Abstract
Integrin-β7 (ITGB7) mRNA is detected in multiple myeloma (MM) cells and its presence is correlated with MAF gene activation. Although the involvement of several integrin family members in MM-stoma cell interaction is well documented, the specific biologic functions regulated by integrin-β7 in MM are largely unknown. Clinically, we have correlated integrin-β7 expression in MM with poor survival outcomes post autologous stem cell transplantation and postsalvage therapy with bortezomib. Functionally, we have found that shRNA-mediated silencing of ITGB7 reduces MM-cell adhesion to extra-cellular matrix elements (fibronectin, E-cadherin) and reverses cell-adhesion–mediated drug resistance (CAM-DR) sensitizing them to bortezomib and melphalan. In addition, ITGB7 silencing abrogated MM-cell transwell migration in response to SDF1α gradients, reduced vessel density in xenografted tumors, and altered MM cells in vivo homing into the BM. Mechanistically, ITGB7 knockdown inhibited focal adhesion kinase (FAK) and Src phosphorylation, Rac1 activation, and SUMOylation, reduced VEGF production in MM–BM stem cell cocultures and attenuated p65-NF-κB activity. Our findings support a role for integrin-β7 in MM-cell adhesion, migration, and BM homing, and pave the way for a novel therapeutic approach targeting this molecule.
Introduction
Multiple myeloma (MM) is a clonal disease of plasma cells that remains, for the most part, incurable despite the advent of several novel therapeutics.1,2 Tumor cells in this disease are cradled within the BM microenvironment by an array of adhesive interactions between the BM extracellular matrix (ECM) components such as fibronectin (FN), laminin, VCAM-1, proteoglycans, collagens and hyaluronan, and a variety of adhesion molecules on the surface of MM cells including integrins, heparan sulfate proteoglycans, and hyaluronan receptors CD44 and RHAMM. These direct adhesive interactions between the BM/ECM and MM cells transduce into the latter antiapoptotic or prosurvival signals leading to drug resistance.3,4 This phenomenon was termed “cell adhesion-mediated drug resistance” (CAM-DR) and it is thought to be one of the major mechanisms by which MM cells escape the cytotoxic effects of therapeutic agents.5,6
Integrins are an extensive family of glycoproteins expressed by many cell types,7,8 including MM cells in which a wide range of integrins (α4, α5, αv, β1, β2, β3, and β7) is detected.9,10 In particular, α5β1 (VLA-5),5,11 α4β1 (VLA-4),5,12-14 and αvβ315 are shown to regulate MM-cell adhesion, migration, homing, invasion as well as drug resistance. The activity of these integrins is regulated both through direct integrins binding to BM-ECM molecules16,17 and through conformational changes induced by inside-out signaling.18-20 The expression of some of these integrins in MM often results from direct cytokine or chemokine stimu-lation and in some instances is driven by MM-associated oncogenes. As such, a recent study by Hurt et al demonstrated that a C-MAF–driven expression of integrin-β7 resulted in enhanced MM-cell adhesion to BM stroma and increased production of VEGF.21 Moreover, transmembrane activator and calcium modulator and cyclophilin ligand interacter (TACI) activation, by the B cell–activating factor (BAFF) and proliferation-inducing ligand (APRIL) binding, also up-regulates C-MAF expression which, in turn, controls cyclin D2 and integrin-β7 gene expression.22 Lastly, gene expression profiling studies detected integrin-β7 mRNA in nearly all MM patients with a particularly high levels in the MS and MF molecular subgroups consistent with a MAF-dependent as well as MAF-independent expression of this integrin.23,24
The integrin-β7 subfamily has 2 known members (α4β7 and αEβ7) that are expressed primarily by leukocytes and have been implicated in secondary lymphoid structure formation, plasma cells homing to gastrointestinal mucosa and inflammatory responses.25-27 Their ligands: the mucosal vascular addressin cell adhesion molecule 1 (MAdCAM-1)28 and E-cadherin are present on the surface of endothelial cells, BM stromal cells,29 and epithelial cells.30 Interaction of α4β7 with MAdCAM-1 allows for tissue-specific migration of circulating lymphocytes into the lamina propria and Peyer patches of the gut, whereas αEβ7 retains intraepithelial lymphocytes within the gut epithelium through E-cadherin binding. Integrin β7 is also reported to be involved in the pathogenesis of several diseases such as colitis,31 diabetic insulitis,32 and lymphoid malignancies including lymphomatous polyposis in mantle cell lymphoma,33 thymic lymphoma,34 and mucosa-associated T- and B-cell non-Hodgkin lymphomas.35 Together, these findings indicate that β7-integrin plays an important role for physiologic functions and pathologic alterations of the immune system. Little, however, is known about the unique biologic roles it plays in MM and whether its functions are essential or redundant to other integrins remains to be elucidated. In this study, we sought to determine the roles of integrin-β7 on MM-cell adhesion, migration, survival, and in vivo BM homing.
Methods
Cell culture and BM stem cells
The human MM cell lines H929, 8226, and U266 were purchased from the ATCC. MM1s (generated by Dr Steven Rosen, Northwestern University, Chicago, IL), OPM2, and INA6 cells were kindly provided by Drs Lawrence Boise (Emory University, Atlanta, GA) and Renate Burger (University of Erlangen-Nuernberg, Germany). Details are available in the supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Flow cytometric analysis
Direct and indirect immunofluorescence flow cytometric analysis was performed using a FACSCalibur flow cytometer (BD Biosciences). The expression of integrin-β7 was monitored using purified rat anti–human integrin-β7 (clone FIB504; BD Biosciences) and AlexaFluor 488 anti–rat IgG (Invitrogen) was used as a secondary Ab. Data were acquired from 10 000 events and staining was compared with matched isotype control Ab.
Quantitative RT-PCR
The mRNA expression of integrin-β7 was detected by RT-PCR in MM cell lines and in sorted CD138+ cells from MM patients as detailed in supplemental Methods.
MM tissue microarray
Myeloma tissue microarrays (TMA) were generated from formalin-fixed paraffin-embedded (FFPE) sections of pretreatment diagnostic BM biopsies (n = 79) and used to evaluate the expression of integrin-β7 (detailed in supplemental Methods).
Lentiviral integrin β7 (ITGB7) shRNA transduction
Lentiviral transduction particles (Sigma-Aldrich) were used to deliver siRNAs expressed from shRNAs for knockdown of ITGB7 gene (NM_000889) into MM1S, INA-6, and H929 MM cells. The sense oligonucleotide sequences for ITGB7 shRNAs and control nontarget scrambled shRNA are shown in Table 1. Lentiviral ITGB7 shRNA and nontarget control shRNA were produced in HEK293T packaging cells, concentrated at different MOIs and then individually added into MM-cell suspensions in the presence of 6 μg/mL polybrene and transduced for 24 hours followed by selection in puromycin (2 μg/mL; Invitrogen) to obtain ITGB7silenced and control ITGB7positive cells. The efficiency of ITGB7 silencing was monitored by flow cytometry and RT-PCR. To reduce the possibility of an off-target effect of an individual shRNA construct, puromycin-resistant cells with the most efficient ITGB7 knockdown, from 2 separate infections with individual and distinct lentiviral ITGB7 shRNAs, were selected for further studies.
Cell adhesion assay
A fluorometric-based assay has be used to evaluate the adherence of ITGB7silenced and control ITGB7positive cells to coated plates with fibronectin (FN), E-cadherin (E-CDH), and human BM stem cells (BMSCs). Briefly, MM cells (5 × 106/mL) were labeled with calcein-acetoxymethyl ester (Molecular Probes) for 30 minutes at 37°C, washed and resuspended in adhesion medium and added to FN, E-CDH, and BMSC-coated plates. After 60-240 minutes, nonadherent cells were removed by gentle washing. Remaining adherent calcein-labeled cells were quantitated in a fluorescence multiwell plate reader (Molecular Devices) using a 494/517nM filter set.
Transwell migration assay and invasion studies
For migration studies ITGB7silenced and ITGB7positive MM cells (5 ×105 cells) suspended in RPMI 1640 media were placed in the upper chambers of transwell plates (pore size 0.8 μm; Costar-Corning) with serial concentrations of SDF-1α (0-20nM) added to 500 μL of RPMI 1640 in the lower chambers. After 4 hours at 37°C, cells that migrated to the lower chambers were labeled with calcein and counted on a fluorescence plate reader (Molecular Devices).
MM-cell invasion was measured by determining their ability to migrate through transwell inserts containing an 8-μm pore-size polycarbonate membrane, over which a thin layer of Matrigel (BD Biosciences) was dried up. Briefly, ITGB7positive and ITGB7silenced cells (1 × 105) resuspended in 300 μL of serum-free media, were added to the upper compartment of the transwell plates with Matrigel-coated inserts and incubated for 24-48 hours at 37°C. Five hundred microliters of RPMI 1640 supplemented with 10nM SDF-1α was added to the lower chamber. Cells that invaded the Matrigel-coated filters were fixed in methanol, stained with 0.2% crystal violet, and counted using an inverted microscope.
ITGB7 blocking Ab and rescue experiments
To exclude the possibility of an off-target effect of the used shRNA constructs, blocking Ab, and rescue experiments were also performed as detailed in supplemental Methods.
VEGF and cytokine ELISA
ITGB7silenced and control ITGB7positive cells were added at 1 × 104 cells per well into 96-well plates coated with or without human BMSCs and incubated at 37°C for 48 hours. Supernatant was collected and assayed for VEGF by ELISA (R&D Systems) and profiled for their cytokines secretions using an ELISA-based assay (SA Biosciences).
Cell apoptosis assay
Survival of MM cells cultured with bortezomib or melphalan in 96-well plates under different conditions (coated with BMSCs or FN or E-CDH) was evaluated by FITC-conjugated Annexin V (Biovision) staining using flow cytometric analysis as previously described.36
Immunoblotting and confocal microscopy
Whole-cell lysates were subjected to SDS-PAGE and transferred to PVDF membrane (Bio-Rad Laboratories). The Abs used for immunoblotting included anti-phospho- focal adhesion kinase (FAK; Tyr397), anti-FAK, anti-phospho-Src (Tyr416), anti-phospho-ERK1/2 (Thr202/Tyr204), anti-ERK1/2, anti-SUMO1, and anti-α-Tubulin (Cell Signaling Technology).
A laser-scanning confocal microscope (LSM 510; Zeiss) was used to assess the distribution and localization of p-FAK. Images were acquired using a 100× Zeiss Plan-Apochromat oil objective and captured by Zeiss image browser using identical laser intensity and gain parameters. ITGB7silenced and ITGB7positive cells were cultured on FN-coated plates and immunostained with anti-actin (AlexaFluor 555 phalloidin), rabbit anti-phospho-FAK (Tyr861), secondary anti–rabbit AlexaFluor 488 and DAPI (Invitrogen).
Rac-1 activation assay
For the detection of activated Rac1 (GTP bound), equal amounts of protein lysates (500 μg) were obtained from ITGB7silenced and ITGB7positive cells cultured in uncoated or FN-coated plates. Lysates were then immunoprecipitated with GST-PAK1 (p21-binding domain corresponding to residues 67-150) agarose beads (20 μg; Chemicon-Millipore) for the pull-down of activated GTP-bound Rac1. Lysates and beads were incubated for 1 hour with agitation at 4°C. Beads were then pelleted by centrifugation (10 seconds, 14 000g, 4°C), washed twice with lysis buffer, resuspended and boiled in 20 μL of 2× Laemmli buffer and subjected to SDS-PAGE and PVDF membrane transfer. Activated Rac1 (GTP-Rac1 bound to PAK1 beads) was detected by immunoblotting with anti-Rac1 Abs (Chemicon-Millipore) and anti-SUMO1 (Cell Signaling Technology).
NF-κB activity
NF-κB activity was investigated using the TransAM NF-κB p65 kit, a DNA-binding ELISA-based assay (Active Motif) and by NF-κB p65 kit immunostaining (details in supplemental Methods).
In vivo studies
Murine xenograft model of human MM.
All animal studies were conducted according to protocols and standard procedures approved by the University of Calgary Animal Care Committee. Animals were killed when their tumors reached 2 cm in largest diameter or when exhibiting any distress symptoms. CB-17 SCID-mice (Charles River Laboratories) were subcutaneously inoculated in the interscapular area with 2 × 106ITGB7silenced or ITGB7positive MM1S cells.37 Tumor sizes were measured every 3 days in 2 dimensions using an electronic caliper, and the tumor volume was calculated using the following formula: V = 0.5 a × b2, where a and b are the long and short diameter of the tumor, respectively.
Myeloma cell BM homing.
Mice with established MM xenografts from ITGB7silenced and ITGB7positive groups were killed whenever their tumors reached 2 cm in largest diameter. Xenografts as well as specimens from the femurs, liver, and spleen of killed animals were rinsed with PBS, fixed with 4% formaldehyde in PBS, dehydrated with ethanol, embedded in paraffin blocks, and sectioned. Sections from femurs, liver, and spleen were stained with Cy-3 labeled rabbit anti–human CD138 Ab (Abcam) for the detection of human MM cells in mice organs. BM homing was reported as the average of the percentages of human CD138+ cells counted on BM sections from femurs of examined SCID mice (n = 3 mice per condition).
Microvessel density.
Serial sections from xenografted tumors were stained for anti–mouse CD31 (Santa Cruz Biotechnology) to evaluate the tumor microvessel density (MVD). MVD was expressed as a percentage according to the following formula: MVD (%) = CD31+ target area/total area examined. The shown MVD is the mean value of 10 randomly selected sections from ITGB7silenced (n = 3) and ITGB7positive (n = 3) xenografts.
In vivo flow cytometry
ITGB7silenced or ITGB7positive MM1S and H929 cells (2 × 106) labeled with Calcein-AM were injected into the tail vein of sedated BALB/c mice (n = 3 per condition) and monitored for their extravasation and homing by in vivo flow cytometry as previously described.18 Mice were anesthetized and placed on a heated stage (32°C) and a laser beam (473 nm) was focused on an appropriate arteriole in the mouse ear for the excitation of calcein-labeled circulating MM cells. Signals were detected by photomultiplier (PMT) tubes through 528 ± 19-nm bandpass filter, and then analyzed with Matlab software. Cell counts were obtained at least every 5 minutes for 40-50 minutes.
Statistical analysis
Statistical significances of differences were determined by using the Student t test. The minimal level of significance was P < .05.
Results
Integrin β7 is highly expressed in MM cells and its expression correlates with poor survival
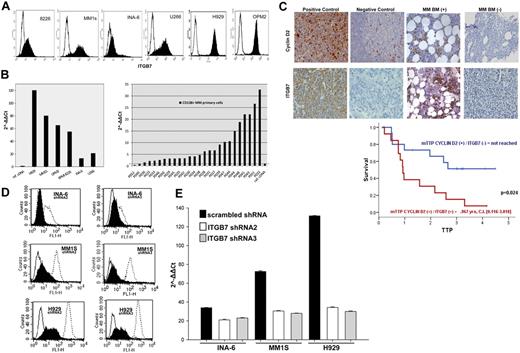
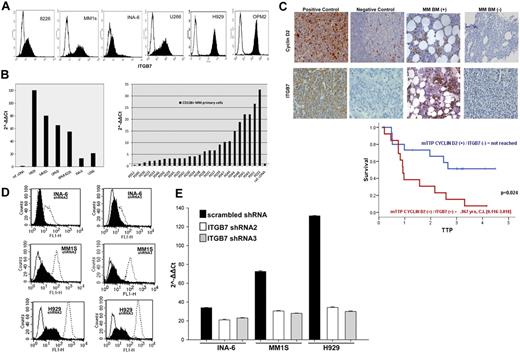
We first screened the expression of integrin-β7 in a panel of MM cell lines (OPM2, MM1S, 8226, U266, H929, and INA6) by flow cytometry. As shown in Figure 1A, integrin-β7 was expressed by all the MM cell lines we have tested, albeit at different levels. qRT-PCR analysis also confirmed ITGB7 mRNA expression in these cell lines with the highest mRNA expression in cells harboring a t(4;14)(H929, OPM2) or t(14;16)(MM1S and 8226; Figure 1B). In primary CD138+ cells sorted from the BM aspirates of individual MM patients, ITGB7 mRNA was detectable (relative to a reference pooled human cDNA) in 24 of 26 (92.3%) of the cases (Figure 1B). A similar frequency and pattern of integrin-β7 expression was also observed in a cohort of patients treated with the Total Therapy protocol and in the MMRC myeloma cell line dataset (supplemental Figure 1A-B). To examine whether integrin-β7 had any impact on disease behavior, we correlated its expression levels with MM patients' survival post frontline stem cell transplantation, in a TMA constructed from their diagnostic BM biopsies (n = 79). By immunohistochemical (IHC) staining, 78.5% biopsies were positive for integrin-β7 with 1 of 3 of these cases coexpressing cyclin D2 and integrin-β7 (Figure 1C, supplemental Figure 1C-D). Patients coexpressing cyclin D2 and integrin-β7 faired very poorly with a median time to progression (TTP) of 0.9 years compared with 57% relapse free at 4 years in patients expressing either one alone or none (Figure 1C). Similarly, we have also interrogated the MM dataset (GSE9782) deposited by Mulligan and colleagues38 for the prognostic impact of ITGB7 mRNA in relapsed and refractory patients treated with salvage bortezomib. High expression (top quartile) of ITGB7 (205718_at Affymetrix probe) also correlated with a significantly shorter survival in this patient cohort (supplemental Figure 2A-C).
Integrin-β7 expression in myeloma cell lines and primary myeloma cells. (A) Flow cytometric analysis demonstrating integrin-β7 expression in MM cell lines (OPM2, INA6, MM1S, 8226, H929, and U266). Open histograms represent isotype IgG1 control, whereas solid histograms indicate integrin-β7 staining. (B) ITGB7 mRNA expression as determined by quantitative RT-PCR in indicated MM cell lines and CD138+ sorted cells from the BM aspirates of MM patients. Data quantification was carried out by the 2−ΔΔct method relative to a reference human cDNA library (Stratagene). (C) Myeloma tissue microarray (TMA) constructed from the BM biopsies of 79 newly diagnosed MM patients was used to evaluate the expression of integrin-β7 and Cyclin D2 by immunohistochemical staining (IHC) and its impact on prognosis. Shown in the insets are representative H&E, Cyclin D2, and integrin-β7 staining of MM patients BM biopsies. Also shown, positive and negative controls (Cyclin D2: positive OPM2 myeloma cell line; negative: human tonsils; Integrin-β7: positive MM1S myeloma cell line; negative: HL60 leukemia cell line). Images were acquired with a bright light Olympus BX5 microscope and multispectral camera (Nuance Fx; CRi) 10× magnification. Kaplan-Meier survival curves indicate the shorter time to progression (TTP) for patients with MM cells coexpressing Cyclin D2 and integrin-β7. (D) ITGB7 silencing with lentiviral mediated delivery of ITGB7-specific shRNAs (shRNA 2 and 3) in MM1S, H929 and INA-6 cells. Shown is integrin-β7 expression in puromycin-selected cells transfected with ITGB7-specific shRNAs (solid histogram: ITGB7silenced) or scrambled oligonucleotides sequences (open histogram, dashed line: ITGB7positive) relative to control IgG1 istoype (open histogram, solid line) as determined by flow cytometry. (E) qRT-PCR confirming ITGB7 silencing in established puromycin-resistant ITGB7silenced versus ITGB7positive cells.
Integrin-β7 expression in myeloma cell lines and primary myeloma cells. (A) Flow cytometric analysis demonstrating integrin-β7 expression in MM cell lines (OPM2, INA6, MM1S, 8226, H929, and U266). Open histograms represent isotype IgG1 control, whereas solid histograms indicate integrin-β7 staining. (B) ITGB7 mRNA expression as determined by quantitative RT-PCR in indicated MM cell lines and CD138+ sorted cells from the BM aspirates of MM patients. Data quantification was carried out by the 2−ΔΔct method relative to a reference human cDNA library (Stratagene). (C) Myeloma tissue microarray (TMA) constructed from the BM biopsies of 79 newly diagnosed MM patients was used to evaluate the expression of integrin-β7 and Cyclin D2 by immunohistochemical staining (IHC) and its impact on prognosis. Shown in the insets are representative H&E, Cyclin D2, and integrin-β7 staining of MM patients BM biopsies. Also shown, positive and negative controls (Cyclin D2: positive OPM2 myeloma cell line; negative: human tonsils; Integrin-β7: positive MM1S myeloma cell line; negative: HL60 leukemia cell line). Images were acquired with a bright light Olympus BX5 microscope and multispectral camera (Nuance Fx; CRi) 10× magnification. Kaplan-Meier survival curves indicate the shorter time to progression (TTP) for patients with MM cells coexpressing Cyclin D2 and integrin-β7. (D) ITGB7 silencing with lentiviral mediated delivery of ITGB7-specific shRNAs (shRNA 2 and 3) in MM1S, H929 and INA-6 cells. Shown is integrin-β7 expression in puromycin-selected cells transfected with ITGB7-specific shRNAs (solid histogram: ITGB7silenced) or scrambled oligonucleotides sequences (open histogram, dashed line: ITGB7positive) relative to control IgG1 istoype (open histogram, solid line) as determined by flow cytometry. (E) qRT-PCR confirming ITGB7 silencing in established puromycin-resistant ITGB7silenced versus ITGB7positive cells.
Integrin-β7 silencing reduces MM-cell adhesion to stromal elements, migration to SDF1α chemokine gradient, and invasion
Integrin-mediated interactions between malignant plasma cells and BM stroma may be crucial for their adhesion and homing to BM niches. To investigate the function of integrin-β7 in MM, its expression was knocked down in 3 MM cell lines (MM1S, H929, and INA6) with lentiviral ITBG7 shRNAs. Five lentiviral ITGB7 shRNAs were generated targeting different regions in the ITGB7 mRNA. Specific ITGB7 down-regulation was confirmed by reduced integrin-β7 mRNA and protein expression in cells infected with shRNA 2 or 3 (hereafter referred to as ITGB7silenced), whereas integrin-β7 was expressed in cell lines infected with control scrambled shRNA lentiviral vector (ITGB7positive; Figures 1D-E; supplemental Figure 3).
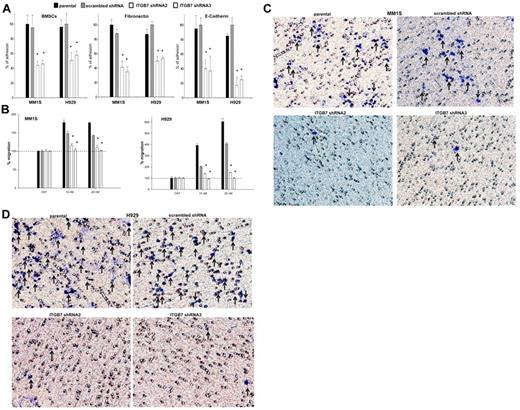
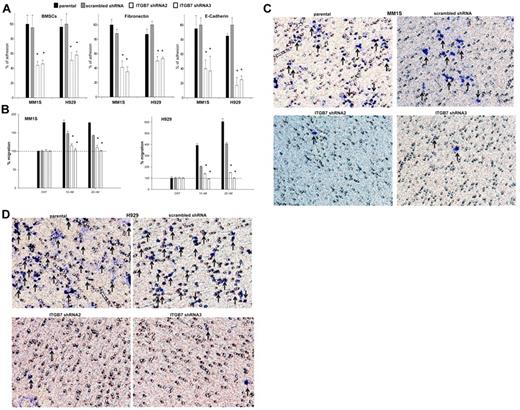
In a calcein-AM fluorescence-based adhesion assay, we next evaluated the effects of ITGB7 silencing on MM-cell adhesion to FN, E-CDH, and human BMSCs. As shown in Figure 2A, ITGB7silenced cells exhibited 50%-70% less adhesion to BMSCs compared with their relative ITGB7positive controls. This effect was even more evident on FN- and E-CDH–coated plates, confirming the role integrin-β7 plays in MM-cell adhesion to stromal elements. Because integrin-β1 heterodimers (in particular α4β1 and α5β1) are known to be involved in MM-cell migration to VEGF, SDF1α, and IGF1 gradients,18,19 we also sought to determine the contribution of integrin-β7 to MM-cell migration. In a transwell migration assay, SDF-1α (10-20nM) induced a 2-fold increase in the migration of ITGB7positive but not ITGB7silenced cells into the lower chambers, consistent with a key role for integrin-β7 in MM cells guided migration toward SDF-1α gradients (Figure 2B). Lastly, as MM cells need to actively penetrate through the subendothelial basement membrane of the BM sinus to migrate in and out of the BM, we examined whether integrin-β7 is required for MM-cell invasion. As shown in Figure 2C and D, ITGB7 silencing also significantly reduced the ability of ITGB7silenced MM1S and H929 cells to invade through a reconstituted membrane (Matrigel) compared with ITGB7positive cells. In INA6 cells, and consistent with their low ITGB7 expression, ITGB7 silencing had a similar effect on these cells adhesion to BMSCs and FN, but a lesser or no effect on these cells' adhesion to E-cadherin or transwell migration (supplemental Figure 4A-C).
Effects of ITGB7 silencing on adhesion, migration, and invasion of MM cells. (A) Adhesion of calcein-AM–labeled ITGB7silenced (ITGB7 shRNA2 and ITGB7 shRNA3) versus ITGB7positive (scrambled shRNA) and parental (nontransfected) MM1S and H929 cells to BMSCs, FN, and E-CDH–coated 96-well microplates. Unattached cells were washed and adherent cells were measured in a fluorescence plate reader. Data are presented as percentage of respective controls (mean ± SD of triplicates from 3 independent experiments). (B) Transwell migration (8-μm pores; Costar) of calcein-AM–labeled ITGB7silenced (ITGB7 shRNA2 and ITGB7 shRNA3) vs ITGB7positive (scrambled shRNA) and parental (nontransfected) MM1S and H929 cells to RPMI serum-free media (cnt) or RPMI supplemented with SDF-1α (10 and 20nM). The fluorescence values, quantitated in a fluorescence multiwell plate reader using the 494/517nM filter set; percentage of migrating cells to SDF-1α versus control (serum-free RPMI) are shown. Data are presented as the mean ± SD of triplicates from 3 independent experiments. (C-D) Shown is a representative transwell Matrigel invasion of ITGB7silenced (ITGB7 shRNA2 and ITGB7 shRNA3) vs ITGB7positive (scrambled shRNA) and parental (nontransfected) MM1S and H929 cells under the conditions described in “Transwell migration assay and invasion studies.” Cells that invaded the Matrigel-coated filters are stained with crystal violet and counted using an inverted microscope. Images were acquired with a bright light Olympus BX5 microscope and multispectral camera (Nuance FX; CRi). 40× magnification.
Effects of ITGB7 silencing on adhesion, migration, and invasion of MM cells. (A) Adhesion of calcein-AM–labeled ITGB7silenced (ITGB7 shRNA2 and ITGB7 shRNA3) versus ITGB7positive (scrambled shRNA) and parental (nontransfected) MM1S and H929 cells to BMSCs, FN, and E-CDH–coated 96-well microplates. Unattached cells were washed and adherent cells were measured in a fluorescence plate reader. Data are presented as percentage of respective controls (mean ± SD of triplicates from 3 independent experiments). (B) Transwell migration (8-μm pores; Costar) of calcein-AM–labeled ITGB7silenced (ITGB7 shRNA2 and ITGB7 shRNA3) vs ITGB7positive (scrambled shRNA) and parental (nontransfected) MM1S and H929 cells to RPMI serum-free media (cnt) or RPMI supplemented with SDF-1α (10 and 20nM). The fluorescence values, quantitated in a fluorescence multiwell plate reader using the 494/517nM filter set; percentage of migrating cells to SDF-1α versus control (serum-free RPMI) are shown. Data are presented as the mean ± SD of triplicates from 3 independent experiments. (C-D) Shown is a representative transwell Matrigel invasion of ITGB7silenced (ITGB7 shRNA2 and ITGB7 shRNA3) vs ITGB7positive (scrambled shRNA) and parental (nontransfected) MM1S and H929 cells under the conditions described in “Transwell migration assay and invasion studies.” Cells that invaded the Matrigel-coated filters are stained with crystal violet and counted using an inverted microscope. Images were acquired with a bright light Olympus BX5 microscope and multispectral camera (Nuance FX; CRi). 40× magnification.
Consistent with the ITGB7 shRNA studies, blocking anti-ITGB7 mAb (FIB504 clone) resulted in a significant reduction in MM-cell adhesion to stromal elements (BMSCs, FN, and E-CDH) and migration (supplemental Figure 5A-B). To further demonstrate that shRNA ITGB7 silencing was responsible for the reduction of MM-cell adhesion and migration, a rescue experiment was performed by constructing a silent mutant ITGB7 (ITGB7mut) that is resistant to ITGB7 shRNA2 and expressing it in ITGB7silenced shRNA2 cells (MM1S and H929). ITGB7silenced shRNA2 cells infected with the silent mutant ITGB7 (ITGB7mut) showed a restored expression of ITGB7, an effect not seen in cells infected with wild-type ITGB7 (ITGB7WT; supplemental Figure 6). Importantly, the expression of ITGB7mut significantly restored the cells adhesive and migratory functions that were suppressed by shRNA-mediated ITGB7 silencing (supplemental Figure 7A-D).
Integrin-β7 regulates VEGF and cytokines production in MM and BMSCs
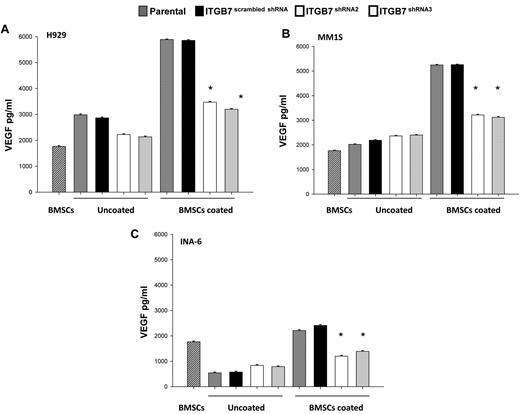
Direct interaction between MM cells and the BM microenvironment is known to induce MM and stromal cell secretion of several cytokines including VEGF.4,39 Therefore, we next investigated whether integrin-β7-dependent cell adhesion, like integrin-β1,19 mediates VEGF secretion. As shown in Figures 3, while MM cells and stroma cells cultured alone did not secrete any significant quantities of VEGF, cocultures of BMSCs with ITGB7positive MM cells lead to a 3-fold increase in VEGF secretion. In contrast, cocultures of BMSCs and ITGB7silenced cells significantly reduced levels of VEGF secretion, albeit not to baseline levels (BMSCs alone), suggesting that in addition to integrin-β7 other adhesion molecules or integrins may be involved in MM-BMSC VEGF secretion. Furthermore, ITGB7 silencing altered MM-cell production of several cytokines and growth factors in MM-BMSC cell cocultures. In particular, ITGB7silenced cells produced significantly lower amounts of IL-1β and MIP-1β suggesting a possible role for integrin-β7 in MM bone disease and osteoclasts activation (supplemental Figure 8A-C).
Effects of ITGB7 silencing on cytokines production in MM-BMSC cocultures. (A-C) ITGB7silenced (ITGB7 shRNA2 and ITGB7 shRNA3) vs ITGB7positive (scrambled shRNA) and parental (nontransfected) H929 and INA-6 MM cells were added into 96-well plates coated with BMSCs or uncoated plates and incubated at 37°C for 48 hours. Supernatant was collected and assayed for VEGF by ELISA-based assay. Data are presented as the mean ± SD of triplicates from 2 independent experiments.
Effects of ITGB7 silencing on cytokines production in MM-BMSC cocultures. (A-C) ITGB7silenced (ITGB7 shRNA2 and ITGB7 shRNA3) vs ITGB7positive (scrambled shRNA) and parental (nontransfected) H929 and INA-6 MM cells were added into 96-well plates coated with BMSCs or uncoated plates and incubated at 37°C for 48 hours. Supernatant was collected and assayed for VEGF by ELISA-based assay. Data are presented as the mean ± SD of triplicates from 2 independent experiments.
Integrin β7 silencing restores MM drug sensitivity
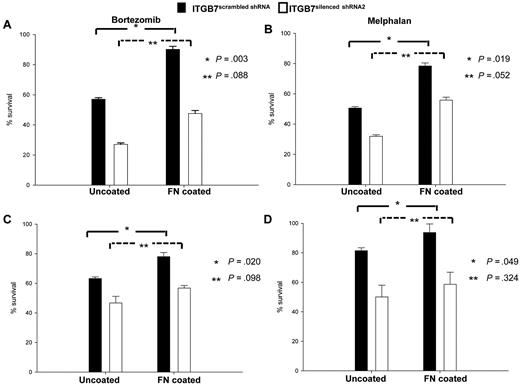
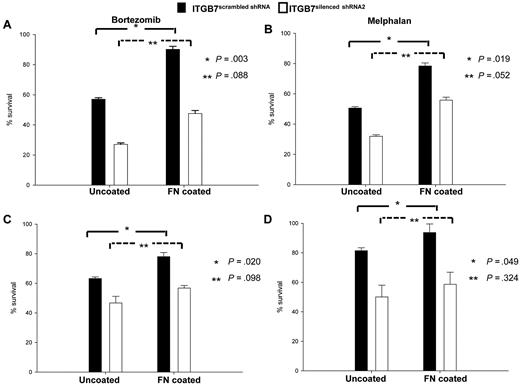
To investigate the involvement of integrin-β7 in CAM-DR, ITGB7positive and ITGB7silenced cells were cultured in uncoated or FN-coated plates in the presence or absence of bortezomib or melphalan. As shown in Figure 4, when cultured in uncoated plates ITGB7silenced cells were more sensitive to the cytotoxic effect of bortezomib and melphalan. In addition, while adhesion to FN protected ITGB7positive cells from the cytotoxicity of bortezomib and melphalan, this effect was partially abrogated in ITGB7silenced cells. Taken together, these results support a role of ITGB7 in delivering a prosurvival signal to MM cells and in conferring drug resistance through cell-adhesion dependent (in FN-coated plates) as well as independent (uncoated plates) mechanisms.
ITGB7 silencing reverses CAM-DR to bortezomib and melphalan. (A-D) ITGB7silenced and ITGB7positive H929 (A-B) and MM1S (C-D) were incubated in 96 well-uncoated or FN-coated plates and cultured for 24 hours in the absence and presence of bortezomib (2.5nM; A,C) or melphalan (20mM; B,D). Annexin V staining was used to evaluate the cytotoxic effects of these agents under the indicated conditions. Shown are the mean ± SD of triplicates from 3 independent experiments (*P < .05; **P > .05).
ITGB7 silencing reverses CAM-DR to bortezomib and melphalan. (A-D) ITGB7silenced and ITGB7positive H929 (A-B) and MM1S (C-D) were incubated in 96 well-uncoated or FN-coated plates and cultured for 24 hours in the absence and presence of bortezomib (2.5nM; A,C) or melphalan (20mM; B,D). Annexin V staining was used to evaluate the cytotoxic effects of these agents under the indicated conditions. Shown are the mean ± SD of triplicates from 3 independent experiments (*P < .05; **P > .05).
Integrin β7-mediated adhesion to FN activates the FAK and p65 NF-κB
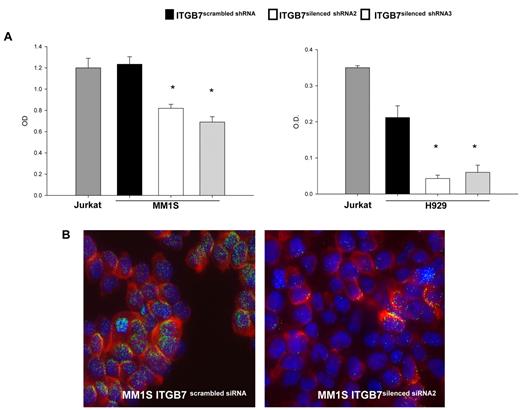
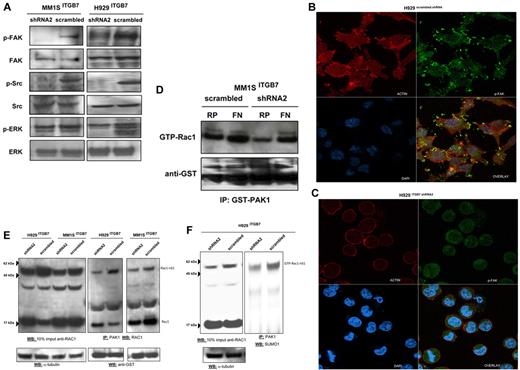
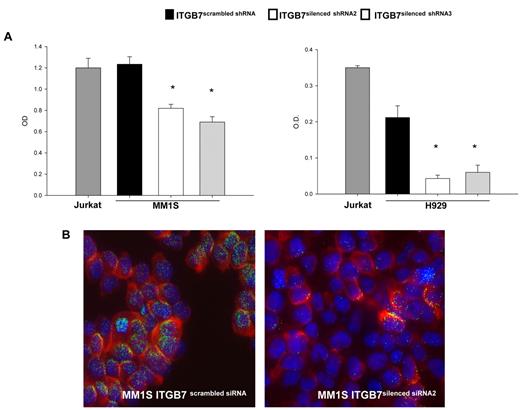
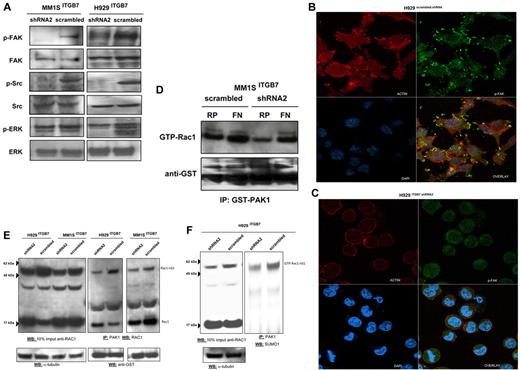
Adhesion of MM cells to FN is reported to increase NF-κB activity and to modulate the expression of several NFκB-dependent genes.13 Using an ELISA-based assay we measured, in nuclear fractions extracted from ITGB7positive and ITGB7silenced MM1S and H929 cells, the NFκB-p65 binding to a p65-specific consensus motif. A significant reduction in NF-κB activity as measured by ELISA and decreased nuclear NFκB-p65 translocation by immunofluorescence staining were observed in ITGB7silenced compared with ITGB7positive cells (Figure 5), consistent with a role for p65 (RelA) in integrin-β7 downstream signaling in MM. Similar results are also shown in supplemental Figure 4C for the INA-6 cells. In addition, ITGB7 silencing significantly attenuated FAK, and Src (Tyr-416) activation in H929 and MM1S cells plated on FN-coated plates and reduced ERK phosphorylation in H929 but not MM1S cells (Figure 6A-C, supplemental 9A-C). ITGB7 knockdown also reduced the activation of Rac1 GTPase (as determined by Rac1-GTP binding to GST-PAK1) in MM1S cells cultured in uncoated (RP) as well as FN-coated plates (Figure 6D). Recent studies have indicated that Rac SUMOylation is required for optimal Rac activation, lamellipodia-ruffle formation, cell migration, and invasion.40 When cultured on FN-coated plates, we have observed a loss of lamellipodia-ruffle formation in H929 ITGB7silenced compared with ITGB7positive cells (Figure 6B-C). This effect was less evident in MM1S cells and was only seen when cells were cultured on poly-lysine–coated plates with FN (supplemental Figure 9A-C). We also noted in Rac1 Western blots (including IP of GTP-Rac1) an up-shifted band (∼ 55 kDa) consistent with the SUMOylation of Rac1 (Figure 6E, supplemental Figure 10). Therefore we next examined whether ITGB7 knockdown reduced Rac1 SUMOylation. As shown in Figure 6E and F and supplemental Figure 10, when pull-downs of GTP-Rac1 were probed for SUMO1, ITGB7 knockdown significantly reduced SUMOylated RAC1.
ITGB7 silencing reduces p65 NF-κB activity in MM cells. (A) NF-κB-p65 transcription factor binding to its consensus sequence on a plate-bound oligonucleotide was measured by ELISA in nuclear extracts from Jurkat, ITGB7silenced (ITGB7 shRNA2 and ITGB7 shRNA3), and ITGB7positive (scrambled shRNA) MM1S and H929 cells cultured on FN-coated plates. Shown results represent means (± SD) of triplicate experiments (* P < .05). (B) Decreased nuclear NF-κB-p65 translocation or localization in ITGB7silenced (right) compared with ITGB7positive (left) MM1S cells cultured on FN-coated plates as detected by immunofluorescence staining with p65 NF-kB Ab (Cy-3 labeled) and DAPI for nuclear visualization. Image acquisition was performed with epifluorescence Olympus BX5 microscope and multispectral color camera (Nuance FX; CRi) with 60× magnification and oil immersion (details in supplemental Materials).
ITGB7 silencing reduces p65 NF-κB activity in MM cells. (A) NF-κB-p65 transcription factor binding to its consensus sequence on a plate-bound oligonucleotide was measured by ELISA in nuclear extracts from Jurkat, ITGB7silenced (ITGB7 shRNA2 and ITGB7 shRNA3), and ITGB7positive (scrambled shRNA) MM1S and H929 cells cultured on FN-coated plates. Shown results represent means (± SD) of triplicate experiments (* P < .05). (B) Decreased nuclear NF-κB-p65 translocation or localization in ITGB7silenced (right) compared with ITGB7positive (left) MM1S cells cultured on FN-coated plates as detected by immunofluorescence staining with p65 NF-kB Ab (Cy-3 labeled) and DAPI for nuclear visualization. Image acquisition was performed with epifluorescence Olympus BX5 microscope and multispectral color camera (Nuance FX; CRi) with 60× magnification and oil immersion (details in supplemental Materials).
ITGB7 binding to FN activates FAK, Src, and Rac-1. (A) Lysates from ITGB7silenced and ITGB7positive MM1S and H929 cells cultured on FN were screened by immunoblotting for the activation of FAK (phospho-Tyr397-FAK), Src (phospho-Tyr416-Src), and ERK (phospho-Thr202/Tyr204ERK1/2) and loading controls. (B-C) p-FAK localization and distribution in ITGB7positive (B) and ITGB7silenced (C) H929 cell cultures on FN-coated plates and measured by laser scan confocal microscopy. Shown are the images of immunostaining with anti-actin (Red: AlexaFluor 555 phalloidin), anti-phospho-FAK (Green: AlexaFluor 488) and DAPI with overlay images. (D) Rac-1 activation (GTP-Rac1 bound to GST-PAK1-p21-binding domain corresponding to residues 67-150) measured by immunoblotting with Rac-1 Ab on GST-PAK1 immunoprecipitated protein lysates (500 μg) from ITGB7silenced and ITGB7positive MM1S cells cultured under the indicated conditions (RP = regular uncoated plate, FN = fibronectin-coated plates) with anti-GST (Cell Signaling Technology) loading control. (E) Activation of Rac1 was measured in lysates from ITGB7silenced and ITGB7positive MM1S and H929 cells cultured on FN. Pulldowns of activated Rac1 (GTP-Rac1 bound to GST-PAK1 beads) were analyzed by Western blot for Rac1 and reprobed with anti-GST as loading control. Blot on the left represent a Western blot for total Rac1 in 10% of the imput lysate used for the immunoprecipiation of activated Rac1. Rac1-nS1 indicates the up-shifted band of Rac1 corresponding to SUMOylated Rac1. (F) Pulldowns of activated Rac1 (GTP-Rac1 bound to GST-PAK1 beads) from H929 ITGB7silenced and ITGB7positive cells cultured on FN were blotted with anti-SUMO1 Ab. Blot on the left represent a Western blot for total Rac1 in 10% of the input lysate used for IP. GTP-Rac1-nS1 indicates the up-shifted band of Rac1 corresponding to SUMOylated Rac1.
ITGB7 binding to FN activates FAK, Src, and Rac-1. (A) Lysates from ITGB7silenced and ITGB7positive MM1S and H929 cells cultured on FN were screened by immunoblotting for the activation of FAK (phospho-Tyr397-FAK), Src (phospho-Tyr416-Src), and ERK (phospho-Thr202/Tyr204ERK1/2) and loading controls. (B-C) p-FAK localization and distribution in ITGB7positive (B) and ITGB7silenced (C) H929 cell cultures on FN-coated plates and measured by laser scan confocal microscopy. Shown are the images of immunostaining with anti-actin (Red: AlexaFluor 555 phalloidin), anti-phospho-FAK (Green: AlexaFluor 488) and DAPI with overlay images. (D) Rac-1 activation (GTP-Rac1 bound to GST-PAK1-p21-binding domain corresponding to residues 67-150) measured by immunoblotting with Rac-1 Ab on GST-PAK1 immunoprecipitated protein lysates (500 μg) from ITGB7silenced and ITGB7positive MM1S cells cultured under the indicated conditions (RP = regular uncoated plate, FN = fibronectin-coated plates) with anti-GST (Cell Signaling Technology) loading control. (E) Activation of Rac1 was measured in lysates from ITGB7silenced and ITGB7positive MM1S and H929 cells cultured on FN. Pulldowns of activated Rac1 (GTP-Rac1 bound to GST-PAK1 beads) were analyzed by Western blot for Rac1 and reprobed with anti-GST as loading control. Blot on the left represent a Western blot for total Rac1 in 10% of the imput lysate used for the immunoprecipiation of activated Rac1. Rac1-nS1 indicates the up-shifted band of Rac1 corresponding to SUMOylated Rac1. (F) Pulldowns of activated Rac1 (GTP-Rac1 bound to GST-PAK1 beads) from H929 ITGB7silenced and ITGB7positive cells cultured on FN were blotted with anti-SUMO1 Ab. Blot on the left represent a Western blot for total Rac1 in 10% of the input lysate used for IP. GTP-Rac1-nS1 indicates the up-shifted band of Rac1 corresponding to SUMOylated Rac1.
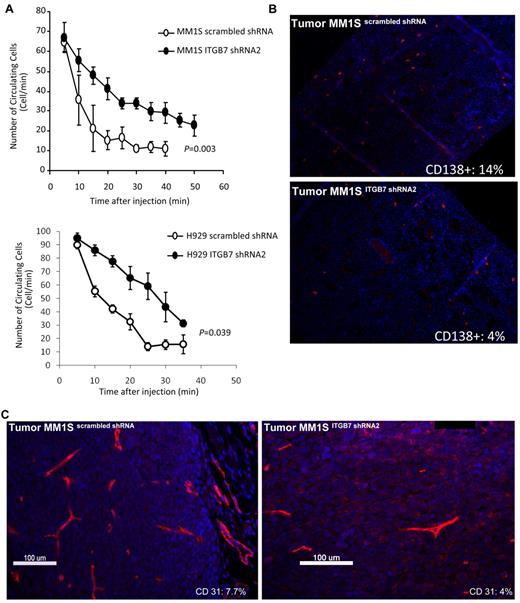
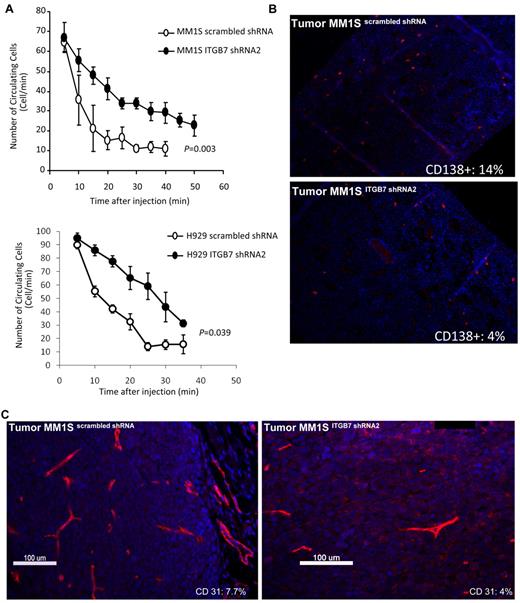
Integrin-β7 silencing reduces in vivo MM cells homing to the BM and decreases tumor vessel density in MM xenografts
We investigated in vivo the effect of ITGB7 silencing on tumor growth and noted no difference in the growth kinetics of the ITGB7positive or ITGB7silenced xenografts in SCID mice. The median time from MM-cell inoculation to mice sacrifice for ITGB7positive was t = 24 ± 2 days compared with t = 26 ± 2 days in ITGB7silenced xenografts, suggesting that integrin-β7 may not be required for MM-cell growth in vivo. Based on our in vitro chemotaxis and transwell migration studies, we next examined whether integrin-β7 plays a role in MM-cell BM homing in vivo. Therefore, using in vivo flow cytometry we first tested the effect of ITGB7 silencing on the number of circulating ITGB7positive versus ITGB7silenced MM1S and H929 cells after mice tail vein injection. As shown in Figure 7A, 20 minutes after cell injection nearly all ITGB7positive cells exited from the circulation, whereas 50% of ITGB7silenced cells were still circulating consistent with delayed organs (including BM) homing. To further examine direct MM-cell homing to the BM, femurs were collected from killed mice and stained with fluorescent anti–human CD138 Ab. As shown in Figure 7B, mice inoculated with MM1S ITGB7silenced cells had delayed engraftment or homing into the BM, compared with ITGB7positive as indicated by the number of human CD138+ cells counted in BM sections (14% ± 2% in ITGB7positive vs 4 ± 1% in ITGB7silenced). No MM cells (human CD138+ cells) were detected in the livers or spleens of killed animals.
ITGB7 silencing reduces in vivo MM-cell extravasation and homing to the BM and decreases xenografted tumors vessel density. (A) ITGB7 silencing significantly reduced in vivo MM-cell homing after BALB/c mice tail vein injection, as detected by in vivo flow cytometry and monitoring the number of circulating calcein-AM–labeled ITGB7silenced (●) and ITGB7positive (○) MM1S and H929 cells over time. (B) Direct homing of CD138+ITGB7silenced (bottom) and ITGB7positive (top) MM1S cells to the BM was detected by IF staining with anti–human CD138-Cy3 labeled Ab and DAPI in mice femoral BM sections, as described in “Methods.” Shown in the inset is the mean ± SD of the percentage of human-CD138+ cells (red: Cy-3) in 500 BM cells counted in bilateral femoral sections from killed SCID mice (n = 3 per condition) (10×). (C) Effect of ITGB7 silencing on microvessel density measured by CD31 staining (Cy-3: red) in ITGB7silenced (right) and ITGB7positive (left) MM1S xenografted tumors (40×). Shown in the inset is the MVD (%) = CD31+ target area/total area examined as described in “Methods.” Images were aquired with an epifluorescence Olympus BX5 microscope and multispectral camera (Nuance Fx; CRi). Automated scaling of fluoresence was performed with the inForm™ analysis software (CRi).
ITGB7 silencing reduces in vivo MM-cell extravasation and homing to the BM and decreases xenografted tumors vessel density. (A) ITGB7 silencing significantly reduced in vivo MM-cell homing after BALB/c mice tail vein injection, as detected by in vivo flow cytometry and monitoring the number of circulating calcein-AM–labeled ITGB7silenced (●) and ITGB7positive (○) MM1S and H929 cells over time. (B) Direct homing of CD138+ITGB7silenced (bottom) and ITGB7positive (top) MM1S cells to the BM was detected by IF staining with anti–human CD138-Cy3 labeled Ab and DAPI in mice femoral BM sections, as described in “Methods.” Shown in the inset is the mean ± SD of the percentage of human-CD138+ cells (red: Cy-3) in 500 BM cells counted in bilateral femoral sections from killed SCID mice (n = 3 per condition) (10×). (C) Effect of ITGB7 silencing on microvessel density measured by CD31 staining (Cy-3: red) in ITGB7silenced (right) and ITGB7positive (left) MM1S xenografted tumors (40×). Shown in the inset is the MVD (%) = CD31+ target area/total area examined as described in “Methods.” Images were aquired with an epifluorescence Olympus BX5 microscope and multispectral camera (Nuance Fx; CRi). Automated scaling of fluoresence was performed with the inForm™ analysis software (CRi).
Finally, based on our observation that ITGB7 silencing reduced VEGF secretion in MM-BMSC cocultures, we evaluated whether ITGB7 was involved in neo-angiogenesis by CD31 immunostaining of serial sections of xenografted MM tumors. As shown in Figure 7C, the number of CD31+ cells was significantly reduced in ITGB7silenced compared with ITGB7positive tumors (CD31 target area as the percentage of the total area examined: 4.1% vs 7.7%, respectively). Taken together, these data are consistent with an essential role for integrin-β7 in MM cells in vivo homing to the BM milieu as well as myeloma-induced neo-angiogenesis.
Discussion
Adhesion molecules mediate plasma cell interaction with BM-ECM components, transducing “outside-in” and “inside-out” signals that regulate MM-cell behavior and gene expression.41 As such, integrins are reported to play a cardinal role in the physiology of malignant plasma cells, including their survival, proliferation, homing into and egress from the BM niches and particularly their resistance to variable therapeutics. To date, functional studies of integrins involved in MM-BM-ECM interactions have predominantly focused on integrins-β1 (α4β1 and α5β1)5,11-14 as well as β2 and β3 (αvβ3)15 and far less integrin-β7.21 Interest in the defined roles integrin-β7 plays in MM pathogenesis remerged with the molecular classification of the disease and the detection of high levels of ITGB7 mRNA in “high-risk” MS and MF myeloma subgroups.23,24 In addition, integrin-β7 was recently identified as a target gene of the oncogene C-MAF which is overexpressed in nearly 50% of MM patients as a result of IgH translocation with t(14;16) or through cytokines (BAFF and APRIL) binding to TACI.21,22
In the current study, we have first demonstrated that integrin-β7 mRNA and protein are expressed in MM cell lines with and without t(14;16) as well as primary CD138+ MM cells in which integrin-β7 mRNA and protein were detectable in 92.3% and 78.5% of the cases, respectively. These results are partially consistent with the previous work by Hurt and colleagues who reported a C-MAF–driven expression of ITGB7 mRNA in 64.3% and 50% of MM cell lines and MM patients, respectively.21 Interrogation of the gene dataset GEO-GSE4581 identified ITGB7 mRNA in 124 of 130 (95.4%) MM patients even in the absence of detectable calls with the C-MAF probes (supplemental Figure 1A). Therefore, these results suggest that in addition to the C-MAF other oncogenes or cytokine-activated transcription factors drive the expression of integrin-β7 in MM cells. As such, the ITGB7 promoter is reported to be responsive to other MM oncogenes like MAFB42 as well to MM cytokines like TGF-β1.43
Functionally and through ITGB7 knockdown, we have demonstrated that integrin-β7 plays a major role in MM-cell adhesion to FN, E-CDH, and to human BMSCs. This reduction in adhesion of ITGB7-silenced cells was particularly evident on E-CDH and to a lesser extent on FN-coated plates. Previous work relying on α4β7 blockade with Act-1 Ab that binds to an unique α4β7, but not to αEβ7 or β7 epitopes, reported a minor role for integrin-β7 in MM-cell adhesion to FN.14 In contrast, studies relying on blocking Abs that bind to a β7 unique epitope (Fib 504 clone) regardless of its heterodimer partners, resulted in a significant reduction in MM-cell adhesion to E-CDH and BMSCs.21 In sum, these results along with our findings suggest that (1) β7-integrin is responsible for MM-cell adhesion to E-CDH and (2) MM-cell adhesion to FN and BMSCs is dependent on several integrins (α4β1, α5β1, etc) including β7 (α4β7, αEβ7). Whether these integrins contribute simultaneously or act individually through a regulated temporal-spatial expression to regulate MM-cell adhesion to BM-ECM components remains to be determined.
Supporting its role in CAM-DR, we have observed that increased integrin-β7 expression on plasma cells correlated with poor survival outcomes in newly diagnosed MM patients treated with high-dose melphalan or in relapsed patients receiving salvage bortezomib in the APEX trial. Similarly, recent updates from the Total Therapy 3 trial reported poor survival in the MF molecular subgroup (characterized by high integrin-β7 expression) despite treatment with the combination of bortezomib and immunomodulatory drugs (IMiDs).44 In line with these clinical observations, we confirmed in vitro the contribution of integrin-β7 to CAM-DR by demonstrating a partial loss of the protective effect mediated by FN adhesion in integrin-β7 silenced cells treated with bortezomib or melphalan. Seminal studies by Damiano and colleagues,5 which focused on the role of α4β1 in CAM-DR, also showed an increase in the expression of integrin-β7 (but not α5) in melphalan-resistant 8226LR5 MM cells. Also of note, the α4-blocking Abs used in their study equally affects CAM-DR mediated by α4β1 as well as α4β7 heterodimers. Mechanistically, integrin-β7 silencing partially attenuated NF-κB activation induced by adhesion to FN as previously reported with α4β113 and significantly reduced the levels of VEGF and several other cytokines produced in MM-BMSC cocultures. Of interest, among these cytokines affected by integrin-β7 silencing, several (IL-1β, MIP-1α, MIP-1β) are implicated in osteoclast-osteoblast-MM-cell interaction leading us to speculate that integrin-β7 might be involved in MM bone disease. Lastly, bortezomib was recently reported to partially overcome CAM-DR by down-regulating α4 expression in MM cells,45 no such effect was observed on integrin-β7 expression (data not shown). Of note, ITGB7 silencing in the H929 cell line (and to a lesser extent in MM1S cells) did potentiate the cytotoxic effect of bortezomib in cells cultured in uncoated plates suggesting that an autocrine soluble or a MM cell-surface ligand may be contributing to the integrin-β7–mediated drug resistance. In summary, based on the results of the current study and others, CAM-DR in MM appears to be mediated through a collaborative effect of several adhesion molecules including integrin-β7. Future treatments aiming at reversing CAM-DR shall take into account this coordinated protective effect of adhesion molecules.
Circulating plasma cells are documented in 35%-70% of MM patients and reported to predict poor survival outcomes.46 In addition, residual focal bone lesions in patients in hematologic remission were recently correlated with disease relapse highlighting the ability of MM cells to spread and migrate in and out of the BM.47 Similarly, previous works by the Pilarski group and others did identify a circulating clonal B-cell population within the peripheral blood of MM patients who was implicated in the migratory spread of the disease.48 Of interest, the adhesive and migratory properties of these clonal B cells were described as CD44 and α4β7-integrin dependent. In the current work, we have first demonstrated in vitro the role of the β7-integrin in MM-cell transwell migration and matrigel basal membrane invasion toward SDF-1α chemokine gradients. We have also shown an integrin-β7–mediated activation of FAK, Src, and the Rho-like GTPase Rac-1 (including Rac1-SUMOylation). More importantly, we have demonstrated in vivo the role of integrin-β7 in MM cells homing to the BM. SCID mice xenografted with ITGB7silenced cells developed localized subcutaneous tumors with growth kinetics similar to mice engrafted with ITGB7positive cells but displayed a significant reduction in their homing to the BM milieu. Measuring the number of circulating MM cells with in vivo flow cytometry in BALB/c mice, we have also shown that ITGB7 silencing resulted in a significant delay in the time needed for MM cells to exit the circulation and home to the BM following tail vein injection. These results demonstrate the direct involvement of integrin-β7 in MM cells homing to the BM and suggest that SDF-1α guided chemotaxis of MM cells to the BM requires integrin-β7 for adhesion to endothelial cells, endothelial basement membrane invasion, and possibly retention of MM cells into the BM niches. Further studies to determine which integrin-β7 heterodimer (α4β7 or αEβ7) and what ligands (VCAM-1, E-cadherin, etc) are involved in these processes are ongoing. Lastly, and consistent with a possible role for integrin-β7 in MM-induced BM neo-angiogenesis, we observed a reduction in VEGF secretion in ITGB7silenced MM-BMSC cocultures and shown a significant decrease in the microvessel density of ITGB7silenced compared with ITGB7positive xenografts.
In conclusion, we have demonstrated that integrin-β7 plays a critical role in MM-cell adhesion, migration, invasion, BM homing, and CAM-DR. Humanized mAbs blocking integrin-β7 are currently investigated for the treatment of chronic colitis49 and gastrointestinal GVHD50 and may warrant further testing in MM.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work has been supported by research grants from the Leukemia & Lymphoma Society of Canada (N.J.B. and P.N.), Alberta Cancer Foundation (P.N. and N.J.B.), Multiple Myeloma Research Foundation (A.C.K. and N.J.B.), Terry Fox Founda-tion (D.A.S. and N.J.B.), and Italian Association of Cancer Research (P.N.).
L.H.B. is a Georgia Cancer Coalition Distinguished Scholar.
Authorship
Contribution: P.N. and N.J.B. designed and performed research, analyzed the data, and wrote the manuscript; P.N., L.R., A.K.A., M.B., K.G., A.C.K., C.L., A.M., and L.H.B. performed research; and P.N., M.B., L.B., D.A.S., P.T., P.D., I.M.G., L.H.B., and N.J.B. analyzed the data and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Nizar Jacques Bahlis, MD, University of Calgary–Division of Hematology, 1403 29th St NW Rm 681, Calgary, AB T2N 2T9, Canada; e-mail: nbahlis@ucalgary.ca.