 or 1.5 × 105 cells/well. The QITP sera were added (1:5 or 1:10) with (0.01mM) or without Qn. NS with 0.01mM Qn was used as a control.10,11 The viable cells were counted using 0.4% Trypan blue (Sigma-Aldrich) after 4 days in culture. To calculate the total number of marker-positive Mks, the cells were also stained for GPIIb, GPIX, and GPIbα expression before analysis by flow cytometry.
Apoptosis assay
CD34+ cells were cultured for 4 days and then treated with QITP sera or NS (1:5) with or without Qn (0.01mM) or 25μM paclitaxel (Sigma-Aldrich) for 20 hours. The cells were stained with either Annexin V-FITC (Miltenyi Biotec) or anti–activated caspase 3-FITC antibody (BD Pharmingen) and analyzed by flow cytometry. Evaluation of complement activity was done using the antibodies eculizumab (Soliris; Alexion Pharmaceuticals) and anti-C3/C3b-FITC (Cedarlane Laboratories).
Polyploidy pattern of mature Mks
The staining of DNA in mature Mks for different ploidy classes was performed as described previously.12 Briefly, the CD34+ cells were cultured with TPO (50 ng/mL) for 4 days and the QITP sera or NS was added (1:5) with or without Qn (0.01mM). After 4-6 days in culture, the cells were collected, washed, stained with anti-human CD41a-FITC, and resuspended in hypotonic citrate buffer (1.25mM sodium citrate, 2.5mM sodium chloride, and 3.5mM dextrose) containing 20 μg/mL of propidium iodide (PI) and 0.1% Triton-X 100 for 15 minutes at room temperature in the dark. RNase was the added to 20 μg/mL, and the cell suspension was incubated for 30 minutes at room temperature. Finally, the intensity of DNA staining by PI in the GPIIb+ population was analyzed by flow cytometry, with ≥ 50 000 cells acquired for each group.
Wright/Giemsa staining and cell-size analysis
Cultured Mks, treated with QITP sera with or without Qn, were centrifuged onto slides and subjected to Wright/Giemsa staining. Slides were imaged using a Zeiss Axioskop microscope and AxioVision Version 3.1 software. For the estimation of cell lobularity, random areas of the slides were chosen and 250 cells counted at 3 locations (750 cells were counted for each group) by an experimenter blinded to the treated samples. Cells were classified as monolobed, bilobed, or multilobed lobed if they had one (2N), 2 (4N), or 4 or more nuclei, respectively. For cell-size calculations, the Mks were stained with anti-CD42a–Alexa Fluor 488 and images were captured by confocal laser scanning microscopy. Cells (a minimum of 94) were selected randomly and their surface areas determined using the ImageJ 1.42 software package (National Institutes of Health).
Proplatelet formation assay
Evaluation of proplatelet formation was conducted essentially as described previously.13,14 Briefly, CD34+ cells were cultured with TPO for 5-6 days and then collected, washed, and seeded in triplicate (1-1.5 × 104 cells/well) in 24-well plates with QITP sera (1:5) in the presence or absence of Qn (0.01mM) and NS (1:5) with Qn (0.01mM). The proplatelet-bearing Mks were counted on day 11 or 12 of culture with a Leica DMIRB inverted light microscope by an experimenter blinded to the treatment conditions. The score was corroborated by a second observer. Proplatelet formation was expressed as the number of proplatelets per 104 Mks.
Statistical analysis
Results
Detection of mature Mks derived from human CD34+ cells in culture with TPO
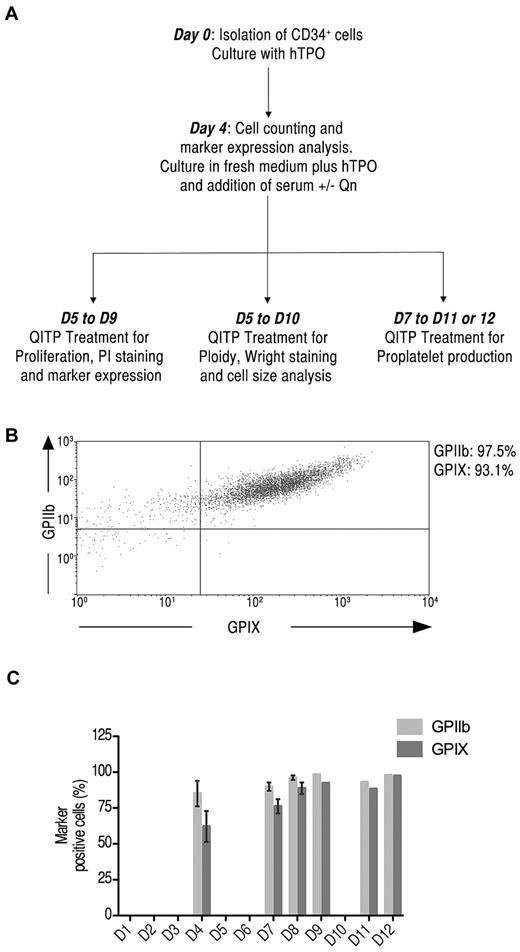
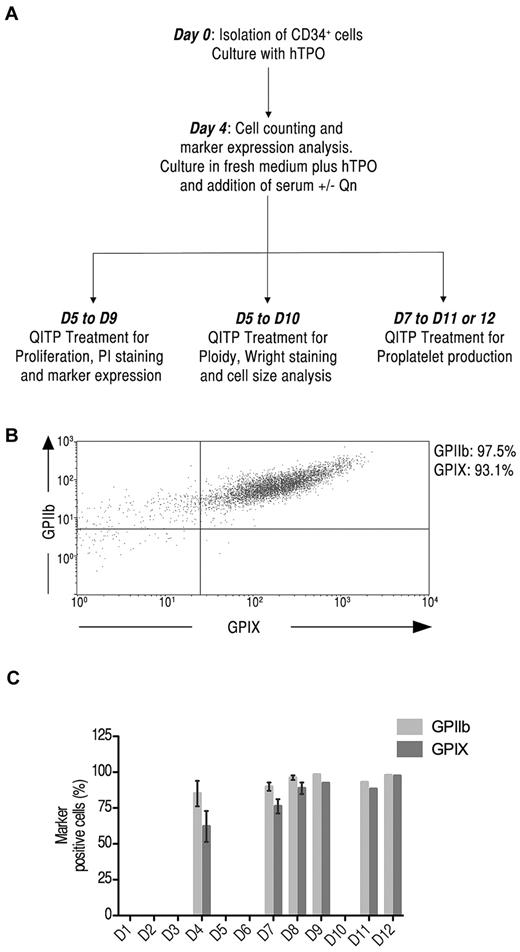
Mks were generated in culture from CD34+ cells isolated from mobilized peripheral blood. A schematic representation of the experimental design is shown in Figure 1A. The kinetics of differentiation of CD34+ cells was monitored at several intervals over 12 days by measuring the expression of the megakaryocytic markers GPIIb and GPIX.15 Figure 1B illustrates the 2-color staining of GPIIb and GPIX expression on Mks as measured by flow cytometry. During the course of differentiation, cell-surface expression of GPIIb and GPIX was significant already on day 4 of culture with GPIIb at 85% ± 15% and GPIX at 62% ± 17% (n = 3). The expression of these markers continued to increase until day 8, reaching 96% ± 3% for GPIIb and 89% ± 8% for GPIX (n = 4) (Figure 1C). Therefore, the number of Mks at day 4 was judged suitable for treatment with QITP sera in subsequent experiments. The expression of CD34 and the granulocytic marker CD15 were also examined. CD34 expression decreased throughout differentiation to < 20% on day 8. CD15 expression remained low, as expected for a largely pure Mk population (supplemental Figure 2C, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Detection of megakaryocytic markers on Mks derived from CD34+ cells in culture. (A) Experimental protocol for the culture conditions used. CD34+ cells were isolated from human mobilized peripheral blood samples cultured in the presence of TPO (50 ng/mL) for 4 days, washed, counted, and cultured for up to 12 days. D indicates days from the start of culture. (B) Cultured cells were stained with anti-human CD41-FITC and CD42a-Alexa Fluor 647 simultaneously and analyzed by flow cytometry. Shown is the dot-plot profile of GPIIb and GPIX double-positive Mks on day 8 of culture. (C) Kinetic expression of GPIIb (light gray) and GPIX (dark gray) on Mks on days 4, 7, 8, 9, 11, and 12 of culture. The graph shows the means ± SD.
Detection of megakaryocytic markers on Mks derived from CD34+ cells in culture. (A) Experimental protocol for the culture conditions used. CD34+ cells were isolated from human mobilized peripheral blood samples cultured in the presence of TPO (50 ng/mL) for 4 days, washed, counted, and cultured for up to 12 days. D indicates days from the start of culture. (B) Cultured cells were stained with anti-human CD41-FITC and CD42a-Alexa Fluor 647 simultaneously and analyzed by flow cytometry. Shown is the dot-plot profile of GPIIb and GPIX double-positive Mks on day 8 of culture. (C) Kinetic expression of GPIIb (light gray) and GPIX (dark gray) on Mks on days 4, 7, 8, 9, 11, and 12 of culture. The graph shows the means ± SD.
QITP antibodies bind to mature human Mks in vitro
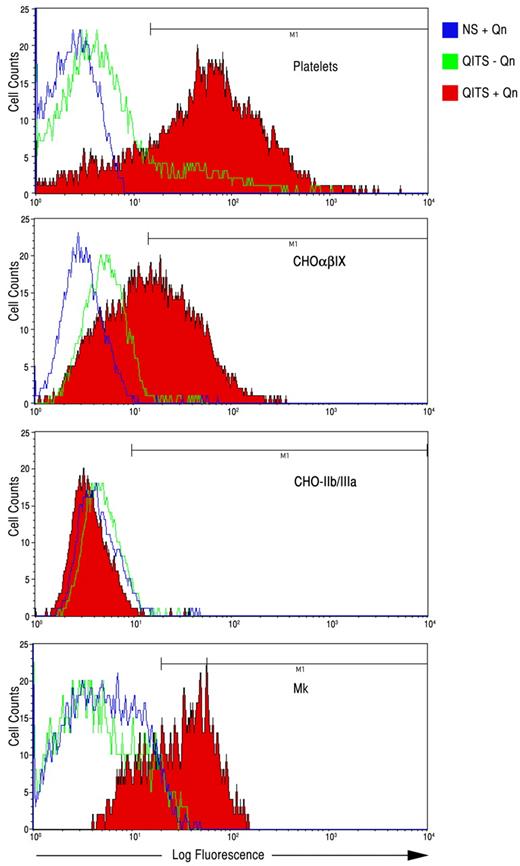
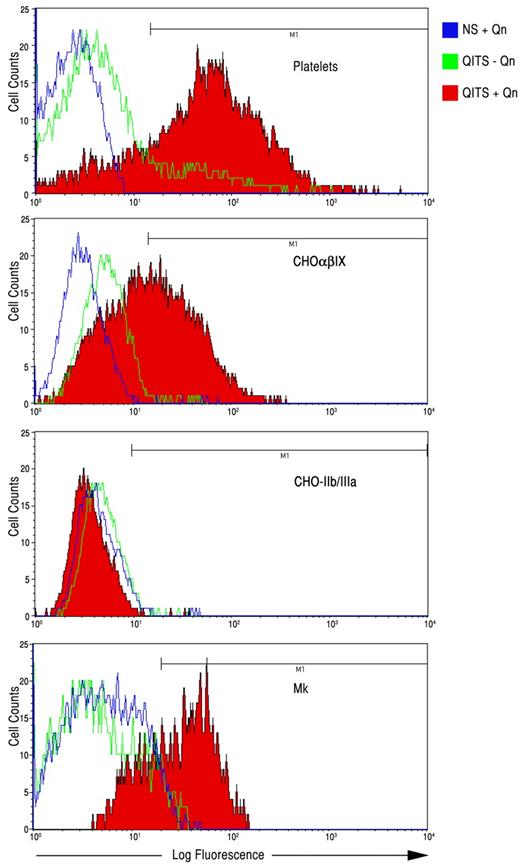
Platelets were incubated with QITP sera from several patients in the presence and absence of Qn to confirm that the sera contained active Qn-dependent antibodies. In the presence of Qn, the QITP antibodies bound 71%-96% of the cells (human platelets) in the test (Figure 2 and Table 1). It has been shown that most Qn-dependent antibodies recognize the GPIb/IX complex, but recognition of GPIIb/IIIa has also been demonstrated.2 To ascertain the reactivity of the sera used in our experiments, we analyzed binding specificity on CHO cells expressing either GPIb/IX or GPIIb/IIIa (Figure 2 and Table 1). Table 1 shows that all sera analyzed bound GPIb/IX in the presence of Qn. Weak, Qn-dependent interaction with GPIIb/IIIa was observed for 2 patients (WJ and JE). Significant interaction with GPIIb/IIIa was detected in one case (patient JG), and this appeared to be Qn independent.
Binding of QITP antibodies to platelets, CHO-GPIb/IX cells, CHO-GPIIb/IIIa cells, and Mks. QITP antibodies (WJ) were bound in the presence (+Qn, red) and absence (−Qn, green) to the platelets, CHO cells expressing human GPIb/IX (CHOαβIX) or GPIIb/IIIa, and cultured Mks derived from CD34+ cells. Control NS plus Qn is shown in blue.
Binding of QITP antibodies to platelets, CHO-GPIb/IX cells, CHO-GPIIb/IIIa cells, and Mks. QITP antibodies (WJ) were bound in the presence (+Qn, red) and absence (−Qn, green) to the platelets, CHO cells expressing human GPIb/IX (CHOαβIX) or GPIIb/IIIa, and cultured Mks derived from CD34+ cells. Control NS plus Qn is shown in blue.
QITP antibodies also bound to mature Mks derived from human CD34+ cells in the presence of Qn ranging from 38%-87% (Figure 2 and Table 1). The sera from 2 patients (JE and JG) bound to Mks to some degree in the absence of Qn (9%-15%, respectively), suggesting that some antibodies present in the sera behaved like ITP auto-antibodies.
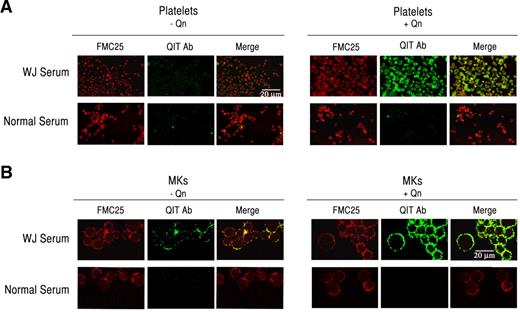
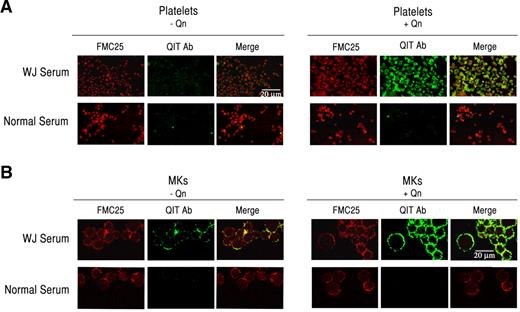
Because Mks interact with QITP antibodies in the presence of the drug, we sought to confirm this interaction by confocal microscopy. Because platelets are known to bind QITP antibodies, they were used as positive controls. Platelets were stained with the anti-GPIX monoclonal antibody FMC25, followed by incubation with QITP serum in the presence and absence of Qn. FMC25 and human IgG were detected with anti-mouse IgG (H+L)–Alexa Fluor 594 (red) and anti-human IgG-Alexa Fluor 488 (green), respectively. NS was used as a control. As shown in Figure 3A, antibodies in the QITP sera did not bind efficiently to platelets in the absence of Qn (left panel), but bound with high affinity in the presence of Qn (right panel). We next incubated mature Mks with QITP sera under the same conditions used for the platelets. In agreement with the flow cytometric data, we observed only weak binding of QITP IgG to GPIX+ Mks in the absence of Qn (Figure 3B middle panel). The addition of Qn, however, resulted in a marked increase in QITP IgG association (Figure 3B green fluorescence), confirming that drug-dependent antibodies are capable of efficiently binding Mks in the presence of the drug.
QITP antibodies bind mature Mks. Human platelets (A) and Mks (B) were incubated with QITP sera or NS without (left panels) and with Qn (right panels) and the anti-GPIX monoclonal antibody FMC25, followed by anti-human IgG–Alexa Fluor 488 (green) and anti-mouse IgG (H+L)–Alexa Fluor 594 (red). Binding of human IgG (green) was considerably enhanced only in the presence of the drug (right panels, middle image). The microphotographs were acquired by confocal laser scanning microscopy (Olympus FV300, 60× oil objective, Fluoview Version 4.3 software). Scale bars indicate 20 μm. Images were cropped and revised using Adobe Photoshop CS5.
QITP antibodies bind mature Mks. Human platelets (A) and Mks (B) were incubated with QITP sera or NS without (left panels) and with Qn (right panels) and the anti-GPIX monoclonal antibody FMC25, followed by anti-human IgG–Alexa Fluor 488 (green) and anti-mouse IgG (H+L)–Alexa Fluor 594 (red). Binding of human IgG (green) was considerably enhanced only in the presence of the drug (right panels, middle image). The microphotographs were acquired by confocal laser scanning microscopy (Olympus FV300, 60× oil objective, Fluoview Version 4.3 software). Scale bars indicate 20 μm. Images were cropped and revised using Adobe Photoshop CS5.
QITP sera affect cell viability and impair Mk production
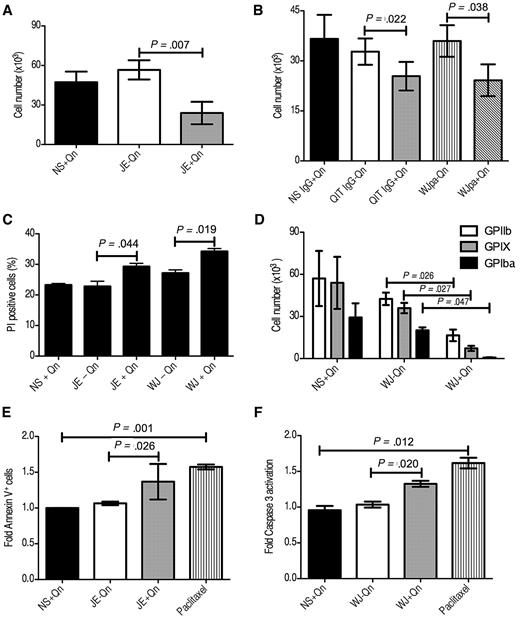
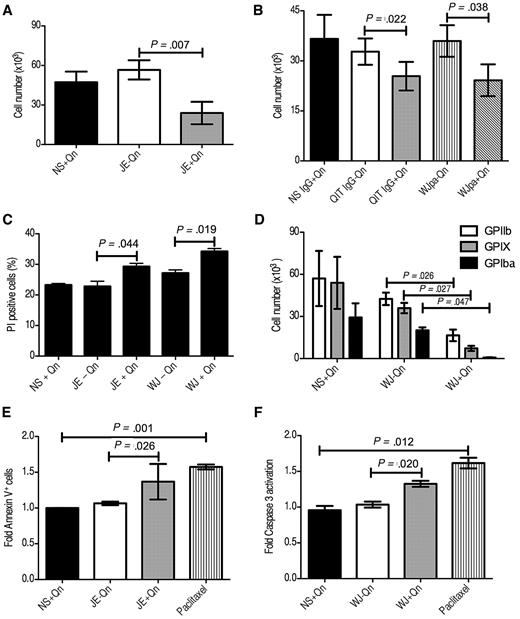
To determine the effect of QITP serum on cultured Mks, CD34+ cells were cultured for 4 days with TPO, then seeded at 1 × 105 cells/well and treated with QITP serum in the presence (0.01mM) or absence of Qn. NS plus Qn was used as a control. Four days after treatment, we observed a significant decrease in the viability of cells incubated with QITP serum plus Qn compared with QITP serum without Qn or NS plus Qn (23.9 ± 14.7 × 103 vs 56.6 ± 12.6 × 103 cells/well, mean ± SD, n = 3; P = .007; NS plus Qn: 47.3 ± 13.8 × 105 cells/well; Figure 4A). Similar results were observed using serum from other QITP patients under the same conditions (summarized in Table 2). To exclude any putative effects of other serum components, we used purified patient IgG (which retained its Qn-dependent binding capacity, see supplemental Figure 1A lower panel) to treat the Mks. Incubation with purified IgG resulted in a decrease in cell viability comparable to that observed for the serum (Figure 4B). Some QITP sera may contain weak, Qn-independent auto-antibodies (Figure 2 and Table 1). To evaluate the influence of auto-antibodies on the viability of Mks, the auto-antibodies present in the serum were removed by absorption onto platelets in the absence of Qn (supplemental Figure 1A upper panel). Absorption of patient serum on platelets may cause activation and release of platelet factor 4 (PF4). Because PF4 is a known inhibitor of Mk differentiation,16 we removed any released PF4 from the serum by absorption onto a Sepharose-heparin column. Incubation of Mks with platelet-absorbed serum (WJpa) also resulted in significant inhibition of Mk viability (Figure 4B), indicating that the effects observed were due to the presence of Qn-dependent antibodies. A similar effect was observed when the number of GPIIb+, nonviable cells (PI+) was determined by flow cytometry. Cells were harvested after 4 days of QITP treatment, stained with anti-GPIIb antibody and PI, and the percentage of double-positive cells calculated. The result (Figure 4C) shows significant differences in the percentage of PI+ cells in the QITP plus Qn samples. To investigate whether the reduction in cell number was due to a decrease in the number of Mks, we chose sera from patient WJ to treat the cells, followed by staining with antibodies against GPIIb, GPIX, and GPIbα. Interestingly, we observed a significant decrease in the number of cells positive for the 3 megakaryocytic markers tested (Figure 4C). Therefore, Mk development and viability was inhibited by QITP serum in the presence of the drug.
Reduction of viable Mks and induction of apoptosis on QITP antibody treatment. Cultured CD34+ cells were treated on day 4 with QITP serum or purified IgG plus Qn or with controls (QITP serum or QITP IgG − Qn or NS or NS IgG + Qn). The total viable cell number was determined after 4 days of treatment by Trypan blue exclusion. Cells were treated with serum (A) or with purified IgG or WJ serum preabsorbed on platelets to remove auto-antibodies (WJpa) (B). (C) Treated Mks stained with anti-GPIIb and PI. The percentage of GPIIb+/PI+ cells was determined by flow cytometry. (D) Viable cells enumerated by Trypan blue exclusion and stained with anti-GPIIb, anti-GPIX, and anti-GPIbα antibodies. The percentage of GPIIb+ (empty bars), GPIX+ (gray bars), and GPIbα+ (solid bars) Mks present in the culture was determined by flow cytometry. The total cell number was calculated by multiplying the number of cells (direct counting) by the percentage of Mks positive for each marker. (E) Cells treated for 20 hours with the indicated sera or with 25μM paclitaxel followed by staining with Annexin V–FITC. The percentage of Annexin V+ cells in the control culture (NS + Qn) was normalized to 1 and the -fold increase in Annexin V+ cells on treatment plotted (n = 4). (F) Cells were treated as in panel E, stained with anti activated caspase 3-FITC antibody, and the -fold increase in activated caspase 3+ cells was plotted (n = 3). The mean ± SD is shown in all cases.
Reduction of viable Mks and induction of apoptosis on QITP antibody treatment. Cultured CD34+ cells were treated on day 4 with QITP serum or purified IgG plus Qn or with controls (QITP serum or QITP IgG − Qn or NS or NS IgG + Qn). The total viable cell number was determined after 4 days of treatment by Trypan blue exclusion. Cells were treated with serum (A) or with purified IgG or WJ serum preabsorbed on platelets to remove auto-antibodies (WJpa) (B). (C) Treated Mks stained with anti-GPIIb and PI. The percentage of GPIIb+/PI+ cells was determined by flow cytometry. (D) Viable cells enumerated by Trypan blue exclusion and stained with anti-GPIIb, anti-GPIX, and anti-GPIbα antibodies. The percentage of GPIIb+ (empty bars), GPIX+ (gray bars), and GPIbα+ (solid bars) Mks present in the culture was determined by flow cytometry. The total cell number was calculated by multiplying the number of cells (direct counting) by the percentage of Mks positive for each marker. (E) Cells treated for 20 hours with the indicated sera or with 25μM paclitaxel followed by staining with Annexin V–FITC. The percentage of Annexin V+ cells in the control culture (NS + Qn) was normalized to 1 and the -fold increase in Annexin V+ cells on treatment plotted (n = 4). (F) Cells were treated as in panel E, stained with anti activated caspase 3-FITC antibody, and the -fold increase in activated caspase 3+ cells was plotted (n = 3). The mean ± SD is shown in all cases.
Treatment of Mks with QITP sera induces apoptosis
Having established that QITP antibodies led to a decrease in Mk viability, we then sought to determine whether these antibodies induced cell death by either complement-mediated cell lysis or induction of apoptosis. The observation that treatment of Mks in serum-free conditions (purified IgG) also caused a significant reduction in cell viability (Figure 4B) suggested that complement involvement was dispensable for the activity of QITP antibodies. This was corroborated by treatment of Mks in the presence of the anti-C5 antibody eculizumab. This antibody prevents the assembly of the membrane attack complex,17 yet its presence did not alter the effect of the QITP serum on cell viability (data not shown). In addition, fixation of the complement protein C3 on the Mks could not be detected by flow cytometry using an anti-C3/C3b monoclonal antibody (data not shown).
To evaluate whether treated Mks undergo apoptosis, we incubated day-4 cells with NS plus Qn or QITP serum with or without Qn for 20 hours. Paclitaxel, a caspase 3–dependent apoptosis inducer,18 was used as a positive control. Figure 4E shows a significant increase in phosphatidylserine present on the cell surface of Mks treated with QITP serum plus Qn or 25μM paclitaxel, as detected by Annexin V binding. In addition, a significant increase in the presence of activated caspase 3 was observed in the treated cells (Figure 4F and supplemental Figure 3). This increase in apoptosis induction was sustained for over 72 hours (data not shown). These results indicate that the reduction in Mk viability was likely due to the induction of apoptosis by Qn-dependent antibodies.
Reduction of GPIX and GPIbα expression on Mks treated with QITP sera
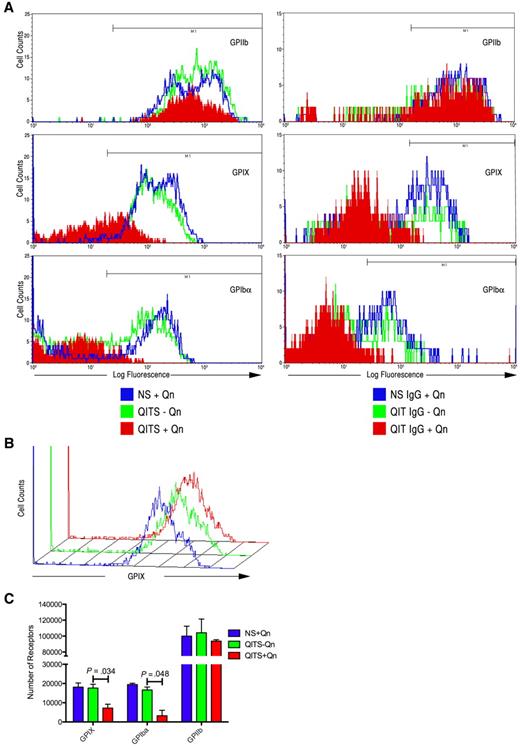
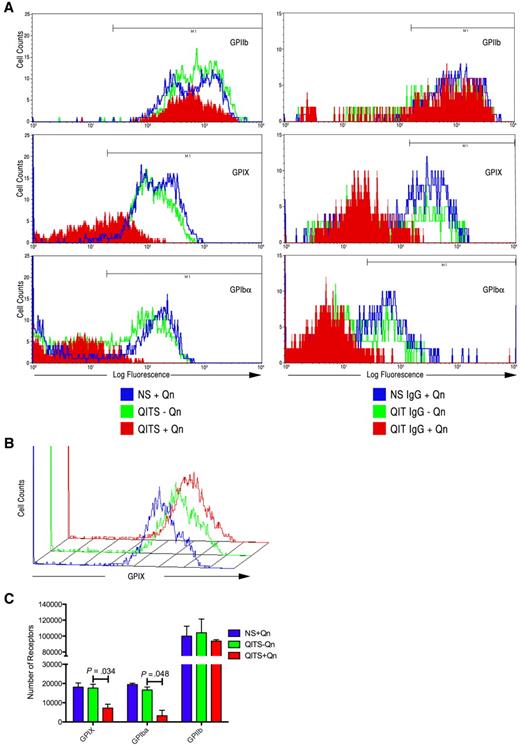
Having shown that both the total number of viable cells and the number of Mks (GPIIb+, GPIX+, and GPIbα+ cells) decreased significantly after treatment with QITP plus Qn, we sought to determine whether QITP antibodies affected Mk maturation. CD34+ cells were treated as described in “CD34+ cell isolation and megakaryocyte differentation culture” and the marker expression percentages compared in the viable cell population. The percentage of GPIIb+ cells remained unchanged after 4 days of QITP plus Qn treatment (Figure 5A left panels and Table 3). Unexpectedly, marked reductions were observed in the expression of both GPIX and GPIbα on the same cells (Figure 5A left panels and Table 3), suggesting that terminal differentiation of Mks was affected by binding of the QITP antibodies. A similar decrease in marker expression was observed after treatment of Mks with purified patient IgG plus Qn (Figure 5A right panels). Binding of anti-GPIX and/or anti-GPIbα antibodies to the GPIb/GPIX complex on Mks could have potentially been affected by steric hindrance because of the presence of QITP antibodies bound to the complex. To validate our observations, we first bound QITP serum to mature Mks, followed by binding of anti-GPIX and anti-GPIbα antibodies, and NS plus Qn and QITP serum minus Qn were again used as controls. As shown in Figure 5B, the presence of QITP antibodies bound to the GPIb/IX complex did not interfere with GPIX or GPIbα antibody binding. These observations were confirmed by determining the mean number of receptors per cell. Figure 5C shows that the number of GPIX and GPIbα receptors was significantly reduced in Mks treated with QITP serum plus Qn. The number of GPIIb receptors, however, remained largely unaffected. Therefore, in the presence of QITP serum plus the drug, mature Mk marker expression was inhibited markedly. The QITP sera used in this study reacted mainly with the GPIb/IX complex (Table 1). To evaluate the effect of a GPIIb/IIIa-interacting antibody, we affinity purified anti-GPIIb/IIIa antibody from patient JE on CHO-GPIIb/IIIa cells. This IgG showed increased affinity for GPIIb/IIIa and greatly reduced binding to GPIb/IX (supplemental Figure 1B left panels). Incubation of day-4 Mks with anti-GPIIb/IIIa antibody in the presence of Qn did not affect expression levels of megakaryocytic markers (supplemental Figure 2A-B).
Inhibition of GPIX and GPIbα expression on treatment of CD34+ cells with QITP sera or QITP IgG. Cultured CD34+ cells were treated on day 4 with QITP serum or purified IgG plus Qn or with controls (QITP serum or QITP IgG − Qn or NS or NS IgG + Qn) and analyzed after 4 days in culture. (A) Left panels: flow cytometric data for GPIIb+, GPIX+, and GPIbα+ Mks treated with NS + Qn (blue), QITP serum − Qn (green), and QITP serum + Qn (red). Right panels: flow cytometric data for GPIIb+, GPIX+, and GPIbα+ Mks treated with NS IgG + Qn (blue), QITP IgG − Qn (green), and QITP IgG + Qn (red). Treatment with QITP serum or QITP IgG + Qn greatly decreased the expression of GPIX and GPIbα. (B) Mature Mks were stained with QITP serum + Qn or with controls (QITP serum − Qn or NS + Qn) for 30 minutes, followed by staining with PE-conjugated anti-human IgG antibody and anti-GPIX antibody conjugated with Alexa Fluor 647. The flow cytometric data show that the binding of the anti-GPIX antibody was not affected by prior incubation with QITP serum + Qn. (C) Number of receptors present on Mks determined as described in the supplemental “Methods.” The number of GPIX and GPIbα molecules on the cell surface was significantly reduced after QITS + Qn treatment. The mean ± SD is shown.
Inhibition of GPIX and GPIbα expression on treatment of CD34+ cells with QITP sera or QITP IgG. Cultured CD34+ cells were treated on day 4 with QITP serum or purified IgG plus Qn or with controls (QITP serum or QITP IgG − Qn or NS or NS IgG + Qn) and analyzed after 4 days in culture. (A) Left panels: flow cytometric data for GPIIb+, GPIX+, and GPIbα+ Mks treated with NS + Qn (blue), QITP serum − Qn (green), and QITP serum + Qn (red). Right panels: flow cytometric data for GPIIb+, GPIX+, and GPIbα+ Mks treated with NS IgG + Qn (blue), QITP IgG − Qn (green), and QITP IgG + Qn (red). Treatment with QITP serum or QITP IgG + Qn greatly decreased the expression of GPIX and GPIbα. (B) Mature Mks were stained with QITP serum + Qn or with controls (QITP serum − Qn or NS + Qn) for 30 minutes, followed by staining with PE-conjugated anti-human IgG antibody and anti-GPIX antibody conjugated with Alexa Fluor 647. The flow cytometric data show that the binding of the anti-GPIX antibody was not affected by prior incubation with QITP serum + Qn. (C) Number of receptors present on Mks determined as described in the supplemental “Methods.” The number of GPIX and GPIbα molecules on the cell surface was significantly reduced after QITS + Qn treatment. The mean ± SD is shown.
Endomitosis is not affected by QITP sera in the presence of Qn
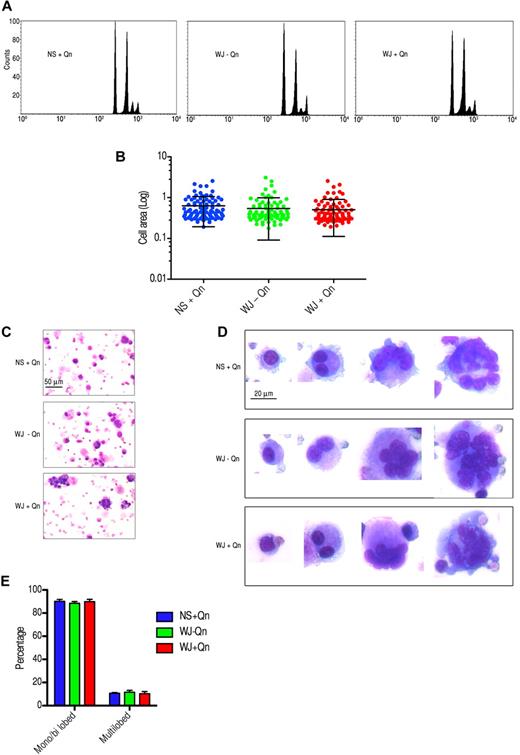
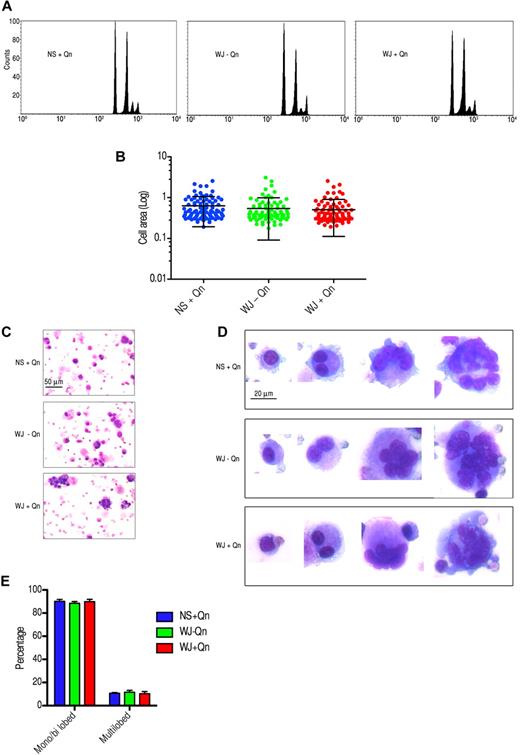
A unique feature of Mks is nuclear replication without cell division at the late stages of maturation, a process called endomitosis.19,20 Day-4 Mks were treated with sera from 3 patients and a normal control and cultured for 4-6 days. To ascertain whether the ploidy status of Mks changed after treatment with QITP serum, the DNA content of GPIIb+ cells was analyzed by 2-color flow cytometry.21 Unexpectedly, no differences in the ploidy distribution of the cells were observed. A representative result from patient WJ is shown in Figure 6A. The increase in size of cultured CD34+ cells was correlated with the maturation of Mks.22,23 To validate the ploidy observations, treated and control Mks were stained with anti-CD42a(GPIX)–Alexa Fluor 488. No significant differences in cell size were observed after treatment with QITP serum plus Qn (0.541 ± 0.046 vs 0.507 ± 0.041; mean of the area in arbitrary units ± SEM, P = .580, n = 94; Figure 6B). Morphologic studies of treated and control day-10 cells revealed Mks at different stages of maturation. Immature Mks containing 1 or 2 nuclei were well represented in the culture. Large cells with several nuclei or with multilobulated nuclei were also present in all groups (Figure 6C). The percentages of immature (monolobed and bilobed) and mature (multilobed) cells were calculated as described in “Counting of cultured Mks.” In agreement with our earlier observations, no significant difference was detected in the 3 groups analyzed (Figure 6D). Therefore, despite affecting the terminal stages of Mk differentiation and late marker expression, the presence of QITP sera plus drug did not impinge on Mk endomitosis, as measured by DNA content, cell size, and nuclear lobularity.
Treatment of Mks with QITP serum plus drug does not affect endomitosis. Cultured CD34+ cells were treated on day 4 with QITP serum + Qn or with controls (QITP serum − QN or NS + Qn) and analyzed after 5 or 6 days in culture. (A) Mks stained with anti-GPIIb antibody and PI in hypotonic citrate buffer. The DNA content of the GPIIb+ population was analyzed by flow cytometry. No differences were observed among the groups analyzed. (B) Mks stained with anti-GPIX antibody conjugated to Alexa Fluor 488 and imaged by confocal microscopy. Cells were chosen randomly (n = 94) and their area calculated using ImageJ software. The graph shows the mean ± SD. No significant differences were observed. (C) Cultured Mks centrifuged onto slides, stained for morphology with Wright/Giemsa staining, and imaged with a Zeiss Axioskop microscope and AxioVision 3.1 software (10× objective using an Axiocam camera; Zeiss). Scale bar indicates 50 μm. (D) Mks at different stages of development (20× objective). Bar indicates 20 μm. Images were cropped and revised using Adobe Photoshop CS5. (E) Percentage of monolobed, bilobed, or multilobed Mks calculated by randomly selecting 750 cells from each treatment group. The graph shows the percentage ± SD.
Treatment of Mks with QITP serum plus drug does not affect endomitosis. Cultured CD34+ cells were treated on day 4 with QITP serum + Qn or with controls (QITP serum − QN or NS + Qn) and analyzed after 5 or 6 days in culture. (A) Mks stained with anti-GPIIb antibody and PI in hypotonic citrate buffer. The DNA content of the GPIIb+ population was analyzed by flow cytometry. No differences were observed among the groups analyzed. (B) Mks stained with anti-GPIX antibody conjugated to Alexa Fluor 488 and imaged by confocal microscopy. Cells were chosen randomly (n = 94) and their area calculated using ImageJ software. The graph shows the mean ± SD. No significant differences were observed. (C) Cultured Mks centrifuged onto slides, stained for morphology with Wright/Giemsa staining, and imaged with a Zeiss Axioskop microscope and AxioVision 3.1 software (10× objective using an Axiocam camera; Zeiss). Scale bar indicates 50 μm. (D) Mks at different stages of development (20× objective). Bar indicates 20 μm. Images were cropped and revised using Adobe Photoshop CS5. (E) Percentage of monolobed, bilobed, or multilobed Mks calculated by randomly selecting 750 cells from each treatment group. The graph shows the percentage ± SD.
QITP antibodies impair proplatelet production by Mks
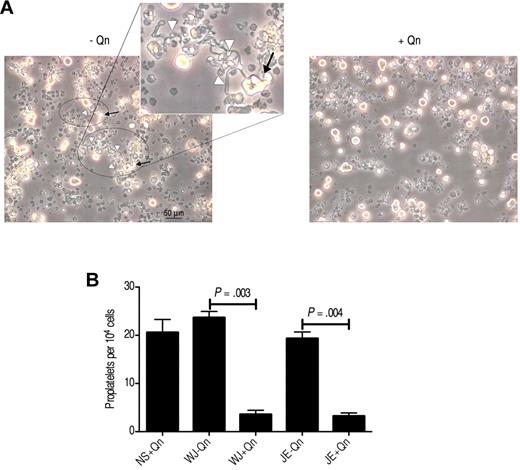
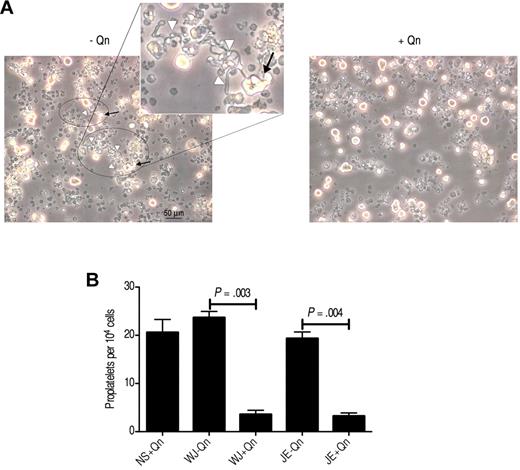
Proplatelets are the immediate precursors of platelets derived from mature Mks both in vitro and in vivo.24,25 We cultured CD34+ cells for 11 or 12 days until the Mks fragmented and extended the cytoplasm to form long protrusions (proplatelets; Figure 7A). To determine whether the antibodies present in QITP serum influence the capacity of Mks to produce proplatelets, we cultured the Mks in the presence of QITP serum and counted the proplatelet-bearing Mks, as described previously.12,13 In contrast to the preceding observations concerning DNA content, nuclear lobularity, and cell size, we observed a marked decrease in the ability of Mks to produce proplatelets (patient WJ: 23.7 ± 1.54 vs 3.6 ± 1.02, P = .003; patient JE: 19.3 ± 1.64 vs 3.2 ± 0.75, P = .004; number of proplatelets/104 cells ± SD, n = 3, with Qn vs without Qn) (Figure 7B). These results suggest a direct effect of QITP sera on the capacity of mature Mks to produce proplatelets.
Reduced proplatelet production by QITP antibodies in the presence of quinine. CD34+ cells were cultured for 5-6 days, and then seeded for treatment with QITP sera plus Qn or with controls (QITP sera − Qn or NS + Qn) and analyzed after 5 or 6 days in culture. (A) Representative image of typical morphologic features of proplatelets taken with a Leica DMIRB inverted microscope (20× objective using a DC200 camera; Leica). Areas containing proplatelets are circled, with proplatelet-bearing Mks indicated by arrows and proplatelet extensions indicated by white arrowheads (see inset for representative features of proplatelets). Bar indicates 50 μm. The images were cropped and revised using Adobe Photoshop CS5. (B) Proplatelets were enumerated and are presented as the number of proplatelets ± SD per 104 cells. Treatment with QITP sera + Qn significantly reduced proplatelet production.
Reduced proplatelet production by QITP antibodies in the presence of quinine. CD34+ cells were cultured for 5-6 days, and then seeded for treatment with QITP sera plus Qn or with controls (QITP sera − Qn or NS + Qn) and analyzed after 5 or 6 days in culture. (A) Representative image of typical morphologic features of proplatelets taken with a Leica DMIRB inverted microscope (20× objective using a DC200 camera; Leica). Areas containing proplatelets are circled, with proplatelet-bearing Mks indicated by arrows and proplatelet extensions indicated by white arrowheads (see inset for representative features of proplatelets). Bar indicates 50 μm. The images were cropped and revised using Adobe Photoshop CS5. (B) Proplatelets were enumerated and are presented as the number of proplatelets ± SD per 104 cells. Treatment with QITP sera + Qn significantly reduced proplatelet production.
Discussion
Binding of auto-antibodies present in patient plasma to Mks has been suspected for decades.5 It was later shown that specific antiplatelet monoclonal antibodies reduced Mk colony formation and interfered with proplatelet formation.14 More recently, it was reported that ITP auto-antibodies from some patients are capable of inhibiting the production of Mks in vitro6,7 by an as yet unknown mechanism. However, there has been a paucity in the literature on the effects of drug-dependent antibodies on Mk biology. One study found no discernible difference in the morphology of Mks in the bone marrow of a QITP patient,26 whereas another showed Mk hyperplasia in a bone marrow aspirate.27 A study by Greinacher et al on eptifibatide-associated thrombocytopenia found only young Mks in the bone marrow of a patient,8 which was consistent with recent damage to mature Mks. This suggests that drug-dependent antibodies may directly affect Mks. Indeed, Greinacher et al showed that antibodies present in the patient decreased Mk viability in vitro.8 Generally, QITP resolves 5-10 days after drug withdrawal, suggesting that Mks may need to mature to replenish the normal platelet population.
QITP has been recognized for over a century and is the prototype for thrombocytopenia caused by several drugs, including quinidine, ranitidine, rifampicin, and vancomycin. In the current study, we used highly pure Mks derived from peripheral blood CD34+ cells to evaluate the effects of Qn-dependent antibodies on the biology of Mks in culture. We have demonstrated that Qn-dependent antibodies are capable of interacting with Mks, which results in induction of apoptosis and an associated decrease in cell viability, inhibition of late Mk marker expression, and a considerable reduction in proplatelet production capacity.
The patient sera used in these experiments predominantly recognized the GPIb/IX complex (only the serum from JE showed significant drug-dependent interaction with GPIIb/IIIa), as determined by binding to CHO cells expressing these complexes. Our flow cytometric data confirmed that, in the presence of the drug, antibodies from QITP patient serum interact robustly with mature Mks, substantiating the suggestion that drug-dependent antibodies bind their antigens irrespective of the cell type on which they are found. Binding of QITP antibodies to Mks was corroborated by confocal microscopy. To determine whether this antibody/Mk interaction has biologic significance, we first sought to ascertain if the presence of QITP serum influenced Mk viability and differentiation. By culturing day-4 Mks with QITP serum in the presence of Qn, we found a substantial decrease in total cell viability. Moreover, there was a significant reduction in the number of viable GPIIb+, GPIX+, and GPIbα+ cells. Consistent with these observations, there was a concomitant increase in cell death (PI+ cells) in the GPIIb+ population. To extend our observations, we investigated whether treatment with QITP sera inhibited Mk differentiation. Interestingly, our data showed a bias against late markers (GPIX and GPIbα) but no changes in GPIIb expression, suggesting that QITP antibodies cause a shift from a GPIIb+GPIX+ to a more GPIIb+GPIX− population. These findings imply that QITP antibodies are capable of inflicting damage to Mks that results in both increased cell death and a shift toward a more immature population. Furthermore, treatment with purified patient IgG induced similar inhibition of both cell viability and late marker expression. Affinity-purified anti-GPIIb/IIIa IgG did not affect marker expression appreciably, suggesting that, at least for this patient, the effects were limited to GPIb/IX-interacting antibodies. Chang et al6 also found that ITP sera containing anti-GPIb antibodies, but not those containing anti-GPIIb/IIIa antibodies, inhibited megakaryopoiesis. Nevertheless, McMillan et al7 showed that ITP sera containing anti-GPIIb/IIIa antibodies also impaired Mk differentiation. It is possible that anti-GPIIb/IIIa ITP auto-antibodies affect Mks differently from anti-GPIIb/IIIa Qn-dependent antibodies.
How may QITP antibodies reduce Mk viability? The effect of QITP IgG in the absence of serum (Figures 4B and 5A) indicates that complement-mediated cell lysis is not the principal cause of cell death. The fact that the anti-complement protein C5 monoclonal antibody eculizumab did not prevent cell death also supports this proposition. We found that QITP serum induces apoptosis in the presence of the drug (Figure 4E-F and supplemental Figure 3). The increase in Annexin V+ and activated caspase 3+ cells was lower than that observed for 25 μm paclitaxel. Nevertheless, it persisted for more than 72 hours, suggesting that the decline in cell viability shown in Figure 4A and Table 2 and the increase in PI+ cells (Figure 4C) were likely due to the induction of apoptosis by QITP serum plus Qn. Antibody-mediated apoptosis was described previously,28 and caspase 3 activation in response to glycoprotein-binding antibodies has already been observed in platelets.29 Moreover, the GPIb/IX complex, through its association with the signaling protein 14-3-3ζ, has been implicated in the induction of platelet apoptosis on VWF binding.30 An analogous process may account for the induction of apoptosis in Mks on association of QITP antibodies with the GPIb/IX complex.
The regular development of Mks encompasses a progressive increase in ploidy,31 nuclear lobularity, and an associated cell enlargement. Although QITP serum affected cell proliferation and marker expression, there were no detectable differences in the ploidy distribution, average cell size, or lobularity of QITP-treated Mks, implying that the association between QITP antibodies and Mks does not hinder endomitosis. Despite normal morphology, proplatelet formation was significantly impaired in treated Mks, indicating that drug-dependent antibodies may also cause thrombocytopenia by interfering with normal platelet production. Failure to produce proplatelets notwithstanding typical endomitosis seems paradoxical; however, the lack of proplatelet formation despite normal megakaryopoiesis has been reported previously after treatment of Mks with IFN-α2b.32
The molecular mechanisms by which QITP antibodies affect proplatelet formation are not clear, but the effects observed are consistent with previous studies. Antibody-mediated inhibition of proplatelet formation was demonstrated by an anti-GPIbα monoclonal antibody.14 Moreover, Balduini et al have shown that antibodies against the GPIb/IX complex have a profound effect on proplatelet formation both in liquid culture and on adhesive matrices.33 Our findings reinforce the view that large, polyploid Mks fail to form proplatelets when the expression of the GPIb/IX complex is reduced. It is reasonable to speculate that anti-GPIb/IX antibodies present in QITP sera may cause aberrant cytoskeletal development during proplatelet formation and consequently affect proplatelet production. Failure to develop proper internal demarcation membranes in Mks has already been observed in GPIbα-null mice.34 Comparison of QITP with ITP antibodies shows some parallels, but also some noticeable discrepancies. In both cases, there is an evident decrease in cell viability on treatment6,7 (Figure 4) and an increase in cell death. Conversely, QITP serum does not interfere with endomitosis but results in a marked decrease in GPIX and GPIbα expression and lower proplatelet formation. As in ITP, the mechanism of thrombocytopenia in QITP is due mainly to peripheral clearance of antibody-coated platelets. Impaired megakaryopoiesis and decreased proplatelet formation in QITP are likely to further contribute to thrombocytopenia. It is possible that the profound thrombocytopenia and sometimes lengthy recovery times35 seen in QITP and other drug-induced thrombocytopenias may be a consequence of both peripheral platelet destruction and inhibition of proplatelet and platelet production by mature Mks.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by program grant number 455395 from the Australian National Health and Medical Research Council.
Authorship
Contribution: J.P. and F.Y. designed and performed experiments, analyzed data, and wrote the manuscript; Z.A. and X.-M.J. performed experiments; R.S. provided scientific input and edited the manuscript; and B.H.C. conceived the idea, designed and supervised experiments, analyzed data, and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Beng H. Chong, MBBS, PhD, FRACP, Level 2, WR Pitney Bldg, Department of Medicine, St. George Clinical School, Gray St, Kogarah, New South Wales 2217, Australia; e-mail: beng.chong@unsw.edu.au.
References
Author notes
J.P. and F.Y. contributed equally to this study.