In his issue of Blood, Reilly and colleagues report a novel inhibitor (PRT-060318) of the tyrosine kinase Syk, expressed in platelets, as a new approach to the management of heparin-induced thrombocytopenia (HIT), using a mouse model of the disease.1
Heparin-induced thrombocytopenia (HIT) is a common form of drug-induced immune reaction, with life-threatening potential, initiated by treatment of patients with unfractionated heparin or low molecular weight heparin.2 Heparin is widely used in the prevention and management of thromboembolic complications in surgery and trauma, in venous thrombotic disease, and in intravenous and intra-arterial lines to maintain patency. Although HIT is a product of immune reaction to the administered heparin, the immunogen is not heparin itself, but a complex of heparin bound to platelet factor 4 (PF4), an abundant platelet protein member of the CXC family of chemokines, alternatively known as CXCL4. PF4 is stored in platelet α-granules, and is released by exocytosis on platelet activation, whereupon it is rapidly bound to negatively charged glycosaminoglycans expressed on the surface of endothelial cells. Heparin, however, also highly negatively charged, binds avidly to PF4 displacing it from its binding to glycosaminoglycans, allowing the complex to enter the circulation where it forms a soluble circulating immunogen.
After the immune response is raised, the PF4-heparin complexes become decorated with immunoglobulin (Ig), and it is these immune complexes that induce the major clinical features of HIT. Particular platelets, through their receptor for IgG FcγRIIA, bind to and become activated by the immune complexes (illustrated in the figure). This causes consumption of platelets leading to thrombocytopenia in the majority of HIT patients, although the drop in platelet count is usually only mild to moderate, and only rarely leads to bleeding complications.3 Thrombosis, however, is the major cause of clinical presentation, occurring in 30% to 60% of patients, with the majority of these associated with venous thrombosis. This may occur in various tissues of the body causing limb gangrene, skin necrosis, and other ischemic conditions. In this way, thrombotic complications contribute to significant morbidity and mortality associated with HIT.
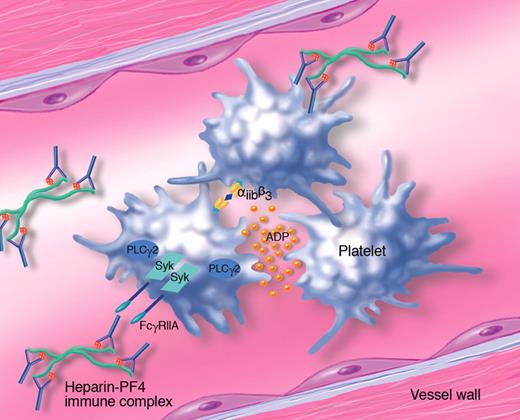
Role of Syk tyrosine kinase in the mechanism of platelet activation and thrombosis in heparin-induced thrombocytopenia (HIT). Platelet factor 4 (PF4) released from platelet α-granules binds with high affinity to heparin through a charge-charge interaction. The heparin-PF4 complex acts as an immunogen, and exposure to heparin after an initial sensitization will lead to the formation of immune complexes, in which the heparin-PF4 aggregates are coated with IgG. These circulating immune complexes activate blood cells expressing Fcγ receptors, including human platelets that express FcγRIIA. After binding to the immune complex, this receptor signals through Syk and activation of phospholipase Cγ2, leading to platelet shape change, aggregation and secretion of granule contents, including ADP. Platelet aggregates form thrombi, which lead to ischemic disease complications characteristic of HIT. (Professional illustration by Marie Dauenheimer.)
Role of Syk tyrosine kinase in the mechanism of platelet activation and thrombosis in heparin-induced thrombocytopenia (HIT). Platelet factor 4 (PF4) released from platelet α-granules binds with high affinity to heparin through a charge-charge interaction. The heparin-PF4 complex acts as an immunogen, and exposure to heparin after an initial sensitization will lead to the formation of immune complexes, in which the heparin-PF4 aggregates are coated with IgG. These circulating immune complexes activate blood cells expressing Fcγ receptors, including human platelets that express FcγRIIA. After binding to the immune complex, this receptor signals through Syk and activation of phospholipase Cγ2, leading to platelet shape change, aggregation and secretion of granule contents, including ADP. Platelet aggregates form thrombi, which lead to ischemic disease complications characteristic of HIT. (Professional illustration by Marie Dauenheimer.)
Because of the thrombotic nature of HIT, with platelet activation being a principal early event, therapy and management of the disease has concentrated on anticoagulation. This needs to be done with agents that do not cross-react with circulating antibodies to the PF4-heparin complex, and therefore direct thrombin inhibitors have been a favored approach, including lepirudin, argatroban, and bivalirudin.2
Here, Reilly et al have exploited our knowledge of the underlying mechanisms of HIT, which include activation of platelets signaling through FcγRIIA, to focus on the use of a novel inhibitor of Syk tyrosine kinase called PRT-060318.1 Syk is a gene with relatively restricted expression profile, limited substantially to B and T lymphocytes and platelets, although there is some expression in neuronal tissues and endothelial cells. Importantly, it had been shown that Syk is essential for signaling and activation of platelets by collagen, downstream of the glycoprotein VI (GPVI) receptor.4 This receptor, a member of the Fcα receptor family, signals similarly to FcγRIIA in that they both recruit Syk through phosphorylation of an immunoreceptor tyrosine-based activation motif (ITAM). It was a highly reasonable hypothesis therefore that targeting Syk pharmacologically may ablate platelet responses to stimulation of FcγRIIA. Because of the restricted expression of Syk, and its pivotal role in B-cell function and development, Syk has already been the subject of inhibitor development, and is currently successfully targeted in the treatment of B-cell lymphoma and immune-mediated disease such as immune thrombocytopenic purpura and rheumatoid arthritis. In the article by Reilly et al, the authors present evidence that their novel inhibitor of Syk, PRT-060318, is also highly effective in managing HIT in a mouse model system, both in vitro and in vivo. The mouse expresses a transgene for human FcγRIIA and also human PF4, to “humanize” the mouse platelets to allow them to respond to immune complexes. The data show clearly that PRT-060318 is highly selective for Syk and is substantially effective at selectively inhibiting Syk-dependent platelet function, such as aggregation in response to GPVI agonism or to a HIT-like antibody. This is the case whether the drug is administered to platelets in vitro or ex vivo after administration of PRT-060318 to mice orally. Importantly, in vivo administration of PRT-060318 was capable of preventing a thrombocytopenic episode in mice administered with a HIT-like antibody. The drug was also capable of blocking thrombotic events in vivo induced by the HIT-like antibody.
This report is therefore potentially a major development in the management of HIT and also in understanding its biology. The major effect of Syk inhibition is likely to be on HIT immune complex–induced platelet activation, but because PF4 needs to be released by platelets in the first place, there may be an additional effect of PRT-060318 on early activation events leading to PF4 secretion. In addition, although the authors report no change in bleeding time with Syk inhibition, careful analysis of this, especially in the presence of residual heparin, will need to take place as one of the early steps in development of this approach in the treatment of HIT. Finally, although the effect is likely principally to be mediated by inhibition of platelet function, in the context of the control of thrombosis, it is possible that effects of Syk inhibition on immune cell function may also play a role in helping to dampen the response in HIT. These are points for further study and analysis, but in the first instance targeting platelet Syk would appear to be a promising novel way forward for management of this difficult clinical problem.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■