Abstract
CD4+ T helper cell differentiation is essential for mounting robust immune responses without compromising unresponsiveness toward self-tissue. Here, we show that different subsets of myeloid cells isolated from human peripheral blood modulate TGF-β–dependent CD4+ T-cell developmental programs ex vivo. Human CD14+HLA-DR−/low myeloid-derived suppressor cells (MDSCs) induce Foxp3+ regulatory T cells, whereas CD14+HLA-DR+ monocytes promote generation of IL-17–secreting RORc+ Th17 cells when cocultured with naive CD4+ T cells. More importantly, not only do these 2 subsets modulate the de novo induction of Tregs and Th17 cells from CD4+ T cells, but MDSCs also catalyze the transdifferentiation of Foxp3+ regulatory T cells from monocyte-induced Th17 cells. The mechanism of such Th17 plasticity is dependent on MDSC-derived TGF-β and retinoic acid. Our results identify a previously unknown feature of the different subsets of CD14+ myeloid cells namely their pivotal role in immune response regulation and plasticity of CD4+ T helper cells. We propose that different subsets of myeloid cells in humans can orchestrate the differentiation of naive CD4+ T cells into effector/regulatory T-cell subsets. The balance between these 2 subsets can impact the outcome of immune reaction from inflammation to tolerance.
Introduction
CD4+ T cells play a pivotal role in regulation of the immune response through induction of key cytokines and can differentiate into several subsets depending on the stimuli in their environment. These subsets are characterized by different cytokine profiles and distinct functions. Until recently, CD4+ T cells were mostly divided into Th1, Th2, and Treg cells; however, a new subset of IL-17–secreting effector T cells, namely Th17 cells, has recently been characterized.1,2 IL-17A, IL-17F, and IL-22 produced by Th17 cells play a critical role in inflammation, autoimmunity, and cancer. Their differentiation is critically dependent on the transforming growth factor-β (TGF-β) and IL-6.3 Both Th17 cells as well as induced regulatory T cells (iTregs) depend on TGF-β for their development/induction, whereas TGF-β together with retinoic acid (RA) is responsible for induction of Foxp3 Tregs from naive T cells in the periphery in the absence of proinflammatory signals.4,5 Tregs play an important role in the control of inflammatory responses6 as well as in the suppression of antitumor immune responses.7-9
Several studies have suggested that the balance of IL-6 and RA in the presence of TGF-β determines development of iTregs or Th17 cells by competition between Foxp3 and RORγt.10-12 It has been shown that all-trans retinoic acid (atRA) can regulate TGF-β–dependent immune responses at least partially through inhibiting IL-6 induction of Th17 cells.13-17
Previous studies have shown that induced regulatory T cells as well as Th17 cells are not at the final stage of their differentiation and have the potential to convert into different subsets of CD4+ T cells depending on the cytokine environment. For example, regulatory T cells stimulated with IL-6 can express IL-17 and down-regulate Foxp3 expression.18 Similarly, already developed Th17 cells can change into IFN-γ–producing Th1 cells or IL-4–producing Th2 cells when stimulated by IL-12 or IL-4, respectively.19-21
Development of iTregs or Th17 cells in mice is shown to be associated with distinct cells of myeloid origin, which express RA and particular cytokines needed for control of these differentiation processes. Several studies have found that mouse intestinal dendritic cells (DCs) induce Th17 cells, whereas macrophages lead to Foxp3+ T-cell generation.22-24 In other studies, the CD103+ DCs were identified as the cells leading to Treg development, whereas the CD103− subset induced inflammatory Th17 cells.14,22-25 However, no human counterpart has been described so far.
Myeloid-derived suppressor cells (MDSCs) were originally described as a heterogeneous population of immature cells derived from myeloid progenitors with suppressive capacity in tumor-bearing mice.26-28 Human MDSCs are not so well characterized because of the lack of specific markers. We have recently shown a novel population of human MDSCs that are CD14+HLA-DR−/low, have arginase activity, and inhibit autologous T cells and NK cells.29,30 These MDSCs convert naive CD4+ T cells ex vivo into Foxp3+–expressing iTreg cells.29,31 In contrast, CD14+HLA-DR+ monocytes have no arginase activity and do not induce Tregs. In general, because these 2 cell types are not well characterized, their relative role in mounting or modulation of immune response remains poorly understood.
Here, we show that human CD14+ HLA-DR+ monocytes and CD14+ HLA-DRlow/− MDSCs isolated from human blood regulate Th17 and iTreg developmental programs and therefore act at the crossroad of Treg-Th17 differentiation. Human CD14+HLA-DR−/low MDSCs induce Foxp3+ Tregs, whereas CD14+HLA-DR+ monocytes induce the generation of IL-17–secreting RORc+ Th17 cells when cocultured with autologous CD4+ T cells. Most importantly, we show that human MDSCs can convert the IL-17+CD4+ T cells, either induced by monocytes or primed in vitro, into Foxp3–expressing CD4+ Tregs through an RA-dependent mechanism. This is supported by the observation that MDSCs overexpress genes involved in RA metabolism and express membrane-bound TGF-β but do not secrete any inflammatory cytokines. Monocytes, however, do not express genes responsible for RA metabolism, but secrete IL-6 and IL-1β, which, along with membrane-bound TGF-β, define Th17 differentiation.
We propose that different subsets of myeloid cells present in human peripheral blood can orchestrate the differentiation of naive CD4+T cells into effector/regulatory T-cell subsets. Furthermore, MDSC have the potential to convert Th17 cells into Foxp3+ iTreg cells hence shifting the immune response from inflammation to tolerance. These findings have important implications for understanding the mechanisms of protective immunity versus tolerance development.
Methods
Cell isolation and sorting
PBMCs were purified by Ficoll density gradient centrifugation (Biochrom) as described previously.29 CD14+HLA-DR−/low or CD14+HLA-DR+ cells were sorted from pre-enriched CD14+ cells using BD FACS Aria II cell sorting system (Becton Dickinson). CD4+ cells were isolated from CD14-depleted (CD14−) fraction of PBMC using microbeads and AutoMACS separation unit (Miltenyi Biotec) according to the manufacturer's instruction. The purity of the cells after separation was > 98%.
Flow cytometric analysis
Cells were surface-labeled with antibodies for 15 minutes at 4°C. Samples for intracellular staining were additionally fixed and permeabilized using Human Foxp3 Buffer Set (Becton Dickinson) according to the manufacturer's protocol. FACS acquisition was performed on LSR-II and results were analyzed with FlowJo Version 9.3.1.2 software (TreeStar Inc). FACS staining was done with following antibodies: CD4-Vioblue (Clone VIT4; Miltenyi Biotec) CD14-Pacific Orange (Clone TüK4; Invitrogen), Foxp3-AlexaFluor648 (Clone 259D/C7; Becton Dickinson), IL-17-PE (Clone SCPL1362; Becton Dickinson), CD103-FITC (Clone Ber-ACT8; Becton Dickinson), and TGF-β-PE (Clone 9016; R&D). Isotype-matched antibodies were used as indicated.
Cytokine bead array
Cell culture supernatants were tested for various cytokines at different time points as indicated. This was done using Cytokine Bead Array (CBA; Bender MedSystems).
Induction of T helper cell subtypes
CD4+ T cells were isolated as described and stimulated with anti-CD3/CD28/CD2 in the presence of CD14+HLA-DR−/low MDSC at a CD4:CD14 ratio of 1:2 for 3 days unless otherwise described. For Th17 cell induction, stimulated CD4+ T cells were cocultured with CD14+HLA-DR+ monocytes at a CD4:CD14 ratio of 1:2 for 6 days.
Isolation of Th17 cells
CD4+ T cells were cocultured with autologous monocytes for 6 days and stimulated with Cytostim and IL-17–secreting Th17 cells were isolated using IL-17-Secretion Assay-Detection Kit (Miltenyi Biotec) according to the manufacturer's protocol. The process was repeated once more to enhance purity.
Determination of mRNA expression by quantitative PCR
CD4+ T-cell subsets, CD14+HLA-DR+, and CD14+HLA-DR−/low cells were purified as described previously. RNA was isolated using RNeasy Micro Kit (QIAGEN). Complementary DNA synthesis was done with iScript Kit (Bio-Rad) and quantitative PCR was performed using the following primers (300 nmol/L each): IL-17RA forward, 5′-GAG CAC ATG CAC CAC ATA CC-3′; IL-17RA reverse, 5′-CGG AAT TGG TTC TGG AGT GT-3′; GATA-3 forward, 5′-GAA CCG GCC CCT CAT TAA G-3′; GATA-3 reverse, 5′-ATT TTT CGG TTT CTG GTC TGG A-3′; T-bet forward, 5′-GAT CAT CAC CAA GCA GGG ACG-3′; T-bet reverse, 5′-TCC ACA CTG CAC CCA CTT GC-3′; RORc forward, 5′-CAT TTT CTG CCT CTG CCT TC-3′; RORc reverse, 5′-TCT TGG CCT TCA TTG TAC CC-3′; IL-17A forward, 5′-AAT TCT GAG GAC AAG AAC TTC CC-3′; IL-17A reverse, 5′-ATA GTC TAA CTG CTT TGG GGA GTG-3′; TGF-β1 forward, 5′-GGG ACT ATC CAC CTG CAA GA-3′; TGF-β1 reverse, 5′-CCT CCT TTG GCG TAG TAG TCG-3′; IL-10 forward, 5′-CAA AAC CAA ACC ACA AGA CAG ACT T-3′; IL-10 reverse, 5′-GAG GAC CAG GCA ACA GAG CA-3′; Cyclophilin A forward, 5′-ATG CTC AAC CCC ACC GTG T-3′; Cyclophilin A reverse, 5′-TCT GCT GTC TTT GGG ACC TTG TC-3′; RDH12 forward, 5′-TCT TGA CCC TTC TGG GGA ATG-3′; RHD12 reverse, 5′-CAA TTT CTC GGG CTC TCT G-3′; RHD16 forward, 5′-AGC TGA GAA ACA GGG ACC AA-3′; RHD16 reverse, 5′-TGG CCC AGA ATT AAC ACA CA-3′; RHD5 forward, 5′-GGG GCT ACT GTG GTC TCC AAA-3′; RHD5 reverse, 5′-CTG CAG GGT TTT CTC CAG AC-3′; RHD8 forward, 5′-CTG TCT CTG GGA AAG CAA GG-3′; RHD8 reverse, 5′-CTG GTG TAT GCA TGG AGG TG-3′; RDH11 forward, 5′- GAG ATG GAT GTG GTG GCT TT-3′; RDH11 reverse, 5′-ATT ACG AGC TTG GGC AGA GAA TT-3′; ALDH8A1 forward, 5′-AGG CTC CTC CCA GGT TAT GT-3′; ALDH8A1 reverse, 5′-CTC AAG TGA TCC CAA CAC CT-3′. Reactions were done in triplicate using SYBR-Green (Bio-Rad) and normalized to endogenous cyclophilin A mRNA level using the δ-δ Ct method.
Statistical analysis
Statistical analysis was performed with GraphPad Prism 5 (GraphPad Software). Data are shown as the mean ± SEM, unless otherwise indicated. Student 2-tailed paired t test was used for comparison between matched paired groups.
Results
Subsets of CD14+ cells induce regulatory T cells or IL-17–secreting T cells
We have previously shown that CD14+HLA-DR−/low cells from human peripheral blood induce CD4+CD25+Foxp3+ regulatory T cells when cultured with autologous CD3/CD28/CD2 stimulated CD4+ T cells.29 In contrast, CD14+HLA-DR+ monocytes did not generate significant levels of Foxp3+ regulatory T cells (iTreg), but induced a high number of IL-17–secreting CD4+ T cells (Figure 1A). We further characterized the iTregs and in vitro primed Th17 cells for expression of specific markers by real-time PCR. The regulatory T cells induced by MDSC expressed Foxp3 and TGF-β, whereas CD4+ T cells cultured with monocytes expressed the Th17-specific transcription factor, RORc, and IL-17 (Figure 1B).
Induction of Treg/Th17 T cells by MDSCs/monocytes. (A) CD4+ T cells were stimulated with anti-CD3/CD28/CD2 beads and cultured in the presence or absence of CD14+HLA-DR−/low or CD14+HLA-DR+ cells. Intracellular staining (ICS) for IL-17 and Foxp3 was performed after 3 days gating on CD4+ T cells. Shown is a representative dot plot of 5 independent experiments. Numbers represent percentage of events within the respective quadrants. (B) qPCR analysis of Foxp3, RORc, and IL-17A expression. CD4+ T cells were stimulated with anti-CD3/CD28/CD2 beads and cultured in the presence of CD14+HLA-DR−/low or CD14+HLA-DR+ monocytes for 3 days. CD4+ T cells were stained for IL-17 and Foxp3 expression. Three different CD4+ T helper subtypes (Foxp3+IL-17− [+/−]/Foxp3−IL-17+ [−/+], and Foxp3−IL-17− [−/−] cells) were isolated using FACS and analyzed for expression of Foxp3, RORc, IL17A, IL-10, and TGF-β mRNA. CD4+Foxp3+IL-17− (+/−) cells were isolated from CD4+ T cells stimulated in the presence of CD14+HLA-DR−/low cells, while Foxp3−IL-17+ (−/+) and Foxp3−IL-17− (−/−) cells were derived from CD4+ T cells stimulated in the presence of CD14+HLA-DR+ monocytes. Expression was set relative to cyclophilin A mRNA. Shown are cumulative results of 2 independent experiments (*P < .05).
Induction of Treg/Th17 T cells by MDSCs/monocytes. (A) CD4+ T cells were stimulated with anti-CD3/CD28/CD2 beads and cultured in the presence or absence of CD14+HLA-DR−/low or CD14+HLA-DR+ cells. Intracellular staining (ICS) for IL-17 and Foxp3 was performed after 3 days gating on CD4+ T cells. Shown is a representative dot plot of 5 independent experiments. Numbers represent percentage of events within the respective quadrants. (B) qPCR analysis of Foxp3, RORc, and IL-17A expression. CD4+ T cells were stimulated with anti-CD3/CD28/CD2 beads and cultured in the presence of CD14+HLA-DR−/low or CD14+HLA-DR+ monocytes for 3 days. CD4+ T cells were stained for IL-17 and Foxp3 expression. Three different CD4+ T helper subtypes (Foxp3+IL-17− [+/−]/Foxp3−IL-17+ [−/+], and Foxp3−IL-17− [−/−] cells) were isolated using FACS and analyzed for expression of Foxp3, RORc, IL17A, IL-10, and TGF-β mRNA. CD4+Foxp3+IL-17− (+/−) cells were isolated from CD4+ T cells stimulated in the presence of CD14+HLA-DR−/low cells, while Foxp3−IL-17+ (−/+) and Foxp3−IL-17− (−/−) cells were derived from CD4+ T cells stimulated in the presence of CD14+HLA-DR+ monocytes. Expression was set relative to cyclophilin A mRNA. Shown are cumulative results of 2 independent experiments (*P < .05).
MDSCs express membrane-bound TGF-β and up-regulate the genes involved in RA metabolism
RA has been shown to enhance TGF-β–dependent induction of regulatory T cells but suppress the development of IL-17–secreting T cells.5 To test this hypothesis in our model, we performed a comparative microarray analysis of CD14+HLA-DR+ and CD14+HLA-DR−/low cells, where we found an up-regulation of several genes involved inRA metabolism (data not shown) in CD14+HLA-DR−/low MDSC. To confirm these results, FACS-sorted CD14+HLA-DR−/low and CD14+HLA-DR+ cells were tested for the expression of multiple genes by real-time PCR. Human CD14+HLA-DR−/low MDSCs had a higher expression level of all the genes tested compared with CD14+HLA-DR+ monocytes. In particular, aldh8 and dhrs9 expression was markedly increased (Figure 2A).
MDSCs express RA genes and membrane-bound TGF-β but do not secrete inflammatory cytokines. (A) MDSCs express genes involved in RA metabolism. CD14+HLA-DR−/low and CD14+HLA-DR+ cells were purified from freshly obtained blood. Gene expression was assayed by qPCR and normalized relative to expression of cyclophilin. Data shown are cumulative results from at least 4 independent experiments. (B) MDSCs express membrane-bound TGF-β. CD14+HLA-DR−/low and CD14+HLA-DR+ cells were purified and cocultured with autologous CD4+ T cells. Cells were stained for membrane-bound transforming growth factor β (mTGF-β; black line) or isotype control (filled histogram) gated on CD14+ cells. Data shown are representative of 2 independent experiments (numbers indicate the MFI ratio for TGF-β staining). (C-F) MDSCs do not secrete inflammatory cytokines. CD14+HLA-DR−/low and CD14+HLA-DR+ cells were cultured in the presence or absence of autologous CD4+ T cells at 2 different ratios [CD4:CD14 cells 1:1 (+) and 1:2 (++)]. Cytokines were tested at various time points using cytokine bead array. CD4+ T cells alone were used as controls. Shown are cumulative results from 2 independent experiments (*P < .05).
MDSCs express RA genes and membrane-bound TGF-β but do not secrete inflammatory cytokines. (A) MDSCs express genes involved in RA metabolism. CD14+HLA-DR−/low and CD14+HLA-DR+ cells were purified from freshly obtained blood. Gene expression was assayed by qPCR and normalized relative to expression of cyclophilin. Data shown are cumulative results from at least 4 independent experiments. (B) MDSCs express membrane-bound TGF-β. CD14+HLA-DR−/low and CD14+HLA-DR+ cells were purified and cocultured with autologous CD4+ T cells. Cells were stained for membrane-bound transforming growth factor β (mTGF-β; black line) or isotype control (filled histogram) gated on CD14+ cells. Data shown are representative of 2 independent experiments (numbers indicate the MFI ratio for TGF-β staining). (C-F) MDSCs do not secrete inflammatory cytokines. CD14+HLA-DR−/low and CD14+HLA-DR+ cells were cultured in the presence or absence of autologous CD4+ T cells at 2 different ratios [CD4:CD14 cells 1:1 (+) and 1:2 (++)]. Cytokines were tested at various time points using cytokine bead array. CD4+ T cells alone were used as controls. Shown are cumulative results from 2 independent experiments (*P < .05).
Because we did not detect any TGF-β in cocultures of MDSCs or monocytes with T cells, we tested the 2 cell populations for membrane-bound TGF-β. Both CD14+HLA-DR−/low MDSCs as well as monocytes expressed membrane-bound TGF-β, with MDSCs up-regulating it over time when cocultured with autologous T cells (Figure 2B).
We also analyzed the cultures by cytokine bead array (CBA) in search of cytokine patterns needed for iTreg as well as Th17 induction. MDSCs did not secrete any of the inflammatory cytokines tested: IL-6, IL-1β, IL-17, and TNF-α were not detected in MDSCs whether cultured alone or cocultured with autologous CD4+ T cells; this is in contrast to monocytes that secreted significant levels of all of these cytokines (Figure 2C-F).
Induction of regulatory T cells and Th17 cells by HLA-DR−/low MDSC and CD14+ HLA-DR+ cells is TGF-β–dependent and influenced by RA
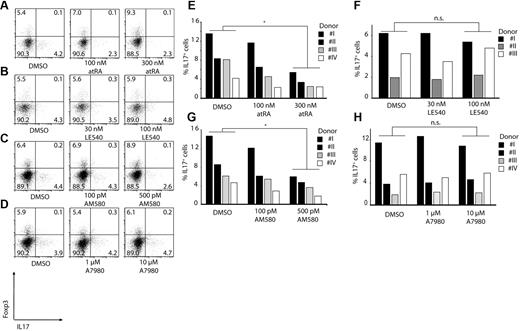
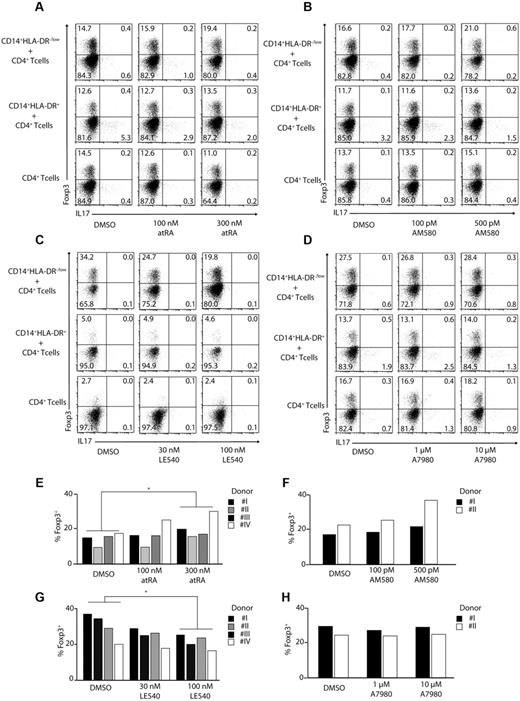
To understand the mechanisms behind the induction of Foxp3+ Treg cells and Th17 cells, we investigated the role of RA (a vitamin A metabolite) in this process. For this, we used LE540, a synthetic RA receptor (RAR-α) antagonist, AM580, an RAR agonist, as well as all-trans retinoic acid (atRA), and added them to cocultures of CD14+HLA-DR−/low MDSCs and CD14+HLA-DR+ monocytes with CD4+ T cells. Increasing concentrations of atRA, as well as AM580, enhanced induction of Foxp3+ regulatory T cells by MDSCs more than 30%, consistent with the role of retinoic acid in Treg development (Figure 3A-B,E-F). In agreement with these results, addition of the RAR inhibitor LE540 reduced the frequency of Foxp3+Treg cells by more than 40% (Figure 3C,G). Similar data were obtained using naive CD45RA+ CD4+ T cells (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Induction of regulatory T cells by MDSCs is RA dependent. CD4+ T cells were stimulated with anti-CD3/CD28/CD2–coated beads and cocultured in the absence or presence of CD14+HLA-DR−/low or CD14+HLA-DR+ cells. As indicated atRA (A,E), AM580 (B,F), LE540 (C,G), or A7980 (D,H) was added to the cocultures at various concentrations. ICS for IL-17 and Foxp3 was performed after 3 days gating on CD4+ T cells. Shown are representative dot plots from 4 (A,C) or 2 (B,D) independent experiments. Numbers represent percentages of events within the respective quadrants. (E-H) Results from single experiments using CD14+HLA-DR−/low cells were plotted as bars gating on CD4+ T cells (*P < .05; n.s. indicates not significant).
Induction of regulatory T cells by MDSCs is RA dependent. CD4+ T cells were stimulated with anti-CD3/CD28/CD2–coated beads and cocultured in the absence or presence of CD14+HLA-DR−/low or CD14+HLA-DR+ cells. As indicated atRA (A,E), AM580 (B,F), LE540 (C,G), or A7980 (D,H) was added to the cocultures at various concentrations. ICS for IL-17 and Foxp3 was performed after 3 days gating on CD4+ T cells. Shown are representative dot plots from 4 (A,C) or 2 (B,D) independent experiments. Numbers represent percentages of events within the respective quadrants. (E-H) Results from single experiments using CD14+HLA-DR−/low cells were plotted as bars gating on CD4+ T cells (*P < .05; n.s. indicates not significant).
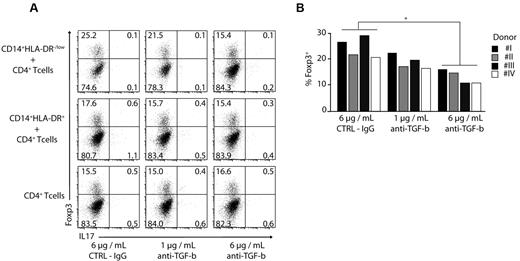
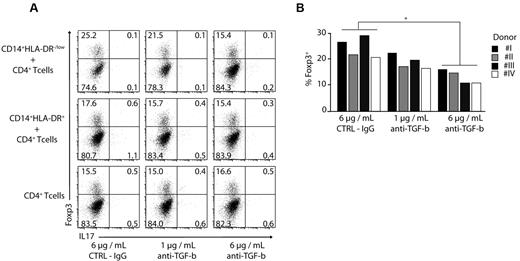
A7980 did not lead to an enhanced expression of Foxp3 indicating that RARγ is not involved in the induction of regulatory T cells (Figure 3D,H). To assess the role of TGF-β in induction of regulatory T cells, we added TGF-β–blocking antibody to MDSC-T cell cocultures. Blocking of TGF-β led to a reduction in frequency of Foxp3+ regulatory T cells by almost 40% in a dose-dependent manner using CD4+ T cells (Figure 4A-B) as well as naive CD45RA+ CD4+ T cells (supplemental Figure 2). Combination of anti–TGF-β and LE540 had a synergistic effect; however, addition of IL1-β, IL-6, and TNF-α did not affect the induction of Tregs (data not shown).
Induction of Foxp3 expression is TGF-β dependent. (A-B) CD4+ T cells were stimulated with anti-CD3/CD28/CD2 beads and cocultured in the absence or presence of CD14+HLA-DR+ and CD14+HLA-DR−/low cells. Anti–TGF-β was added at different concentrations as indicated. The expression of Foxp3 and IL-17 was performed by ICS on day 3 gating on CD4+ T cells. Shown are representative dot plots for 4 independent experiments (A) or results for single donors were plotted as bars gating on CD4+ T cells (B; *P < .05). Numbers represent percentages of events within the respective quadrants.
Induction of Foxp3 expression is TGF-β dependent. (A-B) CD4+ T cells were stimulated with anti-CD3/CD28/CD2 beads and cocultured in the absence or presence of CD14+HLA-DR+ and CD14+HLA-DR−/low cells. Anti–TGF-β was added at different concentrations as indicated. The expression of Foxp3 and IL-17 was performed by ICS on day 3 gating on CD4+ T cells. Shown are representative dot plots for 4 independent experiments (A) or results for single donors were plotted as bars gating on CD4+ T cells (B; *P < .05). Numbers represent percentages of events within the respective quadrants.
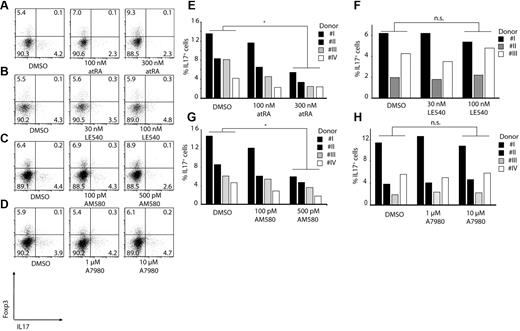
Induction of Th17 cells by monocytes is cytokine-dependent and can be blocked by RA
All the experiments done so far have looked at induction of regulatory T cells after 3 days of coculture. However, we observed that a longer incubation time is required to get the optimal Th17 induction by monocytes. Therefore, we cultured monocytes for 6 days with autologous CD4+ T cells to analyze the induction of Th17 cells in the presence and absence of atRA, AM580, and LE540. Addition of atRA or AM580 had a significant effect on induction of Th17 cells by monocytes, with more than 60% reduction in the IL-17–secreting T cells consistent with the effect of RA on suppression of IL-17 expression (Figure 5A,C,E,G). At the same time, as expected, LE540 (RAR-α antagonist) as well as A7980 (RARγ agonist) did not inhibit or activate Th17-cell generation by monocytes (Figure 5B,D,F,H).
RA can block induction of Th17 T cells. CD3/CD28/CD2-stimulated CD4+ T cells were cocultured with CD14+HLA-DR+ monocytes for 6 days. As indicated atRA (A,E), LE540 (B,F), AM580 (C,G) or A7980 (D,H) was added to the cultures. Shown are representative dot plots for Foxp3 and IL-17 gating on CD4+ T cells (A-D) or single results are shown in bars (E-H; *P < .05; n.s. indicates not significant). Numbers represent percentages of events within the respective quadrants.
RA can block induction of Th17 T cells. CD3/CD28/CD2-stimulated CD4+ T cells were cocultured with CD14+HLA-DR+ monocytes for 6 days. As indicated atRA (A,E), LE540 (B,F), AM580 (C,G) or A7980 (D,H) was added to the cultures. Shown are representative dot plots for Foxp3 and IL-17 gating on CD4+ T cells (A-D) or single results are shown in bars (E-H; *P < .05; n.s. indicates not significant). Numbers represent percentages of events within the respective quadrants.
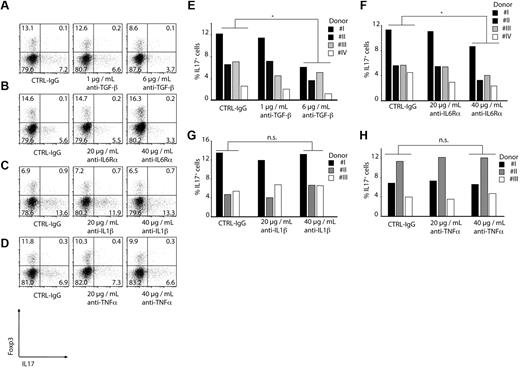
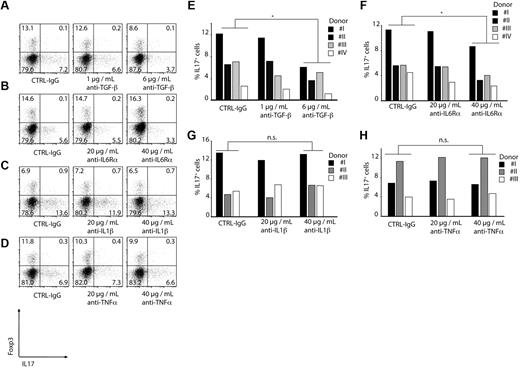
The ability of CD14+HLA-DR+ monocytes to induce IL-17–producing CD4+ T cells was also dependent on TGF-β because blocking of TGF-β reduced the percentage of IL-17–secreting CD4+ T cells by 50% in a dose-dependent manner (Figure 6A,E).
Induction of Th17 T cells is TGF-β and IL-6 dependent. CD4+ T cells were CD3/CD28/CD2-stimulated and cocultured with CD14+HLA-DR+ monocytes for 6 days. As indicated, blocking antibodies were added to the cocultures. Induction of Th17 T cells was measured by ICS FACS analysis gating on CD4+ T cells. Shown are representative dot plots (A-D) or single results for every donor as bars (E-H; *P < .05; n.s. indicates not significant. Numbers represent percentages of events within the respective quadrants.
Induction of Th17 T cells is TGF-β and IL-6 dependent. CD4+ T cells were CD3/CD28/CD2-stimulated and cocultured with CD14+HLA-DR+ monocytes for 6 days. As indicated, blocking antibodies were added to the cocultures. Induction of Th17 T cells was measured by ICS FACS analysis gating on CD4+ T cells. Shown are representative dot plots (A-D) or single results for every donor as bars (E-H; *P < .05; n.s. indicates not significant. Numbers represent percentages of events within the respective quadrants.
Because we found significant concentrations of IL1-β, IL-6, and TNF-α in the cultures of monocytes alone or with T cells, blocking antibodies against these cytokines were used to test their role in Th17 induction. Blocking of IL-1β and TNF-α had no effect on Th17 induction (Figure 6C-D,G-H) whereas blocking of the IL-6Rα in the coculture of monocytes and T cells led to a reduction of Th17 cells by 40% (Figure 6B,F). Similar results were obtained using naive CD45RA+ CD4+ T cells (supplemental Figure 3).
CD14+HLA-DR−/low MDSCs convert Th17 cells to Foxp3+ regulatory T cells
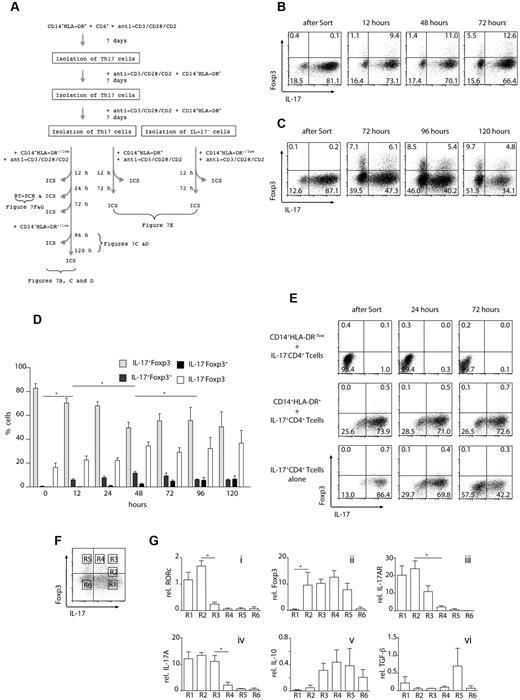
Our data so far indicates that MDSCs and monocytes can differentially regulate naive T-cell responses. Next, we asked whether these cells could affect already differentiated Th17 cells. We performed a series of experiments where CD14+HLA-DR−/low MDSCs were cocultured with already differentiated autologous CD4+ Th17 cells. The outline of experiments is shown in Figure 7A. A Th17-cell population with purity of 80%-90% (Figure 7B-C) was obtained by repetitive incubation of CD4+ T cells in the presence of CD14+HLA-DR+ monocytes and isolation of IL-17+ cells. Th17 cells were cultured with autologous CD14+HLA-DR−/low cells and tested for Foxp3 and IL-17 expression after 12, 48, and 72 hours. As shown in Figure 7B and D, the frequency of IL-17–secreting CD4+ T cells declined as early as 12 hours after coculture and continued to decrease by almost 40% after 72 hours. At that time (12 hours), double-positive IL-17+Foxp3+ cells were detected, which increased by almost 3-fold after 72 hours. In addition, there was a gradual increase in frequency of Foxp3+ IL-17− CD4+ T cells, which doubled after 72 hours. After 48 hours, the frequency of Foxp3+IL-17+ cells decreased, whereas the frequency of the iTregs continued to increase to 8% (Figure 7C-D and supplemental Figure 4). To examine conversion of CD4+ T helper cells at later time points, CD14+HLA-DR−/low MDSCs were added after 72 hours of culture and CD4+ T cells were analyzed for Foxp3 and IL-17 after a total of 96 and 120 hours (Figure 7C-D and supplemental Figure 4). At that time, the frequency of IL-17–secreting CD4+ T cells had declined to 34.1% and the frequency of Foxp3+CD4+ T cells increased to 9.7%.
MDSC convert Th17 T cells to regulatory T cells. (A) Experimental setup: Th17 cells were isolated after 21 days of in vitro culture in the presence of CD14+HLA-DR+ cells. Th17 cells were incubated with CD14+HLA-DR−/low cells and analyzed at the indicated time points for the expression of IL-17 and Foxp3 by ICS. In control experiments, either Th17 cells were incubated with CD14+HLA-DR+ cells or IL-17− CD4+ T cells were stimulated in the presence of CD14+HLA-DR−/low cells. (B) Th17 T cells were incubated with CD14+HLA-DR−/low cells for 12, 24, and 72 hours and then analyzed for the expression of Foxp3 and IL-17 by intracellular FACS staining. (C) Th17 T cells were incubated with CD14+HLA-DR−/low cells. After 72 hours, CD14+HLA-DR−/low cells were added to the cultures and the expression of Foxp3 and IL-17 was analyzed on gated CD4+ T cells at the indicated time points. (D) Cumulative results of FACS analysis shown in panels B and C: Th17 T cells were incubated with CD14+HLA-DR−/low cells for the indicated time points and analyzed for the expression of Foxp3 and IL-17 by intracellular FACS staining. Data represent results from 4 independent experiments. Large-scale magnification is shown as supplemental Figure 2 (*P < .05). (E) IL-17− CD4+ T cells were coincubated with CD14+HLA-DR−/low cells and analyzed for the expression of Foxp3 and IL-17 after 24 and 72 hours (top row). Th17 T cells were incubated with CD14+HLA-DR+ cells and CD4+ T cells were analyzed for the expression of Foxp3 and IL-17 after 24 and 72 hours (middle row). Th17 cells were incubated in the absence of additional CD14+ cells (bottom row). Results shown are representative of at least 2 independent experiments. (F-G) Th17 cells were isolated as described and cultured with CD14+HLA-DR−/low cells for 24 hours. Quantitative PCR was performed on different gates as shown in panel F. Relative mRNA expression levels for the indicated genes in cells isolated from 6 different gates (R1-R6) are shown. Shown are cumulative results of 2 independent experiments.
MDSC convert Th17 T cells to regulatory T cells. (A) Experimental setup: Th17 cells were isolated after 21 days of in vitro culture in the presence of CD14+HLA-DR+ cells. Th17 cells were incubated with CD14+HLA-DR−/low cells and analyzed at the indicated time points for the expression of IL-17 and Foxp3 by ICS. In control experiments, either Th17 cells were incubated with CD14+HLA-DR+ cells or IL-17− CD4+ T cells were stimulated in the presence of CD14+HLA-DR−/low cells. (B) Th17 T cells were incubated with CD14+HLA-DR−/low cells for 12, 24, and 72 hours and then analyzed for the expression of Foxp3 and IL-17 by intracellular FACS staining. (C) Th17 T cells were incubated with CD14+HLA-DR−/low cells. After 72 hours, CD14+HLA-DR−/low cells were added to the cultures and the expression of Foxp3 and IL-17 was analyzed on gated CD4+ T cells at the indicated time points. (D) Cumulative results of FACS analysis shown in panels B and C: Th17 T cells were incubated with CD14+HLA-DR−/low cells for the indicated time points and analyzed for the expression of Foxp3 and IL-17 by intracellular FACS staining. Data represent results from 4 independent experiments. Large-scale magnification is shown as supplemental Figure 2 (*P < .05). (E) IL-17− CD4+ T cells were coincubated with CD14+HLA-DR−/low cells and analyzed for the expression of Foxp3 and IL-17 after 24 and 72 hours (top row). Th17 T cells were incubated with CD14+HLA-DR+ cells and CD4+ T cells were analyzed for the expression of Foxp3 and IL-17 after 24 and 72 hours (middle row). Th17 cells were incubated in the absence of additional CD14+ cells (bottom row). Results shown are representative of at least 2 independent experiments. (F-G) Th17 cells were isolated as described and cultured with CD14+HLA-DR−/low cells for 24 hours. Quantitative PCR was performed on different gates as shown in panel F. Relative mRNA expression levels for the indicated genes in cells isolated from 6 different gates (R1-R6) are shown. Shown are cumulative results of 2 independent experiments.
To see whether it was possible to induce Foxp3+ iTregs from the double-negative cells, IL-17− Foxp3−CD4+ T cells were sorted and cultured with MDSCs for 72 hours. The double-negative cells did not lead to Foxp3+ regulatory T-cell generation and their frequency remained relatively constant as shown in Figure 7E (top row). We also tested whether Th17 cells express Foxp3+ when cultured alone, in the absence of CD14+HLA-DR−/low cells (Figure 7E middle row). However, none of these experimental approaches showed an increase in the frequency of iTregs (Figure 7E bottom row). Therefore, although it is possible that some Th17-negative cells also become regulatory T cells, our data suggests that the majority of the Th17 cells induced by monocytes go through a double-positive stage and become Foxp3+ cells on culturing with CD14+HLA-DR−/low MDSCs.
To investigate the changes in transcriptional activity of CD4+ T cells during the conversion, we repeated the experiments describes, and FACS-sorted CD4+ T helper cells according to Foxp3 and IL-17 expression after 24 hours. Six different cell populations were isolated as shown in Figure 7F and analyzed by RT-PCR for the expression of RORC, Foxp3, IL-17AR, IL-17A, IL-10, and TGF-β. As shown in Figure 7G, up-regulation of Foxp3 (R2) is the first step followed by down-regulation of RORc (R3). Subsequently, IL-17AR (R3-4) and IL-17A (R4) expression are decreased while expression of IL-10 (R3) and TGF-β (R5) is up-regulated.
Blocking RA or TGF-β reduces conversion of Th17 cells into Tregs
To test whether atRA and TGF-β are also important for the conversion of Th17 cells into iTregs, we blocked both pathways using the atRA antagonist LE540 or a TGF-β–blocking antibody (supplemental Figure 5). Addition of LE540 led to a decrease of Foxp3+ as well as Foxp3+IL-17+ cells (supplemental Figure 5C-D). On the other hand, atRA resulted in a slight increase in the frequency of the double-positive cells and a decrease of the Th17 cells (supplemental Figure 5E-F). However, the effect was not statistically significant. Blocking TGF-β led to a significant reduction of Foxp3+IL-17+ cells. In addition, the frequency of double-negative cells increased, suggesting that TGF-β is also important for the stability of Th17 cells (supplemental Figure 5A-B).
Discussion
Here, we show that different subsets of myeloid cells isolated from human PBMC possess the ability to modulate TGF-β–dependent CD4+ T-cell developmental programs ex vivo. We also show that human CD14+HLA-DR−/low MDSCs induce Foxp3+ regulatory T cells with anti-inflammatory properties, whereas CD14+ classic monocytes induce Th17 cells with proinflammatory characteristics. Finally, these 2 subsets do not only modulate the de novo induction of Tregs and Th17 cells from CD4+ T cells, but also catalyze the conversion of Tregs from Th17 cells, indicating the plasticity between these 2 cell subsets.
CD14+HLA-DR−/low MDSCs and CD14+HLA-DR+ monocytes have similarities and differences at a transcriptional level, which is crucial to their potential in triggering 2 different CD4+ T-cell populations. MDSCs and CD14+HLA-DR+ monocytes both express membrane-bound TGF-β known to be important for Th17 as well as Foxp3+ iTreg differentiation. At the same time, ex vivo isolated MDSCs express genes involved in RA metabolism, but they lack the inflammatory cytokines (IL-6, IL-1β, and TNF-α), which in combination with mTGF-β create a favorable environment for Foxp3+ iTreg induction. CD14+HLA-DR+ monocytes, as opposed to MDSCs, lack RA metabolism genes but secrete IL-6 and IL-1β and define Th17 pool differentiation. Interestingly, even addition of atRA or the retinoic receptor agonist AM580 to CD14+HLA-DR+ monocytes had almost no effect on Foxp3 expression in CD4+ T cells, suggesting that other factors are needed to induce Foxp3+ T cells, which are not present when CD4+ T cells are incubated with CD14+HLA-DR+ cells. The balance between RA and IL-6 influences the lineage differentiation of anti-inflammatory or proinflammatory T cells activated in the presence of TGF-β.
Several studies have described the role of intestinal DCs and macrophages in the induction of tolerance and immunity in mice.22,32 Murine CD103+CX3CR1− lamina propria DCs have been shown to originate from macrophage-DC precursors, whereas Ly6Chi monocytes give rise to CD103−CX3CR1+ DCs.24,25 The function of these cells seems to be different as well. Namely, mucosal CD103+ DCs induce Foxp3+ Tregs,33 whereas CD103−CX3CR1+ DCs drive Th17-cell differentiation in the presence of bacteria-derived ATP. Another study24 showed severe intestinal inflammation in mice reconstituted with CD103−CX3CR1+ DCs, indicating the pivotal role of these 2 subpopulations in the regulation of Tregs as well as Th17 cell–mediated immune responses. It has been shown that mucosal DCs mediate the induction of CD103 via TGF-β34,35 in murine models. It has also been demonstrated that the expression of CD103 on DCs is required for the induction of CD4+CD25+ regulatory T cells.14,36 However, neither iTregs nor MDSCs showed any expression of CD103 in our model (supplemental Figure 6). In addition, despite extensive studies in mice, so far no human counterpart for these cell types has been described. One can speculate that MDSCs and monocytes analyzed in our study are comparable with those precursor cells that differentiate into CD103+ and/or CD103− DCs in lamina propria of mice. The observation that cells with similar function are present in human peripheral blood can indicate that such influence on the fate of T cells might be rather a general mechanism and not characteristic solely of the intestinal mucosa.
Previous studies have shown that the differentiation of both induced Tregs and Th17 cells requires TGF-β, indicating that the development of these subsets might be linked.37,38 More recently, direct interactions between the specific transcription factors of these lineages have been described,39 showing a plasticity that allows iTregs to acquire functions of Th17 cells.40,41 However, we were not able to induce IL-17 production in iTregs because isolation of Foxp3+ regulatory T cells was not feasible because of the lack of adequate surface markers. Therefore, different experimental approaches need to be taken to analyze conversion of regulatory T cells to Th17 cells.
In our experiments, not all Th17 cells were converted into iTregs. One possibility is that not enough MDSCs were added to the culture (because of limitation of blood) or that the MDSCs matured while cocultured with T cells (data not shown). Another option is that only a subtype of Th17 cells can be converted into regulatory T cells. These possibilities are being further investigated.
Our study shows another important finding that MDSCs can change the initial Th17 developmental program triggered in CD4+ T cells and lead to transdifferentiation of Th17 cells into Foxp3+ iTregs. Such conversion mechanism can explain the low Th17-cell numbers at tumor sites but at the same time high infiltration of Treg cells. In line with this observation, we have found higher MDSC frequency in the tumor of hepatocellular carcinoma patients, whereas ascitic fluid was enriched in monocytes (data not shown).
It is also not completely clear how Th17 cells switch to Foxp3+ cells in our experimental system. Foxp3 expression is detectable on incubation of Th17 cells with MDSCs within the first 12 hours. Interestingly, this occurs primarily in the IL-17+ and not in IL-17− cells. IL-17− Foxp3+ cells were detected in the cultures only after 24 hours; this might indicate that Foxp3+ iTregs develop primarily from IL-17+ cells through the double-positive stage and not through the double-negative stage. Furthermore, it should be noted that we observed some natural decay of Th17 cells in the absence of additional CD14+ cells over time. Therefore, at this point our data cannot clearly distinguish whether the CD4+ Foxp3+ IL-17+ population is derived from IL-17+ cells or from CD4+IL-17− T cells. However, it should be noted that no Foxp3 IL-17 double-positive cells were induced on coculture of MDSCs with CD4+IL17− T cells (Figure 7E top row), which suggests that CD4+IL-17+ cells were transdifferentiated into Foxp3-expressing CD4+ T cells.
The question of which type of Th17 cells are involved in plasticity also needs to be investigated further. It is possible that IL-17+ T cells still contain intermediate cells with double-differentiation potential, which upon changes in the microenvironment (in this case, MDSCs vs monocytes) will up regulate Foxp3 and switch toward iTreg development. Another possibility is that even terminally differentiated Th17 cells might have the potential to redifferentiate into other subpopulations, in this case into Foxp3+ or Foxp3+IL-17+ cells.42 Several reports have shown the potential of IL-17–producing cells to develop into IFN-γ+ Th1 cells and to drive pathologic processes,19 although the possibility to develop into Foxp3+ Tregs has not been reported. It seems that stability of the Th17 differentiation program as well as of IL-17+ cells widely depends on the microenvironment and timing of response.
MDSCs represent a heterogeneous population of cells that consist of myeloid progenitor cells and immature myeloid cells. Whereas both monocytic and granulocytic MDSCs have been described in mice,43 human MDSC subtypes are not clearly defined. Several studies have described a population of human MDSCs that are CD14−/lin−. These MDSCs are also CD11b+/CD33+ and HLA-DR−/low. Another group of MDSCs described by others and us is also HLA-DR−/low, but CD14+.44 Future studies are needed to determine whether all subtypes express atRA-related genes and whether they play a role in RA-dependent induction of Foxp3 expression in CD4+ T cells.
Our results identify a previously unknown feature of the different subsets of myeloid cells, namely their pivotal role in immune response generation and plasticity of CD4+ T helper cells. The exact mechanism and biologic relevance of our findings still need to be elucidated, although it is plausible to suggest that depending on the prevalence of different myeloid cells in various environments, one can have diverse types of immune response at the same time. Finally, our findings provide evidence that the balance between these 2 subsets of myeloid cells plays a crucial role in immune regulation and has important implications for understanding the mechanisms of protective immunity versus tolerance development.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by a grant to M.P.M. and T.F.G. from the Initiative and Networking Fund of the Helmholtz Association within the Helmholtz Alliance on Immunotherapy of Cancer.
Authorship
Contribution: B.H. performed research and analyzed data; J.G. analyzed data; M.P.M. wrote the paper; and T.F.G. and F.K. designed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for B.H. is Institute for Molecular Medicine and Experimental Immunology, Friedrich-Wilhelms-University Bonn, Bonn, Germany.
Correspondence: Firouzeh Korangy, National Institutes of Health, National Cancer Institute, Bldg 10/12N226, 9000 Rockville Pike, Bethesda, MD 20892; e-mail: firouzeh.korangy@nih.gov or tim.greten@nih.gov.
References
Author notes
T.F.G. and F.K. are joint senior authors.

![Figure 1. Induction of Treg/Th17 T cells by MDSCs/monocytes. (A) CD4+ T cells were stimulated with anti-CD3/CD28/CD2 beads and cultured in the presence or absence of CD14+HLA-DR−/low or CD14+HLA-DR+ cells. Intracellular staining (ICS) for IL-17 and Foxp3 was performed after 3 days gating on CD4+ T cells. Shown is a representative dot plot of 5 independent experiments. Numbers represent percentage of events within the respective quadrants. (B) qPCR analysis of Foxp3, RORc, and IL-17A expression. CD4+ T cells were stimulated with anti-CD3/CD28/CD2 beads and cultured in the presence of CD14+HLA-DR−/low or CD14+HLA-DR+ monocytes for 3 days. CD4+ T cells were stained for IL-17 and Foxp3 expression. Three different CD4+ T helper subtypes (Foxp3+IL-17− [+/−]/Foxp3−IL-17+ [−/+], and Foxp3−IL-17− [−/−] cells) were isolated using FACS and analyzed for expression of Foxp3, RORc, IL17A, IL-10, and TGF-β mRNA. CD4+Foxp3+IL-17− (+/−) cells were isolated from CD4+ T cells stimulated in the presence of CD14+HLA-DR−/low cells, while Foxp3−IL-17+ (−/+) and Foxp3−IL-17− (−/−) cells were derived from CD4+ T cells stimulated in the presence of CD14+HLA-DR+ monocytes. Expression was set relative to cyclophilin A mRNA. Shown are cumulative results of 2 independent experiments (*P < .05).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/117/24/10.1182_blood-2010-11-317321/4/m_zh89991172560001.jpeg?Expires=1769106552&Signature=o1RJF3IBuxHHJjQ~2g4cjxKPwD-cc~9YcyWPd4q6IjrHhGIKK3VXDR03He9H062SUFdbVRVnGEBH-DIrsldQh~hdvHPCwIXLzRfQbzFWn8vzaiBiCniNHmTSerI4alIA7~XQT8d3yIkO3k5LwwiWevL5uYlI87yNaJPYy8d426SWJVb7zM8aiDj6GxsadqAMdsM2vTQnMtzMZNAXQdtJ7dKTnD9F~MIEbzKFmi~65E2E0KZ-M0YxDvq7BRMxbrjg47Fv4fsNFpdZNa9AJ36Z6e7bycSpWNP3WPgLkiAzeZlW1FP1e2qQa2kKJQ8jdemcvZKvzuvTtsO-DGSIJpmWPQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 2. MDSCs express RA genes and membrane-bound TGF-β but do not secrete inflammatory cytokines. (A) MDSCs express genes involved in RA metabolism. CD14+HLA-DR−/low and CD14+HLA-DR+ cells were purified from freshly obtained blood. Gene expression was assayed by qPCR and normalized relative to expression of cyclophilin. Data shown are cumulative results from at least 4 independent experiments. (B) MDSCs express membrane-bound TGF-β. CD14+HLA-DR−/low and CD14+HLA-DR+ cells were purified and cocultured with autologous CD4+ T cells. Cells were stained for membrane-bound transforming growth factor β (mTGF-β; black line) or isotype control (filled histogram) gated on CD14+ cells. Data shown are representative of 2 independent experiments (numbers indicate the MFI ratio for TGF-β staining). (C-F) MDSCs do not secrete inflammatory cytokines. CD14+HLA-DR−/low and CD14+HLA-DR+ cells were cultured in the presence or absence of autologous CD4+ T cells at 2 different ratios [CD4:CD14 cells 1:1 (+) and 1:2 (++)]. Cytokines were tested at various time points using cytokine bead array. CD4+ T cells alone were used as controls. Shown are cumulative results from 2 independent experiments (*P < .05).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/117/24/10.1182_blood-2010-11-317321/4/m_zh89991172560002.jpeg?Expires=1769106552&Signature=xvwueh6dBwczdMBpL1lrRQDEXyWvTrMO9gXndXH0nEBGonnTqdVBAzlZ7b2JymZdaqom4cYh3ty1n1ikBGFIcOZCl5~kBw-Bz~fS5vrCB-6UqHGiDBD8xQiGsocXih~M5p3JIDmdgzl8bquppwIMcCyCiRm2qKbRWPRqu7WicIcGfUttqrMWREgmK9-TbzUB4h4-6p1Q1i50lNUucF0qyxaMZ4EasWxQOz70jq3VVtAlt-O-T6akekyLfEfB6QQpjaEW3l9V8gnHdcnVJ-sqFU7kOWz8vpLZmilU4bmhGa5FPRezpTnYnIe7oMDe48f7SKccYiF5vDHVJbX~e9rfLw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Figure 1. Induction of Treg/Th17 T cells by MDSCs/monocytes. (A) CD4+ T cells were stimulated with anti-CD3/CD28/CD2 beads and cultured in the presence or absence of CD14+HLA-DR−/low or CD14+HLA-DR+ cells. Intracellular staining (ICS) for IL-17 and Foxp3 was performed after 3 days gating on CD4+ T cells. Shown is a representative dot plot of 5 independent experiments. Numbers represent percentage of events within the respective quadrants. (B) qPCR analysis of Foxp3, RORc, and IL-17A expression. CD4+ T cells were stimulated with anti-CD3/CD28/CD2 beads and cultured in the presence of CD14+HLA-DR−/low or CD14+HLA-DR+ monocytes for 3 days. CD4+ T cells were stained for IL-17 and Foxp3 expression. Three different CD4+ T helper subtypes (Foxp3+IL-17− [+/−]/Foxp3−IL-17+ [−/+], and Foxp3−IL-17− [−/−] cells) were isolated using FACS and analyzed for expression of Foxp3, RORc, IL17A, IL-10, and TGF-β mRNA. CD4+Foxp3+IL-17− (+/−) cells were isolated from CD4+ T cells stimulated in the presence of CD14+HLA-DR−/low cells, while Foxp3−IL-17+ (−/+) and Foxp3−IL-17− (−/−) cells were derived from CD4+ T cells stimulated in the presence of CD14+HLA-DR+ monocytes. Expression was set relative to cyclophilin A mRNA. Shown are cumulative results of 2 independent experiments (*P < .05).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/117/24/10.1182_blood-2010-11-317321/4/m_zh89991172560001.jpeg?Expires=1769106553&Signature=1ziWScNo3vmHmLaJMMnV-CnDxorJbF0CZ1KAadtCFGpe95qadA3BSx1lT2xgBVfz1MUcNyRVhtWJZwxdpdrr2AcWeklWckeb6NIfd~SI3clEFWuk0ly3~Kx9z8dd5l1-IteNtn4tQEJvjWruCfgHqUzurusQDflIEXLiPYOJZBsIHJGdpr-R9yDupRT7A8PlnfewmgYzuKHth6YyIVugdPA0MA7VHmAnaFF4VErfe7P6hRcKe8jvRRfLBAnzRA-fP6MMbeNVoYs4DDjMhddNci8fdoG1O~bQ5xlx2D1x630qYHp~JS5hLnAFmUqcpqHq7udZj-P0FS~wZdGp4TjE6Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 2. MDSCs express RA genes and membrane-bound TGF-β but do not secrete inflammatory cytokines. (A) MDSCs express genes involved in RA metabolism. CD14+HLA-DR−/low and CD14+HLA-DR+ cells were purified from freshly obtained blood. Gene expression was assayed by qPCR and normalized relative to expression of cyclophilin. Data shown are cumulative results from at least 4 independent experiments. (B) MDSCs express membrane-bound TGF-β. CD14+HLA-DR−/low and CD14+HLA-DR+ cells were purified and cocultured with autologous CD4+ T cells. Cells were stained for membrane-bound transforming growth factor β (mTGF-β; black line) or isotype control (filled histogram) gated on CD14+ cells. Data shown are representative of 2 independent experiments (numbers indicate the MFI ratio for TGF-β staining). (C-F) MDSCs do not secrete inflammatory cytokines. CD14+HLA-DR−/low and CD14+HLA-DR+ cells were cultured in the presence or absence of autologous CD4+ T cells at 2 different ratios [CD4:CD14 cells 1:1 (+) and 1:2 (++)]. Cytokines were tested at various time points using cytokine bead array. CD4+ T cells alone were used as controls. Shown are cumulative results from 2 independent experiments (*P < .05).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/117/24/10.1182_blood-2010-11-317321/4/m_zh89991172560002.jpeg?Expires=1769106553&Signature=dK71Rlb3-kOS3yDlZrhi~ULFAWy2h1Wv~0rlz5vng8ZHpWy-Q8HjC5Zj~2GFycvQzGOH5OvObdUbbo~7wCVswT5foYzi-LkvrVZbMRNHIHunSE92eMjlewBkVqPPHGbKveeL1rpU8zWlHDGDEqES2bjnK9gzxs61QdQlBUml86PKCV~GCDFzyrrhvCONq2oycorjLlmE2maFWImUh-6ZWfni9W2gyOl3TaOT98ERr5aRW6uCyHI-WaUgTBEDrBiPcUV5B8Y~Ol01AB6T-7Mb84ZNCfpFJZaRMAH9T0QrT7T2Ao~2rOrh3oRsNqe1ov34chl-ajPXP3dPKiIRVbHDHw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)