Abstract
Fibrinogen adsorption on a surface results in the modification of its functional characteristics. Our previous studies revealed that fibrinogen adsorbs onto surfaces essentially in 2 different orientations depending on its concentration in the solution: “side-on” at low concentrations and “end-on” at high concentrations. In the present study, we analyzed the thrombin-mediated release of fibrinopeptides A and B (FpA and FpB) from fibrinogen adsorbed in these orientations, as well as from surface-bound fibrinogen-fibrin complexes prepared by converting fibrinogen adsorbed in either orientation into fibrin and subsequently adding fibrinogen. The release of fibrinopeptides from surface-adsorbed fibrinogen and from surface-bound fibrinogen-fibrin complexes differed significantly compared with that from fibrinogen in solution. The release of FpB occurred without the delay (lag phase) characteristic of its release from fibrinogen in solution. The amount of FpB released from end-on adsorbed fibrinogen and from adsorbed fibrinogen-fibrin complexes was much higher than that of FpA. FpB is known as a potent chemoattractant, so its preferential release suggests a physiological purpose in the attraction of cells to the site of injury. The N-terminal portions of fibrin β chains including residues Bβ15-42, which are exposed after cleavage of FpB, have been implicated in many processes, including angiogenesis and inflammation.
Introduction
Fibrinogen, one of the most abundant proteins in blood, plays a key role in hemostasis, inflammation, wound healing, and additional physiological and pathological processes. Immediately after blood comes in contact with artificial materials or with an injured vessel wall subendothelium, fibrinogen rapidly adsorbs on the surface and interacts with adhered activated platelets and subendothelial proteins. Numerous studies have demonstrated that fibrinogen in solution and fibrinogen adsorbed on various surfaces exhibit different properties.1-4 For example, surface-adsorbed fibrinogen changes its conformation and thus reveals multiple binding sites that interact with the receptors on platelets and leukocytes.5,6 These reciprocal interactions participate in the process of blood clot formation and in the inflammatory response. Platelet adhesion promoted by the deposition of fibrinogen might contribute to the development of the inflammatory response during ischemia reperfusion. The structural properties of fibrinogen play a key role in its interactions with various biomolecules and cell types.
Fibrinogen is a 340-kDa plasma glycoprotein with a complex structure. The fibrinogen molecule consists of 2 identical subunits, each composed of 3 nonidentical polypeptide chains, Aα, Bβ, and γ. These chains are linked together by 29 disulfide bonds and form several structural regions, 2 distal D regions, one central E region, and 2 αC regions.7 Each pair of distal nodules is linked with the central nodule by a triple helical coiled-coil connector composed of the middle portions of all 3 chains. The COOH-terminal parts of the Aα chains (αC regions) fold back to the central E region to form 2 interacting αC-regions. The central nodule contains 2 pairs of polymerization sites (knobs), “A” and “B,” while the complementary polymerization sites (holes), “a” and “b,” are located in the distal γ- and β-nodules, respectively. In addition, these nodules, as well as the αC-regions, contain numerous binding sites that become active after the conversion of fibrinogen into fibrin or the adsorption of fibrinogen on various surfaces.6,8-11
The conversion of monomeric fibrinogen into polymeric fibrin is mediated by thrombin, which binds to the central region of fibrinogen and catalyzes cleavage of the 2 short peptides, the 16-residue fibrinopeptide A (FpA) and the 14-residue fibrinopeptide B (FpB), located at the NH2-termini of the Aα and Bβ chains, respectively.12 This cleavage exposes knobs A and B, which interact with the complementary holes a and b of neighboring molecules to form a fibrin polymer (clot).13 According to the current view, the assembly of a fibrin clot in solution occurs in 2 steps. First, thrombin removes FpA, enabling A-a interactions between individual half-staggered molecules and resulting in the formation of 2-stranded protofibrils. Second, protofibrils aggregate laterally to make thicker fibers that coalesce to form a 3-dimensional network of fibrin clot. It was shown that the lateral aggregation of protofibrils coincides with the removal of FpB, enabling B-b interactions, and that FpB removal is accelerated by forming polymers.14,15 Thus, during fibrin polymerization in solution, the release of FpA occurs very rapidly, whereas the release of FpB is delayed and reaches its maximum when fibrin formation is almost complete. Such a delay results in the sequential release of fibrinopeptides and thereby the sequential activation of the 2 sets of polymerization sites,12,16 which is necessary for normal fibrin assembly.15
It has been suggested that the accelerating effect of polymer formation on FpB cleavage is related mainly to the interaction of the N-terminal parts of the Bβ chains with the dimeric DD regions formed in protofibrils and fibers, bringing FpB into the vicinity of bound thrombin.17-22
So far, fibrin(ogen) characteristics, such as the release of fibrinopeptides and fibrin-fibrinogen interactions, have been mostly studied in solution.23,24 However, adsorbed fibrinogen acquires new properties important for biointeraction compared with fibrinogen in solution. The adsorption of fibrinogen at the site of vascular injury and inflammation or on the artificial surfaces of vascular grafts and other blood-contacting components of medical devices plays a significant role in its interaction with cells and in blood coagulation.25,26 Using infrared multi-internal reflection spectroscopy and monoclonal antibodies against the fibrinogen E and D regions, we previously found that fibrinogen adsorbs on glass, carbon, polyethylene, and polystyrene surfaces in basically 2 different orientations: “side-on” (laying on the surface) and “end-on” (standing on the surface).27-29 While side-on orientation prevails during adsorption from solutions with low fibrinogen concentrations, the other orientation, in which adsorbed fibrinogen is closely packed, occurs with high fibrinogen concentrations. The adsorbed fibrinogen molecules in both cases have a reduced accessibility of thrombin to the E region. Therefore, the aim of the present study was to investigate fibrinopeptide release from fibrinogen layers adsorbed at different surface densities or immobilized on adsorbed fibrin.
Methods
Proteins and reagents
Fibrinogen and thrombin were purchased from Sigma-Aldrich. D-phenylalanyl-L-prolyl-L-arginine chloromethyl ketone (PPACK) and hirudin were purchased from Merck. Solutions were prepared in a 0.05M Tris buffer, pH 7.4, with 0.1M NaCl and 2.5mM CaCl2 (TBS), and in 0.1M phosphate-buffered saline (PBS), pH 7.4. Buffers were filtered through a Millipore 0.22-μm filter.
ATR FT-IR spectroscopy
Attenuated total reflection Fourier transform infrared (ATR FT-IR) spectra were measured using a Bruker IFS 55 spectrometer equipped with a Wilks Scientific ATR attachment. A ZnSe ATR element was spin-coated with a polystyrene film to a thickness of 60 nm. The kinetics of fibrinogen adsorption on the polystyrene surface from fibrinogen solutions was measured in situ in an ATR liquid-flow cell. The amount of adsorbed fibrinogen was estimated by measuring the integral intensity of the fibrinogen amide II band at 1549 cm−1. The intensity-to-mass ratio factor was calculated by comparing spectra of dried adsorbed fibrinogen layers with a calibration curve obtained by deposition and drying of known fibrinogen amounts on the polystyrene-coated ZnSe.
SPR
The surface plasmon resonance (SPR) instrument based on the spectral interrogation of SPR conditions was manufactured at the Institute of Photonics and Electronics, Academy of Sciences of the Czech Republic, Prague. The tested solutions were flowed along the gold surface of SPR chips through the 4-channel flow cell using a peristaltic pump. An increase in the mass of immobilized proteins is expressed by an increase in resonance wavelength λres (nm).
Preparation of adsorbed fibrin(ogen) and fibrinopeptide samples
Fibrin(ogen) structures were prepared on the bottoms of polystyrene wells in 6-well plates (polystyrene non-tissue–treated plate; well diameter 3.5 cm; bottom surface area ∼10 cm2; Falcon Multiwell; Becton Dickinson Labware). A primary adsorbed fibrinogen layer was prepared by the application of a solution of either 20 or 500 μg/mL of fibrinogen in TBS for 150 or 30 minutes, respectively. The wells were then successively washed with TBS, PBS, and incubated in a thrombin solution (2.5 U/mL in PBS) for 60 minutes. Solutions containing released fibrinopeptides were collected at selected reaction times and the samples obtained for high-performance liquid chromatography (HPLC) analysis were frozen and stored. Sample obtained from one well represents one point in the graph. The completeness of release of all accessible fibrinopeptides after 60 minutes under these conditions was proven earlier.30
The second fibrinogen layer was prepared on a primary adsorbed fibrinogen layer treated with thrombin for 60 minutes as follows. The well was washed with PBS and TBS, and the surface-bound thrombin was inhibited by incubation with PPACK (10μM) and hirudin (6 U/mL) for 20 minutes. After washing with TBS, fibrinogen at either 20 or 200 μg/mL in TBS was applied for 120 and 60 minutes, respectively. The surface was washed with TBS and PBS, and treated again with thrombin in PBS (2.5 U/mL). The samples containing released fibrinopeptides were obtained and processed in the same manner as in the case of the first fibrinogen layer. The interaction of surface-bound fibrin with fibrinogen was proven previously.31 Fibrinopeptide release from fibrinogen in solution (3 mg/mL) was performed using 2.5 U/mL of thrombin. The reaction was stopped by trichloroacetic acid (final concentration, 5% m/v) at selected times, the precipitates centrifuged, and the supernatants analyzed by HPLC.
Transmission electron microscopy
The samples of fibrinogen layers for transmission electron microscopy (TEM) were prepared on carbon-coated mica using the same procedure as described in the previous paragraph. Side-on and end-on attachment of fibrinogen at low and at high concentration on the carbon surface was proven earlier.27,29 Saturated primary monolayers of fibrinogen molecules were prepared. Fibrinogen layers were washed with buffer and contrasted with 5% uranyl acetate in water for 5 minutes,32 washed with water, dehydrated with a series of water-ethanol solutions with an increasing ethanol concentrations (0%, 25%, 50%, 75%, 100%), and dried in a flow box.33 The carbon film was floated from the mica at water level and deposited on a microscope grid. The samples were observed using JEOL JEM-1010 and JEOL 200CX transmission electron microscopes at 100 kV.
HPLC analysis
Collected supernatants containing released fibrinopeptides were dried with a centrifugal vacuum concentrator, reconstituted in mobile phase A, filtered using Millipore 0.2-μm centrifugal filters, and analyzed by the reversed-phase HPLC method, essentially according to the method of Suttnar et al.34 Peptide concentrations were obtained using calibration curves of pure substances.
Results
Preparation and characterization of fibrinogen surfaces
To prepare fibrinogen surfaces for the study of the thrombin-mediated cleavage of fibrinopeptides from adsorbed fibrinogen, we first characterized the adsorption of fibrinogen to the polystyrene surface (formation of a primary fibrinogen layer) and the immobilization of a second fibrinogen layer onto the primary layer.
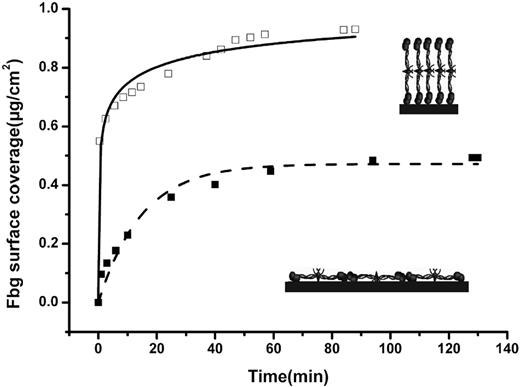
To coat a surface with the primary layer, fibrinogen solutions at high (500 μg/mL) or low (20 μg/mL) concentrations were incubated with the polystyrene surface. The amount of fibrinogen adsorbed on the surface was determined at different time points using ATR FT-IR spectroscopy (Figure 1). Based on previous studies,27-29,32,33 one can assume that when fibrinogen adsorption was performed from the high- or low-concentration solutions, the polystyrene surface was coated with a saturated monolayer of fibrinogen molecules oriented in end-on or side-on manner, respectively.
Adsorption of a primary fibrinogen layer observed by ATR FT-IR spectroscopy. Fibrinogen (Fbg) was adsorbed onto polystyrene from high (500 μg/mL)– and low (20 μg/mL)–concentration fibrinogen solutions (□ and ■, respectively). Inserts show an illustrative arrangement of fibrinogen molecules adsorbed in the end-on (top insert) and side-on orientations.
Adsorption of a primary fibrinogen layer observed by ATR FT-IR spectroscopy. Fibrinogen (Fbg) was adsorbed onto polystyrene from high (500 μg/mL)– and low (20 μg/mL)–concentration fibrinogen solutions (□ and ■, respectively). Inserts show an illustrative arrangement of fibrinogen molecules adsorbed in the end-on (top insert) and side-on orientations.
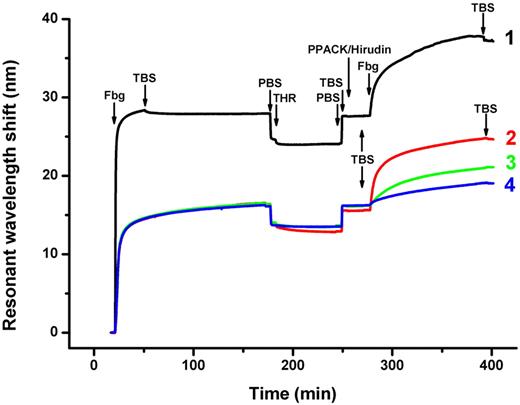
We used SPR to monitor the formation of an additional (secondary) layer of fibrinogen on the primary layer. The SPR signal, which reflects the accumulation of fibrinogen on the surface, revealed that fibrinogen adsorption from the high or low concentration almost reached saturation after 30 or 150 minutes, respectively (Figure 2). The SPR signal revealed the additional immobilization of fibrinogen that presumably occurred through the specific fibrinogen-fibrin interaction. This interaction involves always reactive holes a and b located in the D regions of the added fibrinogen, and the complementary knobs A and B of the E regions of the adsorbed primary layer left exposed by the thrombin treatment. Only a very small drop in the SPR signal was observed when the fibrinogen solution was replaced with TBS (Figure 2 curve 1 right arrow labeled TBS). There was no change in the amount of immobilized fibrinogen when the surface was further contacted with fibrinogen at 500 μg/mL or with a less-concentrated fibrinogen solution of 200 μg/mL (not shown). This suggests that the binding of the secondary fibrinogen layer to the thrombin-treated primary layer, adsorbed from the high concentration of fibrinogen, was specific and reached saturation.
SPR-observed adsorption of primary and secondary fibrinogen layers. A primary layer was formed by the adsorption of fibrinogen onto the gold surface, followed by washing with TBS and PBS; a secondary fibrinogen layer was formed by the immobilization of fibrinogen onto the primary layer, which was treated with thrombin to convert the adsorbed fibrinogen into fibrin. Arrows indicate the replacement of solutions. Curve 1, fibrinogen (Fbg) 500 μg/mL, TBS, PBS; thrombin 2.5 U/mL (THR), PBS, TBS; mixture of PPACK 10μM and hirudin 6 U/mL, TBS; fibrinogen 200 μg/mL, TBS. Curve 2, fibrinogen 20 μg/mL, TBS, PBS; thrombin 2.5 U/mL, PBS, TBS; PPACK 10μM, hirudin 6 U/mL, TBS; fibrinogen 200 μg/mL, TBS. Curve 3, fibrinogen 20 μg/mL, TBS, PBS, TBS; fibrinogen 200 μg/mL, TBS. Curve 4, fibrinogen 20 μg/mL, TBS, PBS; thrombin 2.5 U/mL, PBS, TBS; PPACK 10 μM and hirudin 6 U/mL, TBS; fibrinogen 20 μg/mL, TBS.
SPR-observed adsorption of primary and secondary fibrinogen layers. A primary layer was formed by the adsorption of fibrinogen onto the gold surface, followed by washing with TBS and PBS; a secondary fibrinogen layer was formed by the immobilization of fibrinogen onto the primary layer, which was treated with thrombin to convert the adsorbed fibrinogen into fibrin. Arrows indicate the replacement of solutions. Curve 1, fibrinogen (Fbg) 500 μg/mL, TBS, PBS; thrombin 2.5 U/mL (THR), PBS, TBS; mixture of PPACK 10μM and hirudin 6 U/mL, TBS; fibrinogen 200 μg/mL, TBS. Curve 2, fibrinogen 20 μg/mL, TBS, PBS; thrombin 2.5 U/mL, PBS, TBS; PPACK 10μM, hirudin 6 U/mL, TBS; fibrinogen 200 μg/mL, TBS. Curve 3, fibrinogen 20 μg/mL, TBS, PBS, TBS; fibrinogen 200 μg/mL, TBS. Curve 4, fibrinogen 20 μg/mL, TBS, PBS; thrombin 2.5 U/mL, PBS, TBS; PPACK 10 μM and hirudin 6 U/mL, TBS; fibrinogen 20 μg/mL, TBS.
The immobilization of a secondary layer of fibrinogen on the primary layer adsorbed from the low (20 μg/mL) fibrinogen concentration (presumably in the side-on molecule orientation in the primary layer) was performed similarly, except that the secondary layer was formed by the application of fibrinogen at 2 differing concentrations, high (200 μg/mL) and low (20 μg/mL; Figure 2 curves 2 and 4). The immobilization of fibrinogen from the high concentration in this case also reached saturation. The binding was determined to be tight, as almost no drop in the SPR signal was observed when the fibrinogen solution was replaced with TBS (Figure 2 curve 2 right arrow labeled TBS). In a control experiment, when the primary side-on layer was not treated with thrombin and fibrinogen at a high concentration (200 μg/mL) was applied, some additional binding of fibrinogen was detected (Figure 2 curve 3). However, this binding was approximately 2 times lower than the binding of fibrinogen applied at the same concentration to the thrombin-treated primary layer (Figure 2 curve 2), suggesting that at least half of the latter occurred through the specific fibrinogen-fibrin interaction.
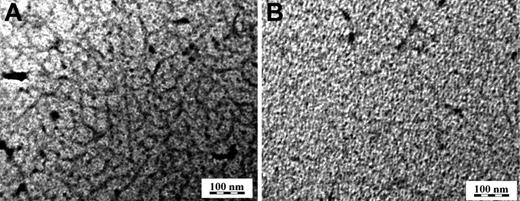
Imaging of the surface fibrinogen-fibrin layers by TEM revealed that their appearance depended on the arrangement of the primary layer adsorbed on the surface. Small protofibrils were observed much more clearly when the primary fibrinogen layer was adsorbed from the low solution concentration of 20 μg/mL, treated with thrombin and thrombin inhibitors, PPACK, and hirudin, and fibrinogen attached by incubation in a 200 μg/mL fibrogen solution (Figure 3A). This is in contrast to the mostly homogeneous layer obtained when the primary layer was adsorbed from the high fibrinogen concentration of 500 μg/mL, treated with thrombin, PPACK, and hirudin, and incubated in a 200 μg/mL fibrinogen solution (Figure 3B). TEM images of primary layers prepared by adsorption from low or high fibrinogen concentration revealed completely coated surfaces (data not shown).
Morphology of fibrinogen-fibrin layers on carbon as observed by TEM. Fibrinogen was adsorbed on carbon-coated mica at a concentration of 20 μg/mL for 150 minutes (A) and 500μg/mL for 30 minutes (B), treated with thrombin at 2.5 U/mL for 60 minutes, a mixture of PPACK at 10μM and hirudin at 6 U/mL for 20 minutes, and fibrinogen at a concentration of 200 μg/mL for 60 minutes. The layers were contrasted with uranyl acetate, dehydrated with a series of water-ethanol solutions, and dried.
Morphology of fibrinogen-fibrin layers on carbon as observed by TEM. Fibrinogen was adsorbed on carbon-coated mica at a concentration of 20 μg/mL for 150 minutes (A) and 500μg/mL for 30 minutes (B), treated with thrombin at 2.5 U/mL for 60 minutes, a mixture of PPACK at 10μM and hirudin at 6 U/mL for 20 minutes, and fibrinogen at a concentration of 200 μg/mL for 60 minutes. The layers were contrasted with uranyl acetate, dehydrated with a series of water-ethanol solutions, and dried.
Thrombin-mediated release of fibrinopeptides from fibrinogen surfaces
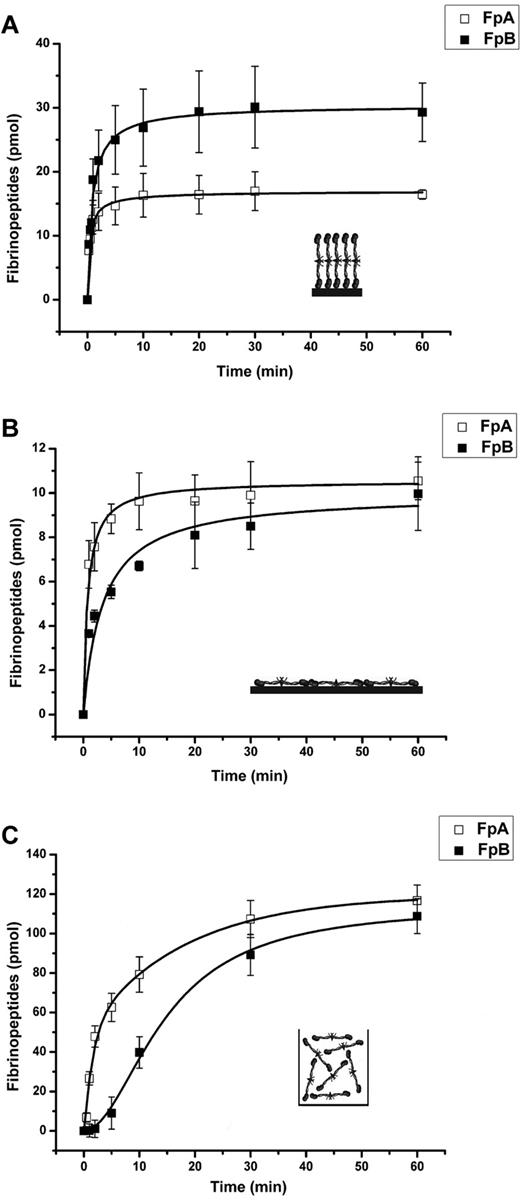
When the primary layer was formed by the adsorption of fibrinogen from the higher concentration (presumably end-on adsorption), the release of both fibrinopeptides occurred very rapidly and reached their maximum after 60 minutes (Figure 4A). The initial rates of release of both fibrinopeptides were similar; however, the amount of released FpA was more than 40% lower than that of FpB (17 vs 30 pmol). In contrast, when the primary layer was formed by the adsorption of fibrinogen from the lower concentration (presumably side-on adsorption), both the initial rate and the amount of FpA released were higher than those of FpB (Figure 4B). Nevertheless, in 60 minutes the amount of released FpB approached that of FpA (∼ 10 pmol). Such kinetics of FpA and FpB release is reminiscent of their release from fibrinogen in solution (shown in Figure 4C for comparison); however, the delay in FpB release characteristic of the latter was not observed with fibrinogen adsorbed from both low and high concentrations. Altogether, these results suggest that the mechanism of thrombin-mediated fibrinopeptide release from adsorbed fibrinogen differs from that of fibrinogen in solution, and that such a mechanism depends on the orientation of the adsorbed fibrinogen.
Kinetics of thrombin-mediated release of fibrinopeptides from the primary fibrinogen layers adsorbed on polystyrene. The amounts of FpA (□) and FpB (■) released from the primary fibrinogen layers adsorbed from the high (500 μg/mL, 30 minutes) and low (20 μg/mL, 150 minutes) fibrinogen concentrations (A and B, respectively) and from fibrinogen in solution (C) was determined by HPLC, as described in “Methods.” The concentration of thrombin in all experiments was 2.5 U/mL. Each point represents a mean value obtained from 3 independent experiments in which the total amount released in one well at the indicated time interval was measured. Inserts in panels a and b show an illustrative arrangement of fibrinogen molecules adsorbed in the end-on and side-on orientations, respectively, and in panel C fibrinogen in solution.
Kinetics of thrombin-mediated release of fibrinopeptides from the primary fibrinogen layers adsorbed on polystyrene. The amounts of FpA (□) and FpB (■) released from the primary fibrinogen layers adsorbed from the high (500 μg/mL, 30 minutes) and low (20 μg/mL, 150 minutes) fibrinogen concentrations (A and B, respectively) and from fibrinogen in solution (C) was determined by HPLC, as described in “Methods.” The concentration of thrombin in all experiments was 2.5 U/mL. Each point represents a mean value obtained from 3 independent experiments in which the total amount released in one well at the indicated time interval was measured. Inserts in panels a and b show an illustrative arrangement of fibrinogen molecules adsorbed in the end-on and side-on orientations, respectively, and in panel C fibrinogen in solution.
The results presented in Figure 4A suggest that not all FpA is accessible for thrombin in the fibrinogen adsorbed from the high concentration. To evaluate the accessibility of FpA, as well as that of FpB, we calculated the amount of fibrinogen adsorbed on the surface of polystyrene wells using the value of the coating density determined by ATR FT-IR spectroscopy (Figure 1). Thrombin cleaved only approximately 37% of FpA and 64% of FpB from the fibrinogen adsorbed from the high concentration and approximately 36% of FpA and 35% of FpB from the fibrinogen adsorbed from the low concentration (Figure 4). Thus, in both adsorbed fibrinogens (adsorbed from both the high and low concentrations) only a portion of the molecules had fibrinopeptides accessible for cleavage by thrombin. Presumably, these molecules were converted into fibrin upon treatment with thrombin, and bound the added fibrinogen to form the secondary fibrinogen layer (or surface-bound fibrinogen-fibrin complexes), as described in the previous section (SPR measurement; Figure 2 curves 1 and 2).
The release of fibrinopeptides from the secondary fibrinogen layers immobilized on fibrin primary layers markedly differed from that of the original primary layers (Figure 5).
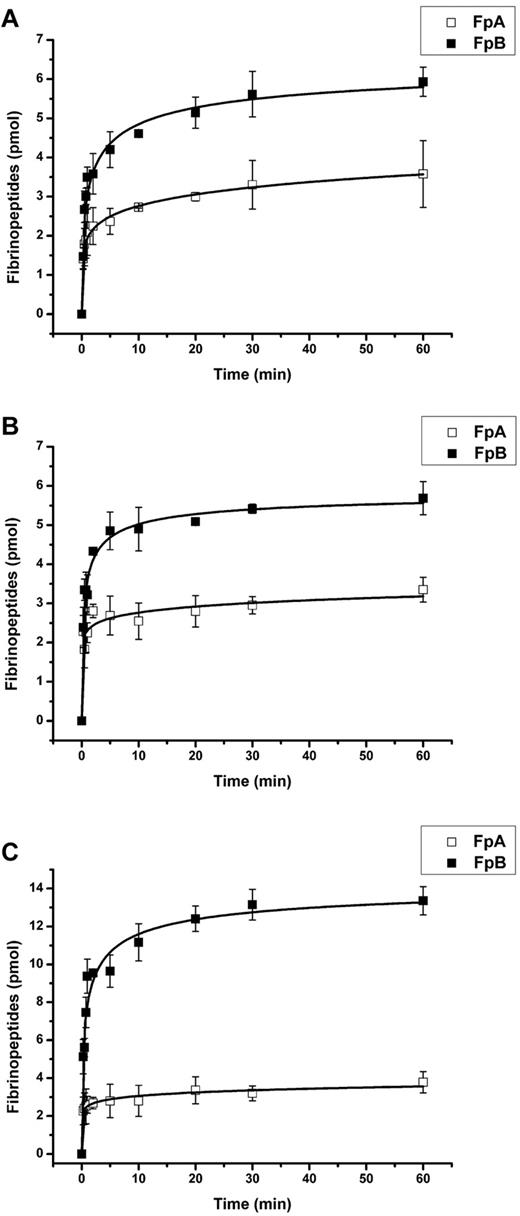
Kinetics of thrombin-mediated release of fibrinopeptides from the secondary layer of surface-bound fibrinogen-fibrin complexes. All fibrinogen-fibrin complexes were formed by the immobilization of the secondary fibrinogen layer on thrombin-treated (2.5 U/mL of thrombin for 60 minutes, followed by a mixture of PPACK and hirudin for 20 minutes) primary fibrinogen layers adsorbed onto polystyrene. (A) The primary layer was adsorbed from 500 μg/mL of fibrinogen for 30 minutes; the secondary layer was immobilized at 200 μg/mL of fibrinogen for 60 minutes. (B) The primary layer was adsorbed from 20 μg/mL of fibrinogen for 150 minutes; the secondary layer was immobilized at 20 μg/mL of fibrinogen for 120 minutes. (C) The primary layer was adsorbed from 20 μg/mL of fibrinogen for 150 minutes; the secondary layer was immobilized at 200 μg/mL of fibrinogen for 60 minutes. The amounts of FpA (□) and FpB (■) released from the secondary layers upon incubation with 2.5 U/mL of thrombin were determined by HPLC, as described in “Methods.” Each point represents a mean value obtained from 3 independent experiments.
Kinetics of thrombin-mediated release of fibrinopeptides from the secondary layer of surface-bound fibrinogen-fibrin complexes. All fibrinogen-fibrin complexes were formed by the immobilization of the secondary fibrinogen layer on thrombin-treated (2.5 U/mL of thrombin for 60 minutes, followed by a mixture of PPACK and hirudin for 20 minutes) primary fibrinogen layers adsorbed onto polystyrene. (A) The primary layer was adsorbed from 500 μg/mL of fibrinogen for 30 minutes; the secondary layer was immobilized at 200 μg/mL of fibrinogen for 60 minutes. (B) The primary layer was adsorbed from 20 μg/mL of fibrinogen for 150 minutes; the secondary layer was immobilized at 20 μg/mL of fibrinogen for 120 minutes. (C) The primary layer was adsorbed from 20 μg/mL of fibrinogen for 150 minutes; the secondary layer was immobilized at 200 μg/mL of fibrinogen for 60 minutes. The amounts of FpA (□) and FpB (■) released from the secondary layers upon incubation with 2.5 U/mL of thrombin were determined by HPLC, as described in “Methods.” Each point represents a mean value obtained from 3 independent experiments.
Discussion
The interaction of fibrin(ogen) with various proteins and cell types, which is of great importance in many vital processes, often occurs after the immobilization of fibrin(ogen) on a surface. Numerous previous studies have characterized fibrin(ogen) properties, including the release of fibrinopeptides and subsequent fibrin formation, mainly in solution. In the present study, we examined the properties of fibrinogen molecules adsorbed on a surface or immobilized on previously adsorbed fibrin. Our results clearly showed that the thrombin-mediated release of fibrinopeptides from adsorbed/immobilized fibrinogen differs significantly from their release from fibrinogen in solution because (1) the delay of FpB release, characteristic of fibrinogen in solution, was not observed with adsorbed/immobilized fibrinogen; and (2) the amount of FpB released from end-on adsorbed fibrinogen or from the secondary fibrinogen layer immobilized on adsorbed fibrin, was significantly higher than that of FpA. Two main questions arise from our results: (1) why is the release of fibrinopeptides from surface-bound fibrinogen so different from that of fibrinogen in solution; and (2) is this of any significant (patho)physiological importance or relevance?
To address the first question, one should consider the possible arrangement of fibrinogen molecules on the surface. When fibrinogen was adsorbed from the lower concentrations in a side-on manner, only approximately one-third of the fibrinopeptides were released by thrombin. It seems that fibrinogen adsorption was random and only this fraction of adsorbed fibrinogen had its fibrinopeptides accessible for thrombin. As in the case with fibrinogen in solution (Figure 4C), the initial rate of FpA release from side-on adsorbed fibrinogen was higher than that of FpB, whereas the final amounts of released fibrinopeptides were similar (Figure 3B). This may have been due to the nonsubstrate interaction of thrombin with adsorbed fibrinogen, which was implicated in the sequential cleavage of fibrinopeptides from fibrinogen in solution.22 At the same time, no delay of FpB release (lag phase) from side-on adsorbed fibrinogen was observed. Because such a delay was proposed to be connected with conformational changes accompanying FpA release21 and protofibril formation,22 such changes seem to have already occurred in adsorbed fibrinogen. This may also have been the reason for the absence of a lag phase in FpB release from the end-on adsorbed fibrinogen or fibrinogen attached to the primary fibrin layer (Figures 4A and 5).
The situation with end-on adsorbed fibrinogen was quite different: the initial rates of FpA and FpB release were similar, whereas the final amount of released FpA was significantly (by 40%) lower than that of FpB. This may be explained by the different accessibility of FpA and FpB for thrombin. Indeed, fibrinopeptides A are located in the central part of the molecule,20,22 which should be difficult to access for thrombin in the end-on–configured adsorbed fibrinogen. The N-terminal segments of the Bβ chains containing FpB were proposed to be flexible9,35 and, according to the results of molecular modeling, may stretch as far as to the outer nodules.22 Thus, the accessibility of FpB in end-on–adsorbed fibrinogen should be superior to that of FpA. Similarly, when the fibrinogen from solution of the high concentration (200 μg/mL) is attached to the primary layer of end-on adsorbed thrombin-treated fibrinogen, the accessibility of FpB for thrombin should also be greater. This was observed in our experiments (Figure 5A).
Our results show that FpB is preferentially released from the secondary fibrinogen layers on the surface of growing fibrins. The tendency for reduced FpA accessibility increased with the increase in the coating concentration (Figure 5B-C). The explanation for such a difference in the accessibility of fibrinopeptides is not entirely clear. One can only speculate that this may be a result of the conformational changes in fibrinogen upon its immobilization or steric hindrance arising from the fibrinogen-fibrin complex formation. The role of the αC region, if any, in the described process is not known and remains to be evaluated. The interaction between fibrin monomers and fibrin(ogen), or vice versa, is characterized by a rather high affinity (KD of ∼ 10−8 M) and a change of standard Gibbs energy of approximately −10 kcal/mol.36 These data demonstrate that the equilibrium is thermodynamically favored for the formation of a stable fibrin-fibrinogen complex. We have shown previously that the overall structure of fibrin arising with the preferential release of FpB by a specific snake venom enzyme is essentially the same as the structure of fibrin initiated by thrombin.37
It should be noted that both our results and those of others have previously indicated no lag phase (delay) in the thrombin-mediated release of FpB from the fibrinogen adsorbed on glass surfaces, and on negatively and positively charged or hydrophobic and hydrophilic surfaces.27,28,31,38 Blombäck and Bark also found no delay in the release of FpB 24 in their experimentation with whole blood and platelet-rich plasma, in which FpB was released almost as quickly as FpA. To explain these observations, they suggested that the binding of fibrinogen to the platelet receptor GPIIb/IIIa may induce conformational changes in bound fibrinogen, resulting in the exposure of a thrombin-susceptible cleavage site and thus facilitating FpB release.
Our data, in accordance with data from many other studies,2,5 suggest that the adsorption of fibrinogen causes changes in its conformation that may have a further impact on its behavior toward other proteins and cell surface receptors. In addition, the TEM experiments revealed that thrombin-treated fibrinogen molecules adsorbed side-on to the surface from the low-concentration fibrinogen solution promoted the formation of a surface-attached fibrin(ogen) network better than the layer of closely packed thrombin-treated fibrinogen molecules adsorbed end-on from the higher concentration (Figure 3).
As to the (patho)physiological relevance of the observed properties of adsorbed/immobilized fibrinogen, one should take into account the following. First, the N-terminal portions of fibrin β chains (amino-acid residues β15-42), which are exposed after cleavage of FpB, have been implicated in a number of important processes, including angiogenesis and inflammation.39-48 Second, the released FpB is a potent chemoattractant,42 and therefore its preferential release may indicate the physiological purpose in the attraction of cells to the site of injury.
It has been shown that the association of soluble fibrinogen with the fibrin clot results in the reduced adhesiveness of such fibrinogen-fibrin matrices toward leukocytes and platelets.49,50 Our finding that FpA is less accessible for thrombin in surface-bound fibrinogen-fibrin complexes may be interpreted as a novel supplementary mechanism preventing the rapid conversion of bound fibrinogen into fibrin, thereby extending its anti-adhesive properties and providing an additional level of protection of thrombi from premature dissolution.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by the Academy of Sciences of the Czech Republic (contract KAN200670701), the Ministry of Health of the Czech Republic (VZ 2373601), and the National Institutes of Health (grant HL-56051).
National Institutes of Health
Authorship
Contribution: T.R. prepared adsorbed fibrin(ogen) and fibrinopeptide samples, designed and performed SPR, TEM, and ATR FT-IR experiments, analyzed the data, and wrote the paper; J.S. performed HPLC analysis and analyzed the data; E.B. designed TEM and ATR FT-IR experiments and analyzed the data; M.H. designed the SPR experiments and analyzed the data; L.M. analyzed the data and wrote the paper; and J.E.D. designed the project, performed SPR experiments, analyzed the data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jan Evangelista Dyr, Institute of Hematology and Blood Transfusion, U nemocnice 1, 128 20 Prague 2, Czech Republic; e-mail: dyr@uhkt.cz.