In this issue of Blood, Pospori and colleagues demonstrate that T cells genetically modified to express a receptor specific for a self-peptide derived from the tumor antigen Wilms Tumor 1 are not deleted in the thymus and undergo activation in the periphery, thus acquiring functionality without vaccination.1
Central tolerance to self is mediated in the thymus by deletion of T cells bearing receptors capable of high-affinity recognition of self-peptides (reviewed in Klein et al2 ). However, the presence of T cells outside the thymus that are reactive against self-antigens (such as those expressed on tumors) indicates that this mechanism is not complete. In addition, low-level TCR signaling by self-antigens in the periphery serves to “tune” the TCR so that T cells respond optimally to foreign antigens.3 Furthermore, during lymphopenic conditions, signaling by self-peptides, in combination with cytokines such as IL-7, contributes to the homeostatic expansion of T cells, resulting in acquisition of some features of activated or memory T cells.4 However, the genes induced in T cells undergoing homeostatic expansion are partially distinct from those induced in T cells expanded by cognate antigens,5 indicating that these programs are not identical. Finally, recent data suggest that self-antigens may trigger conversion of a subset of naive T cells to a memory phenotype even in the absence of lymphopenia.6 Whether such self-peptide–induced “activation” of CD8+ T cells is sufficient to acquire effector function is not clear. Thus, recognition of self by T cells outside of the thymus in both physiologic and pathologic conditions such as lymphopenia plays a critical roll in T-cell maintenance and function.
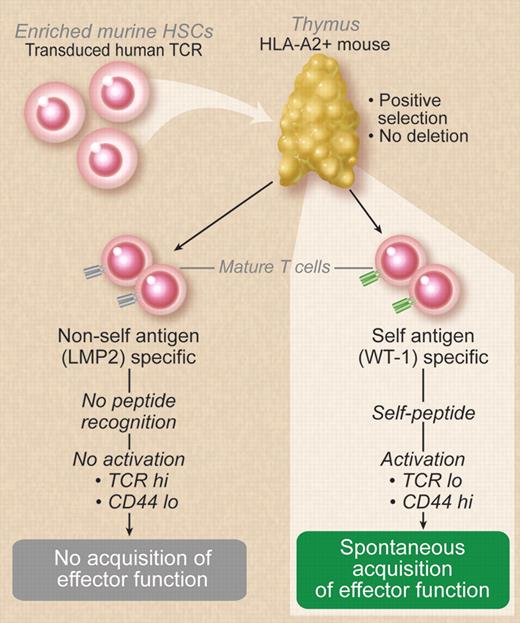
Pospori and colleagues retrovirally transduced enriched murine HSCs with human TCRs recognizing antigens in the context of HLA-A2. These cells were then transplanted into irradiated recipients expressing human HLA-A2 as a transgene resulting in positive selection without deletion and export of human TCR-bearing T cells to the periphery. T cells recognizing WT1 expressed activation markers and were functional without vaccination. This did not occur with nonself-antigen–recognizing T cells. Professional illustration by Debra T. Dartez.
Pospori and colleagues retrovirally transduced enriched murine HSCs with human TCRs recognizing antigens in the context of HLA-A2. These cells were then transplanted into irradiated recipients expressing human HLA-A2 as a transgene resulting in positive selection without deletion and export of human TCR-bearing T cells to the periphery. T cells recognizing WT1 expressed activation markers and were functional without vaccination. This did not occur with nonself-antigen–recognizing T cells. Professional illustration by Debra T. Dartez.
The Wilms tumor 1 (WT1) antigen is over-expressed in a number of malignancies and has been validated as a tumor antigen in preclinical models and in patients.7 Importantly, the WT1 sequence is highly conserved in mammals with shared epitopes that can be appropriately processed and recognized by T cells in both mice and humans. WT1-specific T cells can be indentified in healthy individuals with evidence for increased frequency of WT1-specific T cells in patients with malignancy.8 Because the WT1 gene shows restricted expression in normal adult tissues, including renal podocytes and some CD34+ hematopoietic stem cells, identification of T cells that can recognize WT1 in healthy individuals indi cates that central tolerance to WT1 is incomplete.
Pospori et al use a lentiviral vector to transduce murine HLA-A02 transgenic stem cells with a human TCR that recognizes a dominant HLA-A0201–restricted epitope derived from WT1 (see figure). Importantly, this TCR was generated from the repertorie of an HLA-A02 negative individual, an approach that is used to generate higher affinity TCRs because T cells in these individuals will not be negatively selected in an HLA-02-expressing thymus.9 The transduced murine stem cells were then transplanted into HLA-A02 transgenic mice. Thus, T cells expressing the human WT1-specific TCR can be positively selected in the thymus on HLA-A02 with the possibility that presentation of WT1 peptide derived from the murine protein (with an identical TCR recognition sequence to humans in this case) could result in deletion. However, human TCR-expressing thymocytes (based on human Vβ staining) are present at all stages of CD8 development in the thymus but not in CD4+ T cells. Furthermore, human Vβ-expressing T cells were also identified in the secondary lymphoid tissues, confirming successful escape from deletion and exit from the thymus.
By comparing to another TCR recognizing an EBV-derived peptide from LMP2, Pospori et al were able to identify differences between the behavior of the WT1 self-reactive TCR to a nonself-reactive TCR. Their first observation was that the expression of CD3 was lower on WT1 TCR cells than on LMP2 TCR cells, with lower expression of the Vβ corresponding to the transduced TCR. Further analysis demonstrated dual populations of human Vβ-expressing T cells in the recipients of WT1 TCR-transduced stem cells with CD8lo/TCRlo cells representing a population with a large proportion of CD44hi effector and central memory T cells, suggesting antigen interaction in the periphery. Interestingly, the TCRlo cells are seen even when HLA-0 neg stem cells are used for transduction even though hematopoietic cells should be A2− after transplantation. This surprising observation suggests that nonhematopoietic cells are sufficient for antigen presentation although, as pointed out by Pospori and colleagues, residual A2+ recipient APCs could be driving the conversion.
Perhaps the most compelling data in the paper involve the functionality of the T cells. Whereas the WT1 TCR T cells display in vivo and in vitro effector function, the fully naive LMP2 TCR T cells from the A2 transgenic mice do not. In secondary transfer experiments, the WT1 TCR T cells all become CD44hi and retain functionality. Interestingly, unlike the LMP2 TCR T cells, the WT1 TCR T cells accumulate in the bone marrow, a compartment known to contain cells expressing WT1, where a greater proportion remain CD62L−CD44+ effector cells. However, the final set of data demonstrates that these T cells do not compromise engraftment in serial transplantation experiments. Taken together, these studies indicate that activation of self-antigen–reactive T cells not deleted in thymus results in acquisition of functionality.
There a number of caveats to be considered before it can be concluded that these findings can be generalized. The model uses a high-affinity TCR for a self-peptide that may not be analogous to TCRs derived from the self-MHC–selected repertoire. In addition, it remains possible that the human TCR-expressing cells are activated in a mouse by some process other than self-antigen recognition, although this does not occur with the LMP2 cells, suggesting that this is not the case. It is also possible that the lympopenic conditions present after transplantation contribute to the activated phenotype. Finally, it is interesting to speculate that these results hint at why certain antigens such as WT1 have emerged as good targets in malignancy. Nonetheless, these findings have relevance to the immunobiology of self-reactive T cells in periphery, and have direct implications for immunotherapy for cancer.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■