Abstract
Heparin-induced thrombocytopenia (HIT) is a major cause of morbidity and mortality resulting from the associated thrombosis. Extensive studies using our transgenic mouse model of HIT have shown that antibodies reactive with heparin-platelet factor 4 complexes lead to FcγRIIA-mediated platelet activation in vitro as well as thrombocytopenia and thrombosis in vivo. We tested PRT-060318 (PRT318), a novel selective inhibitor of the tyrosine kinase Syk, as an approach to HIT treatment. PRT318 completely inhibited HIT immune complex-induced aggregation of both human and transgenic HIT mouse platelets. Transgenic HIT model mice were treated with KKO, a mouse monoclonal HIT-like antibody, and heparin. The experimental group received orally dosed PRT318, whereas the control group received vehicle. Nadir platelet counts of PRT318-treated mice were significantly higher than those of control mice. When examined with a novel thrombosis visualization technique, mice treated with PRT318 had significantly reduced thrombosis. The Syk inhibitor PRT318 thus prevented both HIT immune complex-induced thrombocytopenia and thrombosis in vivo, demonstrating its activity in HIT.
Introduction
Heparin-induced thrombocytopenia (HIT), characterized by antibodies to macromolecular complexes formed by heparin and platelet factor 4 (PF4), is the most frequent drug-induced immune thrombocytopenia. Patients with HIT are at an increased risk for thrombosis, a major cause of morbidity and mortality in treated patients. Despite this potential side effect, heparins (unfractionated or low molecular weight) remain the drug of choice in clinical situations where high-intensity therapy is needed along with the ability to rapidly modulate the anticoagulant level.1 The incidence of HIT has therefore not decreased, notwithstanding the introduction of new anticoagulants, primarily because no drug has replaced heparin for the immediate therapy of acute deep vein thrombosis, arterial thrombosis, or extracorporeal circuits during surgery. In addition, indications for its use in the aging population continue to increase.
Multiple factors influence the incidence and severity of HIT. The pathogenesis of the disease is well understood,2-5 although additional progress is being made. Extensive studies in vitro4,6,7 and in vivo using our transgenic mouse model of HIT8 show that antibodies reactive with heparin-PF4 complexes lead to Fc receptor-mediated platelet activation. This activation leads to platelet aggregation, a procoagulant surface, and release of prothrombotic microparticles. In addition, monocytes and other leukocytes bearing Fcγ receptors can become activated by the HIT immune complex (IC), generating tissue factor and resulting in other prothrombotic and proadhesive changes.9-11
Blocking FcγRIIA signaling is an attractive target for therapeutic intervention because FcγRIIA-mediated platelet activation (and possibly concurrent monocyte activation) is central to the disease. FcγRIIA, like other activating receptors, initiates a tyrosine kinase-based signaling pathway after cross-linking with immune complexes. FcγRIIA is unique among the activating Fcγ receptors in that its cytoplasmic tail contains an immunoreceptor tyrosine-based activation motif (ITAM).12 Residues in the ITAM domain become rapidly phosphorylated on receptor engagement and induce cell activation after binding by nonreceptor protein tyrosine kinases, such as spleen tyrosine kinase (Syk).13,14 We hypothesized that inhibition of Syk activity by PRT-060318 (PRT318), a novel Syk inhibitor, would block FcγRIIA-mediated platelet activation in vitro and minimize HIT IC-induced thrombocytopenia and thrombosis in vivo.
Methods
PRT060318 structure and specificity
A novel class of Syk inhibitors was discovered by high-throughput screening of the chemical libraries at Yamanouchi Pharmaceutical Co. The compounds belonging to the class 4-anilino-2-(2-aminoethylamino) pyrimidine-5-carboxamides were optimized by extensive structure-activity relationship studies and synthesis to identify the highly potent and specific Syk inhibitor PRT060318, (2-((1R,2S)-2-aminocyclohexylamino)-4-(m-tolylamino)pyrimidine-5-carboxamide)15 (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). PRT318, also referred to as P142–76, is a derivative of pyrimidine-5-carboxamide (U.S. patent number 6432963).15
The molecular specificity of PRT318 interaction with Syk was evaluated using the Kinase Profiler (Millipore). The intracellular specificity of PRT318 was investigated by determining the phosphorylation of Syk at position Y352, which is known to be phosphorylated downstream of B-cell receptor by src family tyrosine kinases (SFTK),16 in the DHL4 B cell line (DSMZ). Cells cultured in RPMI (Invitrogen) with 10% fetal bovine serum were preincubated with PRT318 for 1 hour before activation with 5 μg/mL anti-IgG (Jackson ImmunoResearch Laboratories) for 30 minutes at 37°C. Cells were pelleted by centrifugation and lysed in the presence of protease and phosphatase inhibitors (Complete protease inhibitor cocktail, PhosSTOP, Roche Diagnostics). Lysates underwent sodium dodecyl sulfate-polyacrylamide gel electrophoresis and were transferred to nitrocellulose membranes. Blots were probed with rabbit anti–phospho-SYK(Y352) (Cell Signaling Technology).
PRT318 activity in platelets
The activity of PRT318 in the presence of several different agonists on platelet aggregation in vitro was evaluated. Human platelet-rich plasma (PRP) was prepared from normal human blood obtained from healthy donors after signed informed consent. Aggregation of PRP was done in a 96-well format assay (SpectramaxPlus plate reader, Molecular Devices) to compare the effect of PRT318 on convulxin versus adenosine diphosphate (ADP). Convulxin (Centerchem), a glycoprotein VI (GPVI) agonist, was used at final concentrations as indicated in the figures. ADP was from Chronolog. Human platelet calcium flux was measured in a 96-well format assay (Flex Station, Molecular Devices) to compare the effects of PRT318 after stimulation by convulxin versus the PAR1 agonist, TRAP-6 (SFLLRN; Peninsula Labs/Bachem).
To evaluate the ability of PRT318 to inhibit platelet aggregation after stimulation by HIT IC, we first prepared heparin-PF4 complexes under conditions that favored development of ultralarge complexes.17 Briefly, recombinant human PF4 (PeproTech) and heparin (H-3393, Sigma-Aldrich) were mixed at a molar ratio of 1.5:1 and incubated at 37°C for 1 hour. Aliquots (5 μL) of the ultralarge complex mixture, containing 5 μg hPF4, were added to PRP and incubated with or without PRT318 (0-3μM) for 15 minutes at 37°C with stirring in a final volume of 250 μL. Aggregation was initiated by the addition of KKO, a monoclonal HIT-like antibody18 (final concentration, 80 μg/mL). Final percentage aggregation was recorded.
SRA
We examined the effect of PRT318 on platelet activation in the presence of human HIT sera using the serotonin release assay (SRA). The SRA is the gold standard for confirmation of platelet-activating HIT antibodies. Briefly, washed 14C-serotonin-loaded platelets were incubated with PRT318 at 1.0μM before incubation for 1 hour at room temperature with 0.1 or 100 IU/mL heparin and HIT sera. Positive control platelets were treated with vehicle only. The platelets were removed by centrifugation, and the 14C-serotonin released in the supernatant was measured by scintillation counting. The percentage of 14C-serotonin in the supernatant relative to total 14C-serotonin in the labeled platelets was determined.19 Positive results are those with more than 20% serotonin released with 0.1 IU/mL heparin concentration and less than 20% with 100 IU/mL heparin concentration.
Studies in vivo
All animal studies were conducted in accordance with the guidelines and approval of the Institutional Animal Care and Use Committees of Thomas Jefferson University and Portola Pharmaceuticals. Both facilities are Association for Assessment and Accreditation of Laboratory Animal Care–accredited. C57Bl/6 (Charles River Labs) mice were used for the dose selection study. The transgenic HIT mice used to determine the effect of PRT318 on HIT IC-induced thrombocytopenia are homozygous for both FcγRIIA and human PF4 and null for mouse PF4.8,20
To determine the effect of PRT318 on HIT IC-induced thrombocytopenia and thrombosis, HIT model mice were treated with KKO (20 mg/kg body weight, intraperitoneally) on day 0. The mice were divided into sex- and weight-matched experimental and control groups. On days 1 to 7, experimental mice (n = 6) received PRT318 (30 mg/kg body weight) orally via gavage twice a day, whereas control mice (n = 6) received vehicle only (sterile water). Both groups received heparin (1600 U/kg body weight, subcutaneously) once daily. Mice were anesthetized by isoflurane inhalation for injections and blood collections. Blood was collected via the retro-orbital plexus using 50 μL ethylenediaminetetraacetic acid-coated microcapillary tubes (Microcaps, Drummond Scientific). Blood was collected 3 days before the antibody injections for baseline values and then on days 1 through 7 after antibody injections. Platelet counts were measured using a Hemavet Analyzer (model 850, CDC Technologies) and monitored over time to assess thrombocytopenia. Mouse plasma samples, collected 2 hours after PRT318 dosing, were precipitated with acetonitrile, and concentrations of PRT318 were quantified by liquid chromatography/tandem mass spectrometry.
To evaluate the effect of PRT318 treatment on thrombosis in vivo, the protocol was modified as follows to take advantage of a novel thrombosis visualization technology. Experimental (n = 3) and control (n = 3) HIT mice were injected with KKO and PRT318 or vehicle only as described in the previous paragraph. For the imaging method, blood was collected in heparin from the retro-orbital plexus of untreated syngeneic donor mice. The donor platelets were washed, pooled, and incubated at 37°C for 10 minutes with Alexa750-labeled anti-GPIX antibody. The labeled platelets were pelleted and resuspended in sterile saline. Both experimental and control mice were infused with 1 × 108 labeled platelets in 100 μL aliquots via the retro-orbital plexus. All mice were then injected with heparin as described in the previous paragraph. Mice were killed 3 hours after heparin treatment, and their organs were perfused with sterile phosphate-buffered saline. Lungs were excised, fixed in 7% paraformaldehyde, and imaged with an Odyssey Imaging System (LI-COR Biosciences). The images were evaluated using Nikon NIS Elements image analysis software Version 3.10 (NIS-Elements, Advanced Research). Thrombi were identified as areas with fluorescence intensity at least 1.5 times above background. The Thrombosis Score represents the number of thrombi multiplied by the average intensity.
Statistical analyses
Data are presented as mean ± SEM unless otherwise indicated. Differences in values for each parameter between groups were analyzed by Student unpaired, 2-tailed t test. Values of P < .05 were considered statistically significant.
Results
PRT318 is a potent and specific inhibitor of Syk
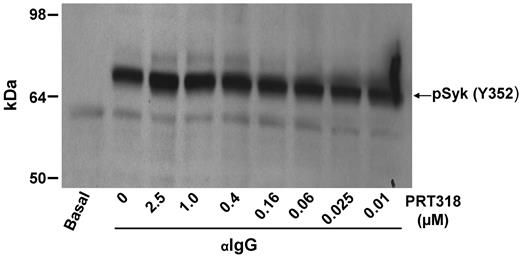
To achieve specific pharmacologic targeting of Syk kinase activity, we used PRT318, a potent inhibitor of purified Syk kinase (50% inhibitory concentration = 4nM). Syk kinase was inhibited by 92%, whereas all other kinases retained more than 70% of their activity when we evaluated PRT318 at a concentration (50nM) more than 10-fold higher than the 50% inhibitory concentration in a broad panel of kinase enzyme assays (Kinase Profiler, supplemental Table 1). The observed kinase inhibitory activity against purified Src kinases, such as Lyn, did not translate into cellular activity in experimental systems containing plasma proteins. Even at a concentration as high as 10μM PRT318, there was no inhibition of Lyn-mediated substrate phosphorylation (Y352 on Syk) in human whole blood (data not shown). Syk(Y352) is known to be phosphorylated downstream of B-cell receptor by SFTKs.16 As shown in Figure 1, PRT318 does not affect cellular SFTKs downstream of ITAM receptor engagement.
DHL4 B cells were preincubated with PRT318 at concentrations indicated for 1 hour before activation with 5 μg/mL anti-IgG for 30 minutes. Lysates underwent sodium dodecyl sulfate-polyacrylamide gel electrophoresis and were transferred to nitrocellulose membranes. Blots were probed with rabbit anti–phospho-SYK(Y352). The blot shown is representative of 2 independent experiments.
DHL4 B cells were preincubated with PRT318 at concentrations indicated for 1 hour before activation with 5 μg/mL anti-IgG for 30 minutes. Lysates underwent sodium dodecyl sulfate-polyacrylamide gel electrophoresis and were transferred to nitrocellulose membranes. Blots were probed with rabbit anti–phospho-SYK(Y352). The blot shown is representative of 2 independent experiments.
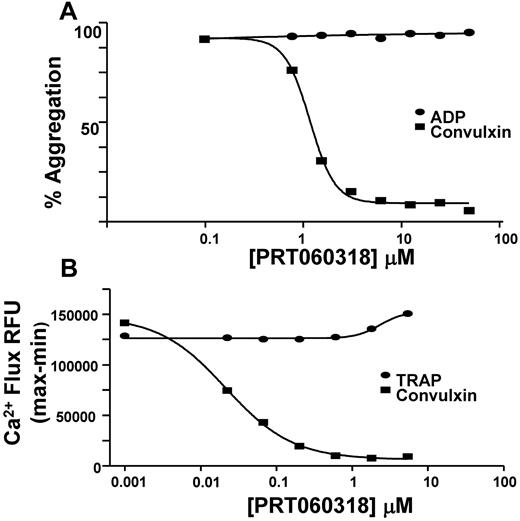
PRT318 was further evaluated in a series of cellular assays. As shown in Figure 2A, PRT318 dose-responsively inhibited convulxin-induced (8 ng/mL) aggregation of human PRP via GPVI/FcRγ, at a 50% inhibitory concentration = 2.5 ± 0.2μM (n = 28). (Note that the inhibitor concentration in plasma and acting on the intracellular enzyme is in micromolar concentrations, whereas molecular activity requires nanomolar inhibitor concentrations.) However, human platelet aggregation stimulated by ADP at 5μM was not affected by PRT318 at any dose tested (up to 50μM). ADP-induced platelet activation requires the activation of G-protein coupled receptors, such as P2Y1 and P2Y12, whereas convulxin signals through GPVI and its associated ITAM-containing FcR γ-chain.
Platelet function studies. (A) Aggregation of human PRP was done to compare the effect of PRT318 on convulxin versus ADP. Aggregation of PRP treated with PRT318 (0-50μM) was determined in response to convulxin (8 ng/mL) or ADP (5μM). (B) Human platelet calcium flux was measured in a 96-well format assay to compare the effects of PRT318 on convulxin versus the PAR1 agonist, TRAP-6 (SFLLRN). Calcium flux in platelets treated with PRT318 (0-16μM) was measured in response to convulxin (125 ng/mL) or TRAP-6 (3μM).
Platelet function studies. (A) Aggregation of human PRP was done to compare the effect of PRT318 on convulxin versus ADP. Aggregation of PRP treated with PRT318 (0-50μM) was determined in response to convulxin (8 ng/mL) or ADP (5μM). (B) Human platelet calcium flux was measured in a 96-well format assay to compare the effects of PRT318 on convulxin versus the PAR1 agonist, TRAP-6 (SFLLRN). Calcium flux in platelets treated with PRT318 (0-16μM) was measured in response to convulxin (125 ng/mL) or TRAP-6 (3μM).
The specificity of ITAM pathway inhibition was shown further by the inability of PRT318 to inhibit intracellular increases in calcium mediated by the thrombin receptor PAR1 in human platelets, even when PRT318 at concentrations as high as 16μM was tested in the presence of 3μM TRAP-6 (Figure 2B). Under similar experimental conditions, PRT318 dose-responsively inhibited increases in intracellular calcium in platelets treated with 125 ng/mL of convulxin.
These studies indicated that PRT318 inhibited platelet activation via GPVI/FcRγ, an ITAM receptor complex, but not via ADP or thrombin, which are G-protein coupled receptors. Having demonstrated PRT318 potency and specificity, we investigated whether PRT318 inhibits platelet activation by the HIT IC via the FcγRIIa-ITAM-SYK axis in vitro and in vivo.
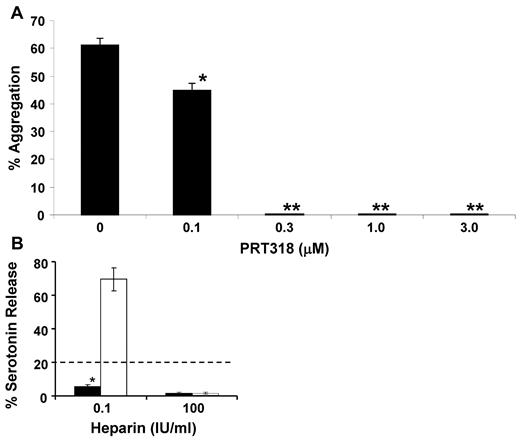
Using human PRP, PRT318 at final concentrations of 0.3 to 3μM completely inhibited HIT IC-induced aggregation (Figure 3A). Without PRT318, HIT IC-induced final aggregation was 50% to 60%. At PRT318 concentrations of ≤ 0.1μM in PRP, or with vehicle only, there was no inhibition of aggregation. These results demonstrate the ability of PRT318 at submicromolar to low micromolar concentrations to prevent platelet aggregation in PRP in response to the actual HIT IC. PRP from HIT model mice was treated with anti-CD9 antibody (Immunotech/Beckman Coulter), which mimics the HIT immune complex acting through FcγRIIA.21 PRT318 concentrations ≥ 0.75μM were completely inhibitory of anti-CD9-induced platelet aggregation (10 μg/mL anti-CD9), whereas 0.33 and 0.5μM showed partial inhibition (data not shown).
HIT IC-mediated platelet aggregation in vitro. (A) Human PRP (250 000 platelets/μL) was incubated at 37°C for 15 minutes with aliquots of a mixture of PF4 and heparin (1.5:1 molar ratio) containing 5 μg PF4. The PRP was then incubated at 37°C for an additional 15 minutes with PRT318 at the concentrations indicated. Aggregation was initiated by the addition of the HIT-like antibody KKO (80 μg/mL). Platelet aggregation was assessed under stirring conditions by the increase in light transmission over time. The final percentage aggregation was recorded. In the absence of the PRT318, HIT IC-induced aggregation in human PRP was 50% to 60%. At PRT318 concentrations of more than or equal to 0.3μM, there was complete inhibition. Data are mean ± SEM; n = 3 each group. *P < .007. **P < .0002. (B) Washed 14C-serotonin-loaded platelets were incubated with PRT318 (1.0μM) or vehicle only before incubation for 1 hour at room temperature with 0.1 or 100 IU /mL heparin and human HIT sera. Positive results are those with > 20% serotonin released at a heparin concentration of 0.1 IU/mL and < 20% with a heparin concentration 100 IU/mL. Data are mean ± SEM; n = 4 for each treatment group. *P < .0001. □ represents control (vehicle only); and ■, PRT treated.
HIT IC-mediated platelet aggregation in vitro. (A) Human PRP (250 000 platelets/μL) was incubated at 37°C for 15 minutes with aliquots of a mixture of PF4 and heparin (1.5:1 molar ratio) containing 5 μg PF4. The PRP was then incubated at 37°C for an additional 15 minutes with PRT318 at the concentrations indicated. Aggregation was initiated by the addition of the HIT-like antibody KKO (80 μg/mL). Platelet aggregation was assessed under stirring conditions by the increase in light transmission over time. The final percentage aggregation was recorded. In the absence of the PRT318, HIT IC-induced aggregation in human PRP was 50% to 60%. At PRT318 concentrations of more than or equal to 0.3μM, there was complete inhibition. Data are mean ± SEM; n = 3 each group. *P < .007. **P < .0002. (B) Washed 14C-serotonin-loaded platelets were incubated with PRT318 (1.0μM) or vehicle only before incubation for 1 hour at room temperature with 0.1 or 100 IU /mL heparin and human HIT sera. Positive results are those with > 20% serotonin released at a heparin concentration of 0.1 IU/mL and < 20% with a heparin concentration 100 IU/mL. Data are mean ± SEM; n = 4 for each treatment group. *P < .0001. □ represents control (vehicle only); and ■, PRT treated.
To determine the efficacy of PRT318 in the presence of human HIT sera, we examined the effect of PRT318 (1μM) on platelet activation using the SRA. The assays were performed on 4 separate platelet donors using 2 different HIT sera. In all cases, PRT318 (1μM) prevented platelet activation in the presence of human HIT sera as shown by the SRA (Figure 3B).
PRT318 prevents HIT in vivo
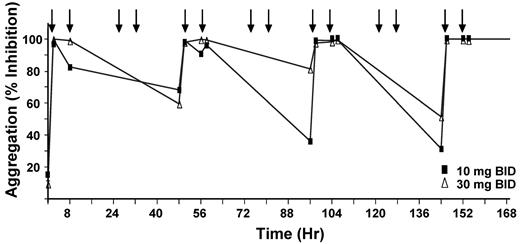
Dose selection study for PRT318 was performed by orally dosing 10 mg/kg or 30 mg/kg twice a day in 2 groups of C57Bl/6 mice (n = 4/group) for 7 days. Platelet aggregation was maximally inhibited by 36% ± 31% and 81% ± 34% (mean ± SD) at 10 mg/kg and 30 mg/kg, respectively (Figure 4). The 30-mg/kg dose was selected for subsequent studies in vivo. At 2 hours after dose, PRT318 was 7.1 ± 1.2μM in plasma from treated mice, consistent with the concentration that blocked FcγRIIA-mediated platelet activation in PRP in vitro.
Dose selection study for PRT318. C57Bl/6 mice (n = 4/group) were orally dosed twice a day for 7 days with either 10 mg/kg or 30 mg/kg of body weight at time points indicated by arrows. PRP from PRT318-treated mice were exposed to convulxin (250 ng/mL), and platelet aggregation was measured as a pharmacodynamic marker of antiplatelet activity. The graph represents the percentage inhibition of aggregation at each time point examined. For clarity, error bars are not shown.
Dose selection study for PRT318. C57Bl/6 mice (n = 4/group) were orally dosed twice a day for 7 days with either 10 mg/kg or 30 mg/kg of body weight at time points indicated by arrows. PRP from PRT318-treated mice were exposed to convulxin (250 ng/mL), and platelet aggregation was measured as a pharmacodynamic marker of antiplatelet activity. The graph represents the percentage inhibition of aggregation at each time point examined. For clarity, error bars are not shown.
In contrast to vehicle-treated mice, which developed the expected thrombocytopenia, PRT318-treated mice showed no significant change in platelet counts after injections of KKO and heparin (Figure 5A). Nadir platelet counts of PRT318-treated mice were significantly higher than control mice (89.7% ± 3.69% of baseline vs 47.8% ± 10.1%; P < .004; Figure 5B).
HIT IC-mediated thrombocytopenia in vivo. HIT model mice were divided into sex- and weight-matched experimental and control groups and treated with the HIT-like antibody KKO (20 mg/kg body weight, intraperitoneally) on day 0. On days 1 through 7, experimental mice received PRT318 (30 mg/kg body weight) orally via gavage twice a day, whereas control mice received vehicle only (sterile water). Both groups received heparin (1400 U/kg, subcutaneously) once daily on days 1 to 7. Blood was collected 3 days before the antibody injections for baseline values and then on days 1 through 7 after antibody injections. Platelet counts were monitored over time to assess thrombocytopenia. (A) Time course of platelet counts expressed as percentage of baseline values. (B) Nadir platelet counts expressed as percentage of baseline values. □ represents control (vehicle only); and ■, PRT treated. n = 6 for each group. *P < .002.
HIT IC-mediated thrombocytopenia in vivo. HIT model mice were divided into sex- and weight-matched experimental and control groups and treated with the HIT-like antibody KKO (20 mg/kg body weight, intraperitoneally) on day 0. On days 1 through 7, experimental mice received PRT318 (30 mg/kg body weight) orally via gavage twice a day, whereas control mice received vehicle only (sterile water). Both groups received heparin (1400 U/kg, subcutaneously) once daily on days 1 to 7. Blood was collected 3 days before the antibody injections for baseline values and then on days 1 through 7 after antibody injections. Platelet counts were monitored over time to assess thrombocytopenia. (A) Time course of platelet counts expressed as percentage of baseline values. (B) Nadir platelet counts expressed as percentage of baseline values. □ represents control (vehicle only); and ■, PRT treated. n = 6 for each group. *P < .002.
The PRT318-treated mice showed no evidence of a bleeding diathesis or other adverse effects. In separate studies using a pig carotid artery, crush thrombosis model PRT318 treatment significantly inhibited platelet deposition without affecting bleeding time.22
PRT318 prevents HIT IC-induced thrombosis in vivo
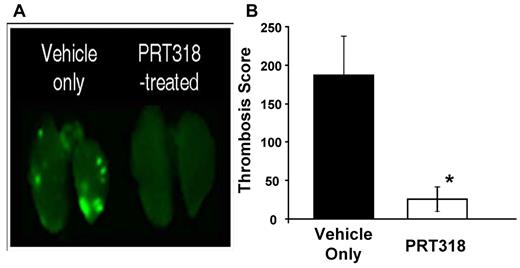
In prior studies, we identified thrombosis in our HIT mouse model using histologic examination of organs from experimental and control mice coupled to semiquantitative analysis.20 However, we noted that it is difficult to quantify thrombosis with this approach as the platelet-fibrin thrombi are randomly dispersed throughout the tissue sections. We have now developed a near-infrared imaging approach to more quantitatively evaluate thrombosis in lungs. As shown in Figure 6A, widely dispersed thrombi of different sizes were observed in the lung vasculature from HIT mice pretreated with vehicle only before receiving heparin and KKO antibody. In contrast, pretreatment of mice with PRT318 markedly reduced HIT IC-induced thrombosis in the lungs. The Thrombosis Score was significantly lower for PRT318-treated mice compared with those mice that received only the vehicle (Figure 6B).
HIT IC-mediated thrombosis in vivo. HIT model mice treated with the HIT-like antibody KKO (20 mg/kg body weight, intraperitoneally) received 2 doses of PRT318 (30 mg/kg body weight) orally via gavage or vehicle only (sterile water). Platelets from donor mice were isolated, labeled with Alexa750-labeled antibodies to GPIX, washed, and injected into HIT model mice one hour before challenge with heparin (1400 U/kg, subcutaneously). Three hours after infusion of heparin, mice were killed, flushed systemically with phosphate-buffered saline, and the lungs were extracted and scanned on a Li-COR Odyssey at 780 nm. (A) Representative images for lungs extracted from mice treated with heparin/KKO in the presence or absence of PRT318. (B) The Thrombosis Score reflects the product of the mean thrombus area (square pixels) and the mean number of thrombi (defined by a signal intensity that is 1.5-fold above the background). The Thrombosis Score is significantly different in vehicle only-treated mice (score = 186.9) versus PRT318-treated mice (score = 25.7). Data are mean ± SEM; n = 6 for each treatment group. *P = .03.
HIT IC-mediated thrombosis in vivo. HIT model mice treated with the HIT-like antibody KKO (20 mg/kg body weight, intraperitoneally) received 2 doses of PRT318 (30 mg/kg body weight) orally via gavage or vehicle only (sterile water). Platelets from donor mice were isolated, labeled with Alexa750-labeled antibodies to GPIX, washed, and injected into HIT model mice one hour before challenge with heparin (1400 U/kg, subcutaneously). Three hours after infusion of heparin, mice were killed, flushed systemically with phosphate-buffered saline, and the lungs were extracted and scanned on a Li-COR Odyssey at 780 nm. (A) Representative images for lungs extracted from mice treated with heparin/KKO in the presence or absence of PRT318. (B) The Thrombosis Score reflects the product of the mean thrombus area (square pixels) and the mean number of thrombi (defined by a signal intensity that is 1.5-fold above the background). The Thrombosis Score is significantly different in vehicle only-treated mice (score = 186.9) versus PRT318-treated mice (score = 25.7). Data are mean ± SEM; n = 6 for each treatment group. *P = .03.
Discussion
Currently, once HIT is suspected or diagnosed, heparin is withdrawn and direct thrombin inhibitor or heparinoid therapy is used. However, thrombosis still occurs and there is a substantial incidence of hemorrhage.23,24 The FcγRIIA/hPF4 transgenic mouse model of HIT is a useful platform for studying possible therapeutic approaches to the disease. We hypothesized that inhibition of Syk would prevent HIT antibody-induced thrombocytopenia and reduce thrombosis.
In this study, we have shown that PRT318 is a potent and specific inhibitor of Syk kinase, which prevents both HIT antibody-mediated platelet activation in vitro and thrombocytopenia and thrombosis in vivo. Although the focus of this work is the effect of Syk inhibition in the preclinical HIT mouse model, it is important to note that Syk inhibitors are being used successfully in humans for other indications, such as B-cell lymphoma,25,26 immune thrombocytopenic purpura,27 and rheumatoid arthritis.28-30
Syk inhibition also affects GPVI/FcRγ signaling in platelets. Spalton et al first reported the effects of a different Syk inhibitor (R406, Rigel; Cellzome), with a distinct chemical structure and specificity profile, on GPVI/FcRγ signaling in platelets.31 Of note, they reported that the effects of inhibiting Syk differed between FcRγ and the hemi-ITAM Clec-2. They also noted that the intracellular specificity of R406 for Syk was evident at extracellular concentrations to which washed platelets were exposed, ranging from 0.1 to 1μM, but specificity for Syk over SFTKs was lost at 10μM. In addition, as reported by Braselmann et al,28 R406 inhibits multiple non-ITAM pathway kinases at submicromolar concentrations. Although the FcγRIIA-GPVI/FcRγ cross-regulatory interaction that leads to receptor proteolytic cleavage described by Gardiner et al is of great interest,32 it is unclear how the beneficial effects of inhibition of Syk signaling in response to the HIT IC would relate to the probable subsequent inhibition of GPVI proteolytic cleavage. The intravascular activation of platelets in HIT occurs without known exposure of the blood to the GPVI agonist collagen.
Our studies document that inhibition of Syk signaling with the orally bio-available PRT318 limits the thrombocytopenic and thrombotic effects of HIT IC in vivo. We focused on the effects of inhibition of Syk in platelets. Similar effects on monocytes and endothelial cells cannot be ruled out yet are probably beneficial with regards to pathology of HIT. It is of note that the antithrombotic effects were readily visualized with a new technique, applicable to other forms of immune thrombocytopenia/thrombosis disorders, (M.S. and W.B., unpublished observations, March 2010).
Taken together, these studies clearly demonstrate that the Syk inhibitor PRT318 is an active agent in HIT. These results support the hypothesis that inhibition of FcγRIIA-Syk signaling with PRT318 or related compounds may have beneficial effects in the treatment of human HIT. Future studies will be needed to address planned short-term use, effects on other Syk-expressing cells, and on hemostasis.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs David Phillips, Mortimer Poncz, Anna Kowalska, and Gow Arepally for helpful discussions and sharing of reagents and Dr Paul Bray, Gene Pullen, Megan Hevelow, and the staff of the Cardeza Special Hemostasis Laboratory for their assistance.
Authorship
Contribution: M.P.R. designed and performed research, analyzed data, and wrote the paper; U.S. designed research, analyzed data, and wrote the paper; P.A., S.M.T., Y.P., F.R.D., and N.N. performed research; A.P. contributed key reagents; M.S. and W.B. designed and performed research and analyzed data; and S.E.M. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: M.P.R. and S.E.M. report receipt of sponsored research support from Portola Pharmaceuticals. U.S., Y.P., F.R.D., N.N., and A.P. are employees of Portola Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Steven E. McKenzie, Thomas Jefferson University, M41F Jefferson Alumni Hall, 1020 Locust St, Philadelphia, PA 19107; e-mail: steven.mckenzie@jefferson.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal