Abstract
In the past 5 years we have witnessed significant advances in both the diagnostic process and optimal therapy for patients with essential thrombocythemia (ET). Insights into the underlying molecular mechanisms have been accompanied by the development of new diagnostic tests and by an improved understanding of the relationship between ET and other related myeloproliferative neoplasms, such as polycythemia vera and primary myelofibrosis. In the first part of this review, we describe how recent molecular and histologic studies can be integrated into a streamlined diagnostic process that is applicable to everyday clinical practice. We also address areas of current diagnostic controversy, including heterogeneity within ET and the phenotypic overlap between ET, polycythemia vera, and primary myelofibrosis. In the second part, we provide an overview of our current approach to the treatment of ET, including risk stratification, choice of cytoreductive agent, and a consideration of special situations such as the pregnant or perioperative patient. Areas of controversy discussed include the identification of those at high risk of complications and therapeutic decisions in the younger patient.
Introduction
Essential thrombocythemia (ET) is a clonal stem cell disorder that shares phenotypic and pathologic similarities with other myeloproliferative neoplasms (MPNs), particularly polycythemia vera (PV) and primary myelofibrosis (PMF). In the last 5 years there has been an acceleration in our understanding of these disorders, after the identification of an acquired JAK2 V617F mutation in approximately 50% of ET patients, along with one-half of those with PMF and the majority with PV.1,2 Subsequently mutations in MPL were reported in approximately 4% of patients with ET or PMF,3-5 and mutations in TET2 have been observed in a variety of myeloid malignancies, including JAK2 V617F-positive and -negative ET.6 In this article, we explore the impact of recent molecular and therapeutic advances on the way we diagnose and manage patients with ET.
How we diagnose ET
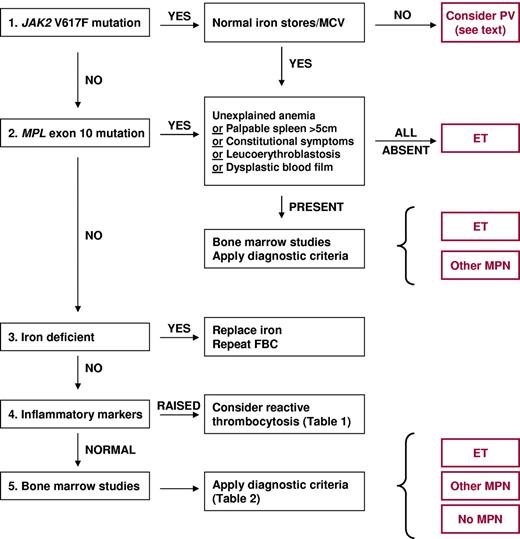
We consider a diagnosis of ET when there is an unexplained and persistent thrombocytosis (platelet count > 450 × 109/L). ET has traditionally been a diagnosis of exclusion, requiring the absence of reactive conditions and other clonal disorders that may present with thrombocytosis (Table 1). The discovery of mutations in JAK2 and MPL now allows for the positive identification of ET in more than one-half of all cases. In our practice, screening for the JAK2 V617F mutation is the initial investigation performed in all patients with suspected ET, followed by screening for MPL exon 10 mutations in V617F-negative cases. TET2 screening is not currently performed because mutations are present in a wide range of other myeloid malignancies,6 screening is not straightforward, and the prognostic significance of TET2 mutations in ET is currently unknown.
In the presence of a pathogenetic mutation in JAK2 or MPL, a diagnosis of ET requires exclusion of PV and PMF. To this end, we find blood film examination and assessment of iron status to be helpful. A normal hemoglobin count in an iron-replete patient is usually sufficient to exclude PV. Although reduced serum ferritin and/or absent bone marrow iron stores may occur in patients with ET,7 we consider the combination of microcytic red cells and a normal hemoglobin count in a JAK2 V617F-positive white patient highly suggestive of iron-deficient PV. In our opinion, PMF can generally be excluded by the absence of significant splenomegaly, unexplained anemia, teardrop poikilocytes, and a leukoerythroblastic blood film. A minority of patients with chronic myelomonocytic leukemia harbor a JAK2 V617F mutation, although such cases generally lack thrombocytosis, with additional features such as leukocytosis, monocytosis, and splenomegaly, suggesting the correct diagnosis.8
In patients with suspected ET who lack JAK2 and MPL mutations, the exclusion of reactive causes is particularly important (Table 1). In studies of unselected patients with thrombocytosis, fewer than 20% harbored a clonal blood disorder.9,10 Therefore a careful history, assessment of inflammatory markers (C-reactive protein and/or erythrocyte sedimentation rate), and bone marrow histology are all recommended (Figure 1). Cytogenetic analysis may also be considered, and we use a panel of fluorescent in situ hybridization probes to detect MPN-associated chromosome abnormalities (detecting additional copies of chromosomes 8 and 9 and deletions of 20q and 13q). However, chromosomal lesions are present in only 5% of patients at diagnosis and lack prognostic significance.11 In the absence of a molecular or cytogenetic marker of clonal hematopoiesis, ET remains a diagnosis of exclusion.
Algorithm for the investigation and diagnosis of thrombocytosis. ET indicates essential thrombocythemia; FBC, full blood count; MCV, mean cell volume; MPN, myeloproliferative neoplasm; and PV, polycythemia vera.
Algorithm for the investigation and diagnosis of thrombocytosis. ET indicates essential thrombocythemia; FBC, full blood count; MCV, mean cell volume; MPN, myeloproliferative neoplasm; and PV, polycythemia vera.
Diagnostic criteria for ET are presented in Table 2. Criteria from the British Committee for Standards in Haematology (BCSH)12 are used in our clinic. These criteria are similar to those of the WHO13 but differ in 3 important respects. First, in the presence of a pathogenetic mutation a diagnosis of ET does not necessarily require bone marrow studies, as other myeloid disorders that present with thrombocytosis and a JAK2 or MPL mutation can generally be excluded by clinical and laboratory features as outlined above. However, it is our practice to obtain bone marrow aspirate and trephine biopsy samples from most patients at diagnosis. This is to provide both confirmatory data (bone marrow generally showing normal cellularity in the presence of giant megakaryocytes with hyperlobated nuclei) and prognostic information, specifically the degree of reticulin fibrosis.11 A bone marrow sample at diagnosis also provides a useful baseline for subsequent comparisons, for example, after myelofibrotic transformation. Second, BCSH criteria do not use bone marrow histology to subdivide ET into “true-ET” and “prefibrotic myelofibrosis” because the existence of the latter as a distinct entity remains controversial and the underlying histologic criteria are difficult to apply reproducibly.14 Third, the BCSH classification includes patients with bone marrow reticulin greater than grade 2 (on a 0-4 scale) who lack other features of PMF or myelofibrotic transformation. Under current World Health Organization (WHO) criteria, such patients cannot be classified as either ET, because they have too much reticulin, or PMF, because they have none of the clinical features required for this diagnosis.
Myelofibrotic transformation of ET
Evolution to myelofibrosis affects a proportion of ET patients, although the reported prevalence varies widely, reflecting differences in study design, therapeutic intervention, and the diagnostic criteria applied. Retrospective studies suggest that myelofibrotic transformation increases with disease duration, affecting 3%-10% in the first decade after diagnosis and 6%-30% in the second decade.15-17 Given the close relationship of post-ET myelofibrosis to PMF, the criteria we use to diagnose these conditions are essentially the same (Table 3). These criteria are similar to other widely used systems13,18,19 but do not include serum lactate dehydrogenase (LDH) because a correlation between increasing LDH and myelofibrotic transformation has yet to be established and increased levels of LDH also are found in most patients with ET or PV.20 It is important to emphasize that the development of reticulin fibrosis on its own does not equate to transformation to myelofibrosis, and the diagnosis of myelofibrotic transformation is reserved for those patients who demonstrate bone marrow fibrosis in association with accompanying clinical and/or laboratory features (Table 3).
Leukemic transformation of ET
Progression to acute myeloid leukemia (AML) occurs in a small minority of patients, with retrospective studies suggesting an incidence of 1%-2.5% in the first decade after diagnosis, 5%-8% in the second decade, and continuing to increase thereafter.15,16,21 However, studies often included patients who had received multiple lines of cytoreductive therapy, including alkylating agents, which are known to increase the rate of leukemic transformation,22,23 thus rendering these findings difficult to interpret. Transformation to AML is diagnosed in the presence of ≥ 20% blast cells in the blood and/or bone marrow. Of note, patients with JAK2 V617F-positive ET may develop AML that is negative for the JAK2 mutation.24-26
Controversies in the diagnosis of ET
Distinguishing ET from PMF
ET is heterogeneous with regards to diagnostic, clinical, and laboratory features, and there has been considerable debate over the existence of distinct subgroups. Some of this heterogeneity, such as variation in diagnostic blood counts and bone marrow cellularity, reflects the presence or absence of mutations in JAK2 or MPL,4,7,14,27 and constitutional genetic differences are also likely to contribute. The current WHO classification proposes that bone marrow histology can be used as a tool to subdivide ET into so-called “true ET” and “prefibrotic myelofibrosis”13 and suggests that true ET is a benign and stable condition whereas prefibrotic myelofibrosis progresses to clinically overt myelofibrosis.28 However many of the histologic criteria used to define prefibrotic myelofibrosis, such as megakaryocyte morphology, are subjective and difficult to apply reproducibly, even by experienced hematopathologists.14 We therefore avoid the terms true ET and prefibrotic myelofibrosis in our practice.
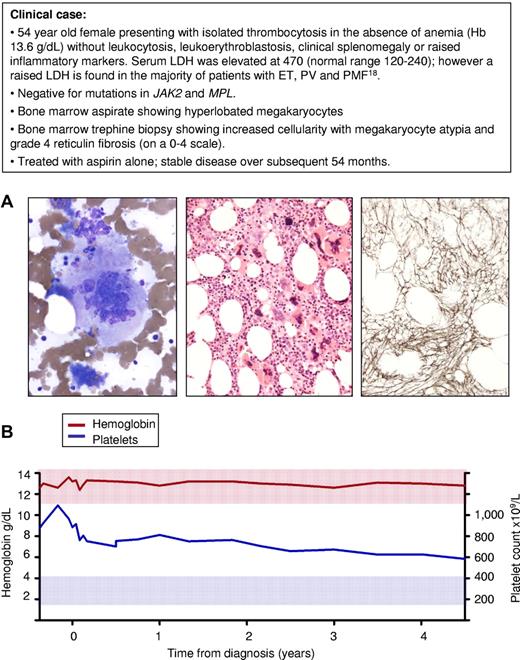
A second area of debate relates to patients with an isolated thrombocytosis who show an increase in bone marrow reticulin fibrosis at diagnosis but lack any other features of PMF (Figure 2). Such patients clearly have an MPN but cannot be classified as having either ET or PMF according to WHO criteria.13 Importantly, such cases are not unusual, with data from the PT-1 trial indicating that 15%-20% of ET patients harbor grade 3 or occasionally grade 4 reticulin fibrosis at diagnosis (on a 0-4 scale, with grade 4 indicating the presence of collagen fibrosis), in the absence of other features of PMF.29 Increased bone marrow fibrosis at diagnosis is associated with greater rates of myelofbrotic transformation, thrombosis, and hemorrhage but no change in overall survival.29 The lack of a survival difference, together with the small number of complications even at greater reticulin levels, supports the concept that patients presenting with an isolated thrombocytosis but elevated reticulin have a relatively benign prognosis. We therefore follow BCSH guidelines according to which such patients are diagnosed and treated as ET.
Isolated thrombocytosis and bone marrow fibrosis in the absence of clinical features to support a diagnosis of primary myelofibrosis. (A) Bone marrow samples obtained at diagnosis from a 54-year-old female patient with thrombocytosis in the absence of additional features of PMF: left, bone marrow aspirate stained with Wright-Giemsa showing a large megakryocyte with a hyperlobated nucleus; middle, bone marrow trephine biopsy stained with hematoxylin and eosin showing loose clusters of megakaryocytes containing both large megakaryoctes with hyperlobated nuclei and smaller forms with cloud-like or hyperchromatic nuclei; right, silver-stained bone marrow section showing grade 4 reticulin fibrosis (on 0-4 scale). (B) Blood counts during the course of 54 months from diagnosis showing stabilization of the platelet count and no change in hemoglobin level. Red and blue shaded areas represent the normal range for hemoglobin and platelet count, respectively.
Isolated thrombocytosis and bone marrow fibrosis in the absence of clinical features to support a diagnosis of primary myelofibrosis. (A) Bone marrow samples obtained at diagnosis from a 54-year-old female patient with thrombocytosis in the absence of additional features of PMF: left, bone marrow aspirate stained with Wright-Giemsa showing a large megakryocyte with a hyperlobated nucleus; middle, bone marrow trephine biopsy stained with hematoxylin and eosin showing loose clusters of megakaryocytes containing both large megakaryoctes with hyperlobated nuclei and smaller forms with cloud-like or hyperchromatic nuclei; right, silver-stained bone marrow section showing grade 4 reticulin fibrosis (on 0-4 scale). (B) Blood counts during the course of 54 months from diagnosis showing stabilization of the platelet count and no change in hemoglobin level. Red and blue shaded areas represent the normal range for hemoglobin and platelet count, respectively.
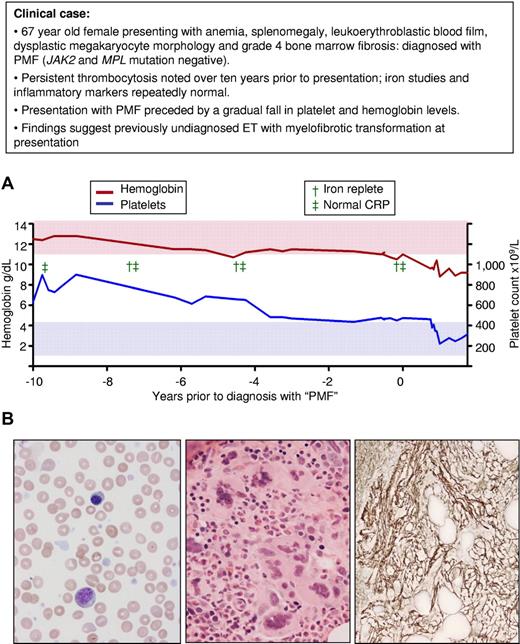
A third area of controversy relates to the traditional view of ET and PMF as separate entities. More recently it has been suggested that PMF represents presentation in accelerated phase of a previously undiagnosed MPN, usually ET.1,14,30 This concept is supported by several lines of evidence: (1) PMF is clinically indistinguishable from myelofibrotic transformation of ET; (2) the prevalence of JAK2 and MPL mutations are similar in ET and PMF; (3) laboratory (eg, cytogenetic changes) and clinical features (eg, increased rate of leukemic transformation) suggest PMF represents accelerated phase disease; and (4) patients with PMF may have thrombocytosis for many years before they are diagnosed (Figure 3). Of interest, the patient illustrated in Figure 3 had a platelet count of 450-500 × 109/L at diagnosis of PMF but was subsequently found to have had counts lose to 1000 × 109/L during the preceding 10 years. This finding indicates that ET patients presenting with marginally elevated platelet counts may represent either early-phase disease (platelet count on the way up) or late-phase disease (platelet count on the way down).
Presentation with myelofibrotic transformation of previously undiagnosed ET. (A) Platelet and hemoglobin levels during a 10-year period before presentation with PMF in a patient at the age of 67 years; a marked thrombocytosis between 8 and 10 years before diagnosis is followed by a gradual decrease in platelet count and progressive anemia in the absence of iron deficiency or inflammation. (B) At clinical presentation, left, Wright-Geimsa–stained blood film showing leukoerythroblastosis and tear-drop red cells; middle, hematoxylin and eosin–stained bone marrow trephine biopsy showing clusters of dysplastic megakaryocytes; and right, silver-stained bone marrow trephine biopsy showing grade 4 reticulin fibrosis (on 0-4 scale). Red and blue shaded areas represent the normal range for hemoglobin and platelet count, respectively.
Presentation with myelofibrotic transformation of previously undiagnosed ET. (A) Platelet and hemoglobin levels during a 10-year period before presentation with PMF in a patient at the age of 67 years; a marked thrombocytosis between 8 and 10 years before diagnosis is followed by a gradual decrease in platelet count and progressive anemia in the absence of iron deficiency or inflammation. (B) At clinical presentation, left, Wright-Geimsa–stained blood film showing leukoerythroblastosis and tear-drop red cells; middle, hematoxylin and eosin–stained bone marrow trephine biopsy showing clusters of dysplastic megakaryocytes; and right, silver-stained bone marrow trephine biopsy showing grade 4 reticulin fibrosis (on 0-4 scale). Red and blue shaded areas represent the normal range for hemoglobin and platelet count, respectively.
These various controversies over the boundary between ET and PMF are likely to be resolved in time as we gain greater molecular insights into these disorders. However, the current lack of a universally agreed classification will impair comparisons between clinical studies. In our view the WHO criteria place too much reliance on subjective histologic criteria, the variable application of which by different centers will result in a lack of comparability between patient cohorts. By contrast, the broader definition of ET adopted by BCSH guidelines has the advantage that it is easier to apply in a reproducible manner.
Distinguishing ET from PV
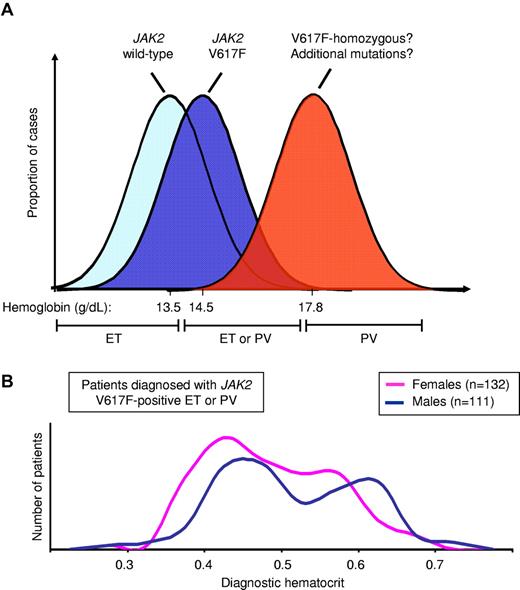
The distinction between ET and PV is, in theory, simple—patients with PV have an overt erythrocytosis that is lacking in ET. Unfortunately, in practice matters are more complex. Mounting evidence demonstrates that patients with JAK2 V617F-positive ET represent a forme fruste of PV and exhibit PV-like features.7,30 ET and PV therefore form a phenotypic spectrum and there has been considerable debate over where to draw the line between them. Indeed, there are inherent problems in the use of continuous variables, such as hemoglobin, hematocrit, or red cell mass, to make this binary distinction, because the group of patients with borderline values will inevitably include both disorders, as illustrated in Figure 4A.
Distribution of hematocrit level in JAK2 V617F-positive disease. (A) Diagrammatic representation of hemoglobin levels in patients with JAK2 V617F-negative ET, V617F-positive ET and V617F-positive PV, showing mean hemoglobin levels for patients with V617F-positive and negative ET (from Campbell et al7 ) and V617F-positive PV (from Cambridge cohort); hemoglobin levels in the mid-range will include patients with both ET and PV. (B) Hematocrit levels at diagnosis from a cohort of patients attending the Cambridge MPN clinic and diagnosed with JAK2 V617F-positive ET or PV, showing considerable overlap in hematocrit between ET and PV in both male and female patients.
Distribution of hematocrit level in JAK2 V617F-positive disease. (A) Diagrammatic representation of hemoglobin levels in patients with JAK2 V617F-negative ET, V617F-positive ET and V617F-positive PV, showing mean hemoglobin levels for patients with V617F-positive and negative ET (from Campbell et al7 ) and V617F-positive PV (from Cambridge cohort); hemoglobin levels in the mid-range will include patients with both ET and PV. (B) Hematocrit levels at diagnosis from a cohort of patients attending the Cambridge MPN clinic and diagnosed with JAK2 V617F-positive ET or PV, showing considerable overlap in hematocrit between ET and PV in both male and female patients.
We take a pragmatic approach and base a diagnosis of PV on the presence of a JAK2 mutation and a raised hematocrit (with or without supporting features such a low serum erythropoietin), an approach consistent with both WHO and BCSH guidelines.13,31 We recognize that, unless markedly elevated, hematocrit does not accurately predict an increased red cell mass,32 that serum erythropoietin levels do not distinguish PV from ET,7 and that ET patients, predominantly those with JAK2 V617F-positive disease, may harbor an increased red cell mass despite a normal hematocrit.33,34 However, in the presence of a normal hematocrit and normal iron stores, the clinical significance of a raised red cell mass is unclear, and so we do not measure red cell mass in our ET patients.
Further progress in distinguishing ET from PV is likely to require a better understanding of their molecular pathogenesis. It has recently been reported that the distinct phenotypes of JAK2 V617F-positive ET and PV reflect differential STAT1 activation,35 an observation that could lead to clinically useful biomarkers.
How we treat ET
Modification of cardiovascular risk factors
In our practice, all patients are screened for the presence of established cardiovascular risk factors, including hypertension, diabetes, smoking, hypercholesterolemia, and obesity, and treated where indicated according to local guidelines. The broad efficacy of the cholesterol-lowering statin drugs in the prevention of atherosclerotic disease has raised the possibility that such agents may be useful in ET, although this has yet to be tested in a prospective study.
Antiplatelet therapy
A large randomized trial in PV demonstrated a reduction in thrombotic events in those taking aspirin, without a concomitant increase in the risk of hemorrhage.36 Retrospective studies have suggested a similar protective effect in ET,37,38 although the authors of one recent study suggested that low-risk patients may not derive benefit from antiplatelet therapy.39 On the basis of current evidence, we recommend aspirin for all ET patients unless contraindicated. Although there are few data concerning the use of newer antiplatelet agents such as clopidogrel, their proven track record in atherosclerotic disease suggests they are appropriate for ET patients unable to tolerate aspirin.
Indications for cytoreductive therapy
The best established risk factors for thrombotic complications in ET are age older than 60 years or a history of previous thrombosis,16,40,41 and patients with these risk factors probably benefit from cytoreductive therapy.42 Risk factors for atherosclerotic disease also predict for thrombosis in ET patients,41,43,44 although it is unclear whether their presence necessitates cytoreductive therapy in the absence of high-risk ET (age > 60 years or history of thrombosis). The degree of thrombocytosis is not a reliable indicator of thrombotic risk, although very high levels may predict for hemorrhagic complications.16,41,45-48 Meta-analysis has confirmed an increased risk of venous and arterial thrombosis in JAK2 V617F-positive compared with V617F-negative ET.49,50 Studies have also suggested that the size of the JAK2-mutant clone may be of prognostic significance in MPN patients, although data for ET patients are limited27 Increased leukocyte count or increased bone marrow fibrosis at diagnosis are also independent predictors of thrombotic complications.15,29,48
We stratify ET patients on the basis of thrombotic risk (Table 4) and recommend cytoreductive therapy for all high-risk patients. Cytoreduction is also considered in those with previous serious hemorrhage (eg, requiring hospitalization or red cell transfusion) that is thought to be related to ET. Microvascular complications, such as erythromelalgia, generally settle with antiplatelet therapy alone,38 and cytoreductive therapy is only considered for refractory cases. Those with indicators of atherosclerotic disease in the absence of high-risk ET are assessed on a case-by-case basis, taking into account the severity of each cardiovascular risk. For example in the absence of high-risk features, cytoreductive therapy would not generally be recommended for a patient with well-controlled hypertension but would be considered in someone with poorly controlled diabetes or a strong family history of early onset atherosclerotic disease. The role of the platelet count in directing cytoreductive therapy remains contentious. Many physicians still recommend cytoreductive therapy in patients with platelets > 1500 × 109/L, although it may be reasonable to use a greater cut-off in younger patients. There are, as yet, no prospective clinical data on the utility of novel thrombotic risk factors, such as JAK2 mutation status, mutant allele burden, leukocyte count, and bone marrow fibrosis. We therefore do not currently use these factors to guide therapeutic decisions.
Patients without high-risk features can be divided into low risk (age < 40 years) and intermediate risk (age 40-60 years). Cytoreductive therapy is unlikely to offer a significant protective effect for those with low risk of disease, in whom the a priori risk of thrombosis is small. There is currently little evidence available to guide treatment decisions in the intermediate risk group. The ongoing PT-1 trials (http://www.haem.cam.ac.uk/pages/pt1/), which comprise a randomized trial of hydroxycarbamide and aspirin versus aspirin alone for intermediate-risk patients and an observational study of low-risk patients treated with aspirin alone, will provide prospective data to help clarify therapeutic decisions for these patients.
Choice of cytoreductive agent
Hydroxycarbamide (also known as hydroxyurea) is the only cytoreductive agent proven to reduce thrombotic events in a randomized controlled trial42 and remains our recommended first-line therapy for the majority of patients requiring treatment (Table 5). Concerns have been raised, however, about a possible increased risk of leukemic transformation with this agent. Clinical studies have given conflicting results,23,51-54 further confounded by inclusion of patients who have received multiple cytotoxic agents, lack of proper controls, retrospective data collection, and relatively short follow-up. Further evidence cited in support of a mutagenic role for hydroxycarbamide includes a possible association with deletions of 17p (harboring the TP53 locus),51,55-57 increased risk of skin neoplasia during prolonged therapy,58 and possible clastogenic activity in vitro.59 By contrast, hydroxycarbamide appears nonleukemogenic in the treatment of sickle cell disease60 and does not appear to increase in vivo mutation rates in sickle cell or MPN patients.61 At this time it is unclear whether single agent hydroxycarbamide is leukemogenic; however, any increased risk is likely to be small and should be balanced against the reduction in thrombotic complications because thrombosis remains the major source of morbidity in ET.
Anagrelide reduces the platelet count by inhibition of megakaryocyte differentiation,62 and we currently use it as second-line therapy for patients in whom hydroxycarbamide is inadequate or not tolerated. Combined therapy with anagrelide and hydroxycarbamide has also been used successfully in our clinic in circumstances in which hydroxycarbamide alone has failed to control the platelet count. Anagrelide does not affect the white cell count, but anemia is common and often progressive.29 Up to one-third of patients cannot tolerate anagrelide because of side effects, many of which result from its vasodilatory and positive inotropic actions, including palpitations and arrhythmias, fluid retention, heart failure, and headaches.19,63 The use of this drug requires particular caution in elderly patients or those with pre-existing cardiac disease. Although anagrelide is not cytotoxic and therefore unlikely to be leukemogenic, the PT-1 trial demonstrated that anagrelide plus aspirin was inferior to hydroxycarbamide plus aspirin in high-risk ET patients. Despite equivalent control of the platelet count, anagrelide-treated patients experienced greater rates of arterial thrombosis, major hemorrhage, and progression to myelofibrosis and were more likely to be intolerant of their therapy.19 In contrast to hydroxycarbamide, anagrelide therapy was also associated with an increase in bone marrow reticulin over time.29 Comparison of patients in the PT-1 (comparison of hydroxycarbamide vs anagrelide19 ) and Italian (comparison of hydroxycarbamide vs no cytoreductive therapy42 ) prospective studies suggests that anagrelide provides partial protection from thrombosis.64 The reduced efficacy of anagrelide compared with hydroxycarbamide in thrombosis prevention was limited to those with JAK2 V617F-positive ET, probably reflecting increased sensitivity of these patients to the cytoreductive effects of hydroxycarbamide.7 However, the increased risk of myelofibrotic transformation associated with anagrelide was observed in V617F-positive and negative patients.
Preliminary reports of the final results of the ANAHYDRET (Anagrelide versus Hydroxyurea in ET) trial claim to show that anagrelide is not inferior to hydroxycarbamide in the treatment of ET.65 However, compared with PT-1, the number of patients enrolled was small, the duration of follow-up relatively short (539 patient-years compared with 2653 patient-years in PT-1), and considerably fewer end-point events were recorded.30 It therefore seems highly unlikely that the ANAHYDRET study had the statistical power necessary to detect the differences observed in the PT-1 study. In addition, the “noninferiority” design of the ANAHYDRET study seems inappropriate, especially after the marked inferiority documented by the PT-1 trial. Such designs define noninferiority up to a predetermined limit of “tolerable inferiority,” but often this limit is entirely arbitrary or selected with an eye on obtaining regulatory approval, and risks failing to detect clinically relevant differences between the treatment arms. Indeed, the whole ethical basis for conducting noninferiority trials has recently been called into question.66
Recombinant interferon α is free from leukemogenic or teratogenic effects, is effective at controlling the platelet count in ET, and may reduce JAK2 mutant allele burden.67,68 It should be noted, however, that interferon has not been shown to protect from thrombotic complications, and the PT-1 trial reminds us that 2 agents that result in equivalent control of the platelet count may nonetheless be associated with very different thrombotic risk.19 Interferon therapy is still considered by many as experimental, and its use in the MPN is not licensed in Europe or North America. Therapy is often associated with significant side-effects, and although pegylated interferon may be more convenient, toxicity appears similar to the native compound.67,69 Interferon is used in our clinic for young patients (typically those < 40 years), for those wishing to start a family, or for those in whom hydroxycarbamide may be inappropriate. We try and avoid the use of interferon in older patients, who are generally less able to tolerate this agent.
Radioactive phosphorus and alkylating agents such as busulphan are effective at controlling the platelet count but are associated with an increased risk of progression to acute leukemia, particularly when used sequentially with hydroxycarbamide.23,52 Both agents can be given intermittently with long intervals between doses and may be useful in treating older patients who are unable to attend the clinic on a regular basis. Pipobroman, a piperazine derivative, is effective at reducing the platelet count in ET, although there is little direct evidence for thrombosis prevention.70 Pipobroman is chemically similar to the alkylating agents and is associated with an increased risk of leukemic transformation when used in the long-term treatment of PV.71 In our clinic, the use of pipobroman and other alkylating agents is restricted to older patients (typically those > 75 years), where they are used as second- or third-line agents for those who are unable to tolerate hydroxycarbamide, for example, because of nonhealing leg ulcers.
Response to therapy
The traditional goal of cytoreductive therapy in ET has been resolution of symptoms and normalization of the platelet count. However, retrospective studies have identified an association between leukocytosis and thrombosis,48 raising the possibility that controlling the white cell count may be important. Recent prospective data from the PT-1 trial have shed further light on this issue, with analysis of blood counts revealing that a leukocyte (but not platelet) count above the normal range during follow-up predicted for thrombosis in the subsequent 60 days (P.J.C., C. MacLean, P.A.B., G. Buck, K. Wheatley, C. N. Harrison, A.R.G., manuscript in preparation). We now use freedom from ET-related symptoms together with normal platelet and leukocyte counts as our primary therapeutic endpoints, although it should be noted that this approach has yet to be validated in a prospective clinical study.
Special considerations: pregnancy
First-trimester fetal loss complicates 25%-50% of pregnancies in ET patients, with other complications such as intrauterine growth retardation, stillbirth, and preeclampsia also occurring more frequently.72-77 Such complications occur irrespective of the platelet count before conception but appear to be more common in those with JAK2 V617F-positive disease.74,77 Whether the use of aspirin or cytoreductive agents can improve pregnancy outcome is uncertain; researchers have reported contradictory results.72-77 Moreover, the use of aspirin during pregnancy does not benefit non-ET patients with a history of recurrent miscarriage.78 However, a large meta-analysis of non-ET preeclampsia patients suggested that aspirin use in pregnancy is safe for mother and fetus,79 and we therefore recommend aspirin for all pregnant ET patients (unless otherwise contraindicated).
We consider the use of cytoreductive therapy for all pregnant women with a history of thrombosis. We also consider cytoreductive therapy for those with previous obstetric complications, such as stillbirth or recurrent miscarriage. Because the platelet count often decreases during pregnancy, we do not consider a platelet count of greater than 1500 × 109/L as an absolute indication for cytoreductive therapy, and such patients are considered on a case-by-case basis.
Although hydroxycarbamide has been used during pregnancy, usually without adverse effects for mother or fetus, it is teratogenic in nonhuman mammals80 and should therefore be avoided. Anagrelide can cross the placenta with unknown effects on fetal development and should also be avoided. Interferon α is nonteratogenic and is the agent of choice should cytoreductive therapy be required. Although studies in ET are lacking, thromboprophylaxis appears safe in pregnancy81 and may be considered for patients with a history of thrombosis or recurrent pregnancy loss; in those with a previous occurrence of thrombosis, treatment should be continued for at least 6 weeks postpartum. Overall, pregnancy does not appear to affect the natural history of ET.72 Pregnant ET patients should ideally be managed in a center at which regular fetal monitoring can be performed, with good communication between the obstetric, hematology, and anesthetic departments. In animal studies, hydroxycarbamide is associated with reduced spermatogenesis and genetic damage to spermatogonia.80 We therefore advise male patients requiring cytoreductive treatment to switch to interferon α before attempted conception.
Special considerations: surgery
Although perioperative thrombotic and bleeding complications appear increased in ET patients, it is not clear whether this can be ameliorated by therapeutic intervention.82 In general, we stop antiplatelet agents 7-10 days before major surgery or surgery to critical sites and reintroduce as soon as the surgeon is confident of hemostasis. Postoperative thromboprophylaxis is recommended according to usual guidelines for the specific procedure. For patients receiving cytoreductive therapy who are undergoing elective surgery, the blood count is optimized preoperatively, and interruptions in therapy are kept to a minimum. For patients not receiving cytoreductive treatment, temporary therapy is considered on a case-by-case basis, after assessment of the patient's thrombotic risk profile, degree of thrombocytosis (because greater platelet counts may predict for hemorrhage47 ), and the nature of the surgery. Splenectomy in ET patients generally results in a sharp increase in platelet count that may lead to thrombotic and hemorrhagic complications. Unless otherwise contraindicated, we commence cytoreductive therapy before splenectomy in ET patients with an increased or high-normal platelet count, aiming for a platelet count in the mid-normal range before surgery. Postoperative thromboprophylaxis and daily monitoring of bloods counts are also recommended.
How we treat advanced-phase disease
The problems and complications associated with myelofibrotic transformation of ET are similar to de novo PMF, and we manage the conditions in the same way. Therapy of post-ET AML is often limited by the age and comorbidities and supportive treatment is often the most appropriate strategy. Overall the prognosis of AML secondary to an MPN is very poor. Younger patients who achieve remission with AML induction therapy are considered for allogeneic bone marrow transplantation.
Controversies in the management of ET
The choice of cytoreductive therapy in the younger patient
Hydroxycarbamide has proven efficacy in the prevention of thrombosis, the major source of morbidity in this condition, but concerns remain about potential leukemogenicity.57 Because the incidence of leukemic transformation in ET increases with disease duration,15,16 this concern is particularly relevant to younger patients who may require therapy for several decades. Interferon α is not leukemogenic, but efficacy data are lacking, and some patients experience intolerable side-effects. Anagrelide is not cytotoxic and likely not mutagenic. However, the PT-1 trial demonstrated inferiority of anagrelide to hydroycarbamide in the prevention of thrombosis and identified an increased risk of myelofibrotic transformation with anagrelide therapy.19
It is our current practice to recommend a trial of interferon α to patients younger than the age of 40 years who require therapy. Approaches to maximizing tolerance include slow escalation from a low starting dose (eg, 1MU 3 times a week initially), administration at bedtime with prophylactic acetaminophen (paracetamol), dose reduction once control is achieved (to the lowest dose that affords blood count control), and support from specialist nursing staff between visits to the clinic. If interferon α is not tolerated, the pros and cons of hydroxycarbamide versus anagrelide are discussed in detail with each patient. For those receiving anagrelide, we recommend baseline bone marrow studies to assess reticulin fibrosis with follow-up bone marrow histology every 2-3 years while therapy continues. Cessation of anagrelide should be considered in those with increasing reticulin fibrosis because anagrelide-associated fibrosis is reversible in some cases.29
Indications for cytoreductive therapy in ET versus PV
As outlined previously in this article, ET and PV form a phenotypic continuum with significant overlap in hemoglobin and platelet levels. These observations call into question the rationale for different therapeutic strategies in these 2 disorders, particularly the use of a target hematocrit level to guide treatment in PV but not ET. The primary aim of therapeutic intervention in both ET and PV is the prevention of thrombosis. Whereas thrombotic risk appears greater in V617F-positive compared with V617F-negative ET,49,50 retrospective data suggest comparable thrombosis rates in V617F-positive ET and PV.83 Common predictors of thrombosis in ET and PV include older age, thrombotic history, and increased white cell count,16,40,41,84,85 with hematocrit identified by some but not all studies as an important predictor of thrombosis in PV.86,87 Given the similarities between V617F-positive ET and PV, the time may be approaching when these disorders are stratified and managed as a single disease entity, with older age, history of previous thrombosis, or an increased hematocrit indicating high-risk disease and mandating cytoreductive therapy. Given the lower thrombosis rates in V617F-negative ET, it may be that indications for therapy could safely be relaxed in this group. Clearly further prospective trials are necessary before these goals can be realized, with studies enrolling both ET and PV patients allowing for prospective comparison of complication rates and assessment of novel risk factors such as mutation status, leukocyte count and bone marrow fibrosis.
Future directions
In the past 5 years we have witnessed dramatic advances in our understanding of the pathogenesis, classification, and therapy of ET. The identification of additional molecular markers is likely to be needed to clarify the overlap between ET, PV, and PMF. Novel risk factors such as leukocyte count, JAK2 mutation status, and bone marrow fibrosis may provide more precise therapeutic stratification, an approach that awaits validation in prospective studies. With regards to therapy, direct comparison of interferon α and hydroxycarbamide seems timely. JAK2 inhibitors may prove useful for accelerated-phase disease88 but given the excellent prognosis of chronic phase ET, it is unclear whether there is likely to be a role for JAK2 inhibitors in the majority of ET patients.
Acknowledgments
We thank Dr Penny Wright for helpful comments on bone marrow histology and our colleagues in the NCRI MPN study group for many helpful discussions.
Work in the authors' laboratories is supported by Leukemia and Lymphoma Research UK, Cancer Research UK, the Kay Kendal Leukemia Fund, the National Institute for Health Research Cambridge Biomedical Research Center, and the Leukemia & Lymphoma Society of America. P.J.C. is supported by a Wellcome Trust Senior Clinical Research Fellowship and P.A.B. by a Kay Kendall Leukemia Fund Intermediate Fellowship.
Wellcome Trust
Authorship
Contribution: P.A.B. and A.R.G. wrote the manuscript; P.J.C. contributed to the writing of the manuscript; W.N.E. contributed to the writing of the “How we diagnose ET” section; and all authors reviewed and revised the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Prof Tony Green, Cambridge Institute for Medical Research, Hills Rd, Cambridge CB2 0XY, United Kingdom; e-mail: arg1000@cam.ac.uk.