Abstract
The expression of CD56 antigen in acute promyelocytic leukemia (APL) blasts has been associated with short remission duration and extramedullary relapse. We investigated the clinical significance of CD56 expression in a large series of patients with APL treated with all-trans retinoic acid and anthracycline-based regimens. Between 1996 and 2009, 651 APL patients with available data on CD56 expression were included in 3 subsequent trials (PETHEMA LPA96 and LPA99 and PETHEMA/HOVON LPA2005). Seventy-two patients (11%) were CD56+ (expression of CD56 in ≥ 20% leukemic promyelocytes). CD56+ APL was significantly associated with high white blood cell counts; low albumin levels; BCR3 isoform; and the coexpression of CD2, CD34, CD7, HLA-DR, CD15, and CD117 antigens. For CD56+ APL, the 5-year relapse rate was 22%, compared with a 10% relapse rate for CD56− APL (P = .006). In the multivariate analysis, CD56 expression retained the statistical significance together with the relapse-risk score. CD56+ APL also showed a greater risk of extramedullary relapse (P < .001). In summary, CD56 expression is associated with the coexpression of immaturity-associated and T-cell antigens and is an independent adverse prognostic factor for relapse in patients with APL treated with all-trans-retinoic acid plus idarubicin–derived regimens. This marker may be considered for implementing risk-adapted therapeutic strategies in APL. The LPA2005 trial is registered at http://www.clinicaltrials.gov as NCT00408278.
Introduction
Several investigators have suggested a relationship between the expression of CD56 (neural adhesion factor) antigen in the surface of leukemic blasts and both short remission duration1,2 and development of extramedullary relapse3 in patients with acute promyelocytic leukemia (APL). However, this relationship has not been yet established. In fact, only one of these studies was performed in a relatively large population of APL patients receiving a state-of-the-art treatment with all-trans retinoic acid (ATRA) and anthracycline-based chemotherapy.2 With regard to the incidence of CD56-positive (CD56+) APL and the association with other clinical and biologic variables, very little information has been published.1-5
In this study, we set out to assess the frequency of CD56 expression, its relationship with a broad variety of clinical and hematologic features, as well as its prognostic value in a large series of patients with newly diagnosed APL who were enrolled in 3 consecutive trials of the Programa Español para el Tratamiento de Enfermedades Hematológicas (PETHEMA) and Hemato-Oncologie voor Volwassenen Nederland (HOVON) groups.
Methods
Eligibility
Patients enrolled in the consecutive multicenter PETHEMA LPA96 and LPA99 trials and PETHEMA/HOVON LPA2005 were required to have a diagnosis of de novo APL with demonstration of the t(15;17) or PML/RARA rearrangements. More details about general exclusion and inclusion criteria have been reported elsewhere.6 Of the 1208 patients included in the 3 trials (LPA96, n = 172; LPA99, n = 560; LPA2005, n = 476), 651 patients (54%) had available the percentage of APL leukemic promyelocytes expressing CD56 surface antigen and were evaluable for the present study. Informed consent was obtained from all patients. In accordance with the Declaration of Helsinki, the protocol was approved by the Research Ethics Board of each participating hospital.
Therapy of APL
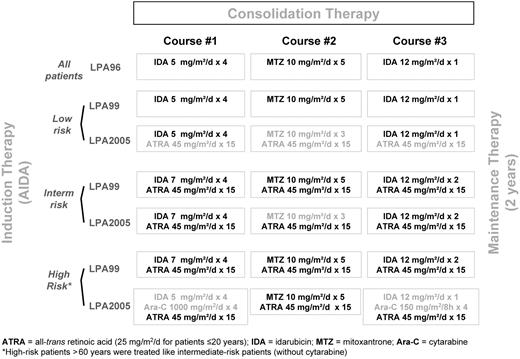
Induction therapy consisted of oral ATRA and idarubicin given as an intravenous bolus on days 2, 4, 6, and 8 (ATRA plus idarubicin, ie, AIDA regimen). In the LPA99 and LPA2005 trials, patients older than 70 years of age received only the 3 first doses of idarubicin.6,7 All patients in complete remission (CR) received 3 monthly consolidation courses with anthracycline-based chemotherapy. The consolidation schedule in the 3 consecutive protocols has been previously described6,7 and is shown in the Figure 1. After completion of consolidation, patients who tested negative for PML/RARA were started on maintenance therapy, as described elsewhere,6 with intermittent ATRA and low-dose chemotherapy with 6-mercaptopurine and methotrexate for 2 years.
Multiparameter flow cytometry
Inmunophenotyping was performed on bone marrow samples collected at APL diagnosis. Leukemic cell analysis was performed at local or reference laboratories by standard inmunofluorescence methods by the use of monoclonal antibodies directed against CD2, CD7, CD9, CD11b, CD13, CD15, CD19, CD33, CD34, CD56, CD117, and HLA-DR surface antigens. The inclusion of anti-CD56 in the panel antibodies was performed at the center's discretion. Multiparametric flow analysis was performed by the use of 3 or 4 colors on a flow cytometer. Leukemic promyelocytes were gated on the basis of their unique side-scatter/CD45-positive and/or CD33hi/homogeneous immunophenotypic profile, in both hyper and hypogranulated cases; CD45hi expression with SSClo was used to further exclude T cells and other lymphocytes from the gate. In addition, CD3 and/or CD5 antigens were routinely included in the diagnostic set to rule out contamination of the gated leukemic promyelocytes by mature T cells. Following the EGIL criteria,8 a sample was defined as positive if ≥ 20% of leukemic promyelocytes expressed a specific antigen in the cell surface. The only exception was the CD34 marker, for which a cutoff level of ≥ 10% expressing cells was required in line with previous reports on APL.4,9 Expression of CD56 was systematically assessed with sensitive (eg, phycoerythrin) fluorochrome-conjugated antibody reagents.
Definitions and study end points
Remission induction response was assessed according to the revised criteria by Cheson et al10 For morphologic assessment of leukemia resistance, it was required that sufficient time had passed to allow for full terminal differentiation of the malignant promyelocytes (up to 40-50 days). Molecular remission was defined as the disappearance on an ethidium bromide gel of the PML/RARA-specific band visualized at diagnosis by use of reverse transcription polymerase chain reaction (PCR) assays with a sensitivity level of one cell in 10−4. Molecular persistence was defined as PCR positivity in 2 consecutive bone marrow samples collected at the end of consolidation therapy. Molecular relapse was defined as reported elsewhere.11 Genetic diagnosis of APL with the use of reverse transcription PCR, anti-PML staining, or cytogenetic tests was required for the diagnosis of overt hematologic relapse. Central nervous system (CNS) relapse was confirmed by lumbar puncture and cytologic examination of cerebrospinal fluid, which was performed only in patients with clinically suspected CNS relapse. Extramedullary relapse in other localization (eg, skin) was confirmed by histopathology.
Data were collected and registered prospectively. Forty patient and disease characteristics were examined to establish their relationship to CD56 expression. We analyzed the characteristics listed in Tables 1 and 2. In addition we analyzed also the following variables: total body surface; liver and spleen enlargement; coagulopathy; hemorrhagic syndrome at presentation; serum levels of lactate dehydrogenase, creatinine, uric acid, alkaline phosphatases, and total bilirubin; peripheral blood blast count and blast cell percentage; bone marrow aspirate cellularity, peroxidase reactivity, and blast cell percentage; and CD13, CD19, and CD33 surface antigen markers.
Diagnosis and gradation of the differentiation syndrome was made according to the previously defined criteria.12 Coagulopathy was defined as a prolonged prothrombin time and/or activated partial thromboplastin time in addition to hypofibrinogenemia and/or increased levels of fibrin degradation products or D-dimers. Patients were classified as having t(15;17) with or without additional chromosomal abnormalities accordingly to previously defined criteria.13 The patient performance status at diagnosis was measured using the Eastern Cooperative Oncology Group (ECOG) scale. Risk of relapse was established at diagnosis according to a predictive model on the basis of patient leukocyte and platelet counts at diagnosis, as reported elsewhere.14
Statistical analysis
The χ2 test, with Yates correction if necessary, was used to analyze differences in the distribution of categorical variables between patient subsets. The Student t test was used to analyze continuous variables following a normal distribution and the Mann-Whitney U test for data that failed the normality test. Unadjusted time-to-event analyses were performed by use of the Kaplan-Meier estimate15 and, for comparisons, log-rank tests.16 The probability of relapse also was estimated by the cumulative incidence method (for marginal probability).17,18 Overall survival (OS) was calculated from the date of starting induction therapy, whereas cumulative incidence of relapse (CIR) and disease-free survival (DFS) were calculated from the date of CR. In the analysis of DFS, relapse, development of secondary myelodysplastic syndrome or acute leukemia (t-MDS/t-AL), and death in CR were considered uncensored events, whichever occurred first. For cumulative incidence analysis, death in CR and development of t-MDS/t-AL were considered as a competing cause of failure. For all estimates in which the event “relapse” was considered as an end point, overt morphologic and molecular relapse, as well as molecular persistence at the end of consolidation, were each considered as uncensored events. Patient follow-up was updated on March 15, 2010. Characteristics selected for inclusion in the multivariate analysis were those for which there was some indication of a significant association in univariate analysis (P < .1) and, if available, those for which previous studies had suggested a possible relationship. Multivariate analyses were performed by use of Cox model for DFS and OS19 and Fine and Gray model for CIR.20 Missing data were substituted by the mean values from patients in whom data were available.21 All P values reported are 2-sided. Computations were performed by use of the 3D, 4F, 1L, LR, and 2L programs from the BMDP statistical library (BMDP Statistical Software), and R 2.9.2 software package for CIR and Fine and Gray model.
Results
Patient characteristics according to CD56 expression
Between December 1996 and December 2009, 651 consecutive patients in whom the results of the analysis of CD56 surface antigen expression at diagnosis were available are the subject of the study (79 patients in the LPA96, 330 in the LPA99, and 242 in the LPA2005 trial). Patients were from 85 institutions from Spain, The Netherlands, Poland, Argentina, and the Czech Republic (see supplemental Appendix, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Median follow-up of the series was 70 months (range, 3-158 months) from diagnosis.
Seventy-two of 651 patients (11%) showed expression of CD56 ranging from 20% to 100% (median, 70%). The main clinical and biologic characteristics of CD56+ APL patients are shown in Tables 1 and 2. Concerning the demographic and clinical characteristics, the white blood cell (WBC) count at baseline was greater among patients with CD56+ APL (P = .03), whereas serum albumin levels were lower (P = .002). There was a trend toward a greater proportion of patients with ECOG performance status grade 2-3 in the CD56+ group (P = .06; Table 1).
Regarding other biologic features of APL, patients with CD56+ APL presented more frequently with BCR3 isoform (P < .001), CD2+ (P < .001), CD34+ (P < .001), CD7+ (P < .001), HLA-DR+ (P = .001), CD15+ (P = .004), and CD117+ (P = .02). There was also a trend toward a greater frequency of microgranular morphology (P = .09), CD11b+ (P = .07), and CD9+ (P = .06; Table 2).
Induction results
Overall, 595 of the 651 evaluable patients achieved morphologic CR (91.4%). As shown in Table 3, 61 of 72 patients (85%) achieved CR in the CD56+ subgroup, compared with 534 of 579 patients (92%) in the CD56− group (P = .04). No significant differences were observed in the distribution of the different causes of death between the CD56+ and CD56− cohorts. The incidence and severity of differentiation syndrome were similar among patients with CD56+ and those with CD56− APL (Table 3).
After multivariate analysis, the regression model for induction death selected the following adverse factors: abnormal creatinine level (P < .0001), WBC count greater than 10 × 109/L (P < .0001), age older than 60 years (P < .0001), male sex (P = .0004), ECOG more than 1 (P = .009), and CD56 positivity (P = .02).
Postremission outcomes
Sixty relapses occurred among the 595 patients who had achieved CR (4 molecular persistence, 15 molecular relapses, and 41 clinical relapses), 12 among 61 CD56+ patients and 48 among 534 CD56− patients. Seven relapses involved extramedullary sites (6 in CNS and 1 in skin), of which 4 occurred among CD56+ patients. In addition, 13 patients died in CR (1 in the CD56+ group), and 10 patients developed t-MDS/t-AL (2 in the CD56+ group).
Relapse rate.
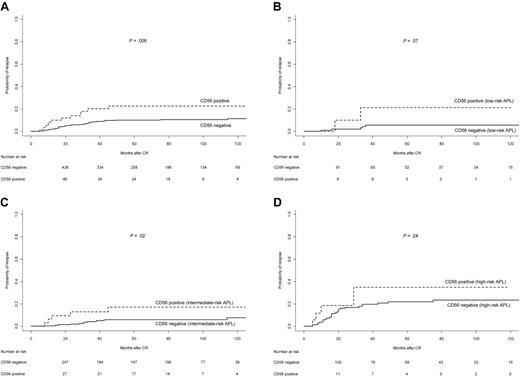
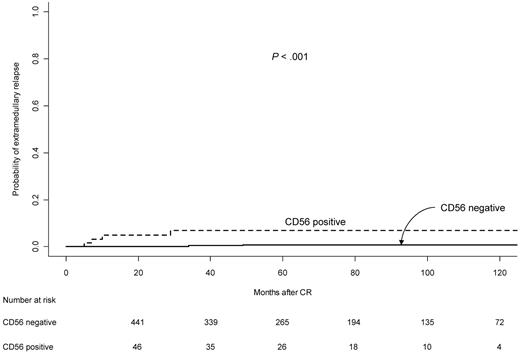
The 5-year CIR rate in the CD56+ cohort was 22%, whereas for CD56− APL it was 10% (P = .006; Table 3 and Figure 2A). For patients in the LPA96 trial, the 5-year CIR rates for CD56+ and CD56− patients were 50% and 10%, whereas in the LPA99 trial they were 18% and 11%, and in the LPA2005 they were 25% and 7%, respectively (P = .003, P = .29, and P = .01, respectively). According to relapse risk groups, the 5-year CIR rates for CD56+ and CD56− patients were 21% and 5% (P = .07) in the low-risk group, 17% and 6% (P = .02) in the intermediate group, and 35% and 22% (P = .24) in high-risk patients (Figures 2B-D). In the multivariate analysis, CD56 expression retained the independent predictive value along with the WBC counts (Table 4). The 5-year cumulative incidence of extramedullary relapse was significantly greater in CD56+ patients compared with those CD56− (7.0% vs 0.7%, P < .001; Table 3 and Figure 3).
Cumulative incidence of relapse in APL patients according to CD56 expression. (A) Overall series; (B) low-risk patients; (C) intermediate-risk patients; and (D) high-risk patients.
Cumulative incidence of relapse in APL patients according to CD56 expression. (A) Overall series; (B) low-risk patients; (C) intermediate-risk patients; and (D) high-risk patients.
Cumulative incidence of extramedullary relapse in APL patients according to CD56 expression.
Cumulative incidence of extramedullary relapse in APL patients according to CD56 expression.
DFS and OS.
The 5-year DFS rates were 73% in the CD56+ cohort and 85% in the CD56− cohort (P = .03). The probability of remaining alive after 5 years was 78% in the CD56+ group and 84% in the CD56− group (P = .09; Table 3).
Discussion
This study shows a prevalence of CD56+ APL in 11% of newly diagnosed patients with APL. The expression of CD56 antigen was correlated with the BCR3 isoform and the coexpression of other surface antigens, such as CD2, CD34, HLA-DR, and CD7. The results presented here confirm that the expression of CD56 antigen is an independent risk factor for predicting relapse in patients with APL treated with ATRA and anthracycline-based regimens, along with the APL relapse-risk score, which is a composite of WBC and platelet counts.14 In addition, CD56+ APL had a significantly higher risk of extramedullary relapse.
The present study analyzes the clinical significance of CD56 expression in a significantly larger series of APL patients compared with previous studies, in which only the Gruppo Italiano Malattie Ematologiche dell'Adulto (GIMEMA) study included a sizable number of patients (100 patients). As in previous studies, we used the cut-off level of 20% of leukemic promyelocytes expressing CD56 antigen to define CD56+ APL.1-3 The frequency of 11% of CD56+ APL here reported is not dissimilar to the12% to 15% values found in other studies.2-4 It should be noted that in the current multicenter study, as in previous studies that analyzed the prognostic impact of CD56 in APL,1,2 immunophenotypic analyses were not performed centrally, preventing a systematic standardization of flow cytometry. A further limitation of our study is the possible selection bias because not all centers performed cytometric analysis that included anti-CD56 in the diagnostic panel. This limitation leads to a considerable reduction in the sample size to almost half compared with the total series.
Concerning the biologic features of CD56+ APL, our study shows a significant correlation with the BCR3 isoform1,5 and CD34 coexpression,3 as has been previously observed. Furthermore, we found an association between CD56+ APL and expression of additional immaturity-associated markers, such as CD117 and HLA-DR antigens, as well as natural killer (NK) and T-cell antigens, such as CD2 and CD7, which have not been previously reported. The higher frequency of coexpression of stem-cell and NK-cell antigens in CD56+ APL may suggest that in some of these cases the APL might have arisen in progenitors that have not undergone lineage restriction.4,22 Interestingly, we found a trend indicating an association with the M3 variant morphology, which has been previously related to CD2 and CD344,5,9 but never CD56 expression. The relationship between CD56 expression and WBC counts confirmed in our series had been suggested in previous studies, although without statistical significance, probably because of the small sample size in those studies.1,2 However, we were unable to confirm the relationship between fibrinogen levels and CD56 expression suggested by others.1
The higher induction mortality rate in CD56+ patients observed in our series treated with the AIDA regimen confirms a similar observation previously reported,1 although many of these patients did not receive a state-of-the-art treatment. Probably, the higher induction mortality rate in CD56+ APL was attributable to its association with other recognized adverse factors for induction response23 ; however, multivariate analysis showed that this immunophenotypic feature had an independent prognostic value.
As suggested in previous studies,1-3 we demonstrated that CD56 expression has an impact on the relapse rate and also confirmed its independent prognostic value as in the GIMEMA study.2 Interestingly, the CD56 expression was able to distinguish a subset of patients with a greater risk of relapse in the intermediate-risk category, whereas its usefulness in low-risk patients needs to be confirmed in larger series. Moreover, the prognostic value of CD56 in high-risk APL seems insignificant. Of note, the expression of CD56 was associated with a higher relapse rate in all 3 PETHEMA trials, although the differences were significant in the LPA96 and LPA2005 trials, but not in the LPA99.
The reason why an elevated CD56 expression leads to a greater risk of relapse remains uncertain but at least 2 hypotheses can be proposed. First, as we have alluded to previously, CD56+ APL may emerge from a more immature undifferentiated and pluripotent leukemic stem cell that is less sensitive to the combination of ATRA and anthracyclines. CD56 expression has been associated with the expression of the multidrug resistant marker P-glycoprotein in patients with acute myeloid leukemia with t(8;21).24 Unfortunately, in the current study the expression of the multidrug resistance markers has not been assessed. Second, the implication of CD56 expression in the development of extramedullary relapses found in our study has already been hypothesized in a previous study by Ito et al.3 In this study, 3 of the 4 CD56+ APL patients developed extramedullary relapse, compared with none of the 24 CD56− patients. Of note in this respect, the expression of CD56 has been associated with extramedullary involvement in nonpromyelocytic acute myeloid leukemia.25-29
In addition to confirm the prognostic value of CD56 expression, this study also provides new insights into the clinical features of CD56+ APL. CD56 expression is associated with some clinical and biologic features, such as increased WBC counts, BCR3 isoform, and coexpression of immaturity and NK-cell antigen markers. It should be noted that, despite this association with WBC counts, the regression model for relapse risk selected both as independent adverse factors, WBC count greater than 10 × 109/L and CD56 positivity. If the independent prognostic value of CD56 expression in APL cells is confirmed, it should be considered for designing future risk-adapted strategies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Alberto Orfao for the critical review of the section addressing the immunophenotypic analyses and Leticia Llopis for data collection and management.
This study was supported in part by the Fundación para la Investigación Hospital Universitario La Fe-Ayudas Bancaja (grant 2006/0137) and Red Temática de Investigación Cooperativa en Cáncer (RD06/0020/0031).
A complete list of participating institutions and clinicians appears in the online supplemental Appendix.
Authorship
Contribution: P.M. and M.A.S. conceived the study and analyzed and interpreted the data; P.M., B.L., and M.A.S. wrote the paper; P.M. performed the statistical analyses; and C.R., E.V., S.B., J.G., M.G., A.H., J.E., J.B., J.D.G., C.R., M.T., V.R., J.B., F.M., G.M., J.d.l.S., I.P., M.P.-E., I.K., J.M.R., and L.E. included data of patients treated in their institutions, reviewed the manuscript, and contributed to the final draft.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Miguel A. Sanz, Hospital Universitario La Fe, Avenida Campanar 21, Valencia 46009, Spain; e-mail: msanz@uv.es.