Abstract
Major limitations of currently investigated αβT cells redirected against cancer by transfer of tumor-specific αβTCR arise from their low affinity, MHC restriction, and risk to mediate self-reactivity after pairing with endogenous α or βTCR chains. Therefore, the ability of a defined γ9δ2TCR to redirect αβT cells selectively against tumor cells was tested and its molecular interaction with a variety of targets investigated. Functional analysis revealed that a γ9δ2TCR efficiently reprograms both CD4+ and CD8+ αβT cells against a broad panel of cancer cells while ignoring normal cells, and substantially reduces but does not completely abrogate alloreactivity. γ9δ2TCR-transduced αβT cells reduced colony formation of progenitor cells of primary acute myeloid leukemia blasts and inhibited leukemia growth in a humanized mouse model. Thereby, metabolites of a dysregulated mevalonate pathway are targeted and the additional application of widely used biphosphonates is crucial for in vivo efficacy most likely because of its modulating effect on cytokine secretion of γ9δ2TCR-transduced αβT cells. Expression of NKG2D ligands and F1-ATPase contributed to the activity of γ9δ2TCR-transduced αβT cells but were not mandatory. In summary, γ9δ2 TCRs are an attractive alternative to broadly redirect αβT cells against cancer cells with both an improved efficacy and safety profile compared with currently used αβTCRs.
Introduction
The major challenge in the field of adoptive immunotherapy is the generation of tumor-reactive αβT cells which can be applied to a broad variety of cancer patients. To facilitate the rapid generation of tumor-reactive αβT cells, it has been proposed that αβT cells can be reprogrammed with genes encoding for a tumor-specific αβTCR or a chimeric receptor.1 Several such receptors are already being used to redirect αβT cells in phase 1 clinical trials.1,2 However, reprogramming αβT cells with defined αβTCRs is substantially hampered by their restriction to HLA types, thus limiting the number of patients who can be treated with one αβTCR. In addition, pairing of introduced with endogenous αβTCR chains can induce life-threatening autoreactivity.3,4
One attractive alternative to mediate a selective antitumor reactivity with a high-affinity TCR might arise from the ability of γδT cells to mediate antitumor reactivity while ignoring a healthy environment.5-7 Isolated γ9δ2T cells efficiently kill tumor cells of hematologic malignancies and from solid tumors.7 However, the function and proliferation capacity of γδT cells is frequently heavily impaired in cancer patients8 making autologous γδT cells less attractive for immune interventions. On the other hand, as end-stage cancer patients can easily elicit αβT-cell immune responses against, for example, viral Ags,9,10 αβT cells might serve as carriers for broadly tumor-reactive γδTCRs.
The recognition of mevalonate metabolites (phosphoantigens)11 which are overexpressed in a broad range of tumor cells has been suggested as an important mechanism by which multiple γ9δ2TCR can sense malignant transformation as the recognition involves TCR domains which are conserved in most γ9δ2TCRs.12-14 In addition, γ9δ2TCR G115 has been also suggested to bind to a complex of Apolipoprotein AI (ApoAI) and F1-ATPase,15 a complex mitochondrial enzyme found on the surface of many malignant cells.16 This knowledge might allow a rational design of γδT cell–based immunotherapies. Therefore, we investigated whether a defined γ9δ2TCR can be efficiently expressed in αβT cells, mediate tumor-specific proliferation of αβT cells, and redirect both effector CD8+ and helper CD4+ αβT-cell subsets against a broad panel of tumor cell lines while ignoring normal cells in vitro and in vivo.
Methods
Results
Functional expression of γ9δ2 TCR in peripheral blood αβT cells
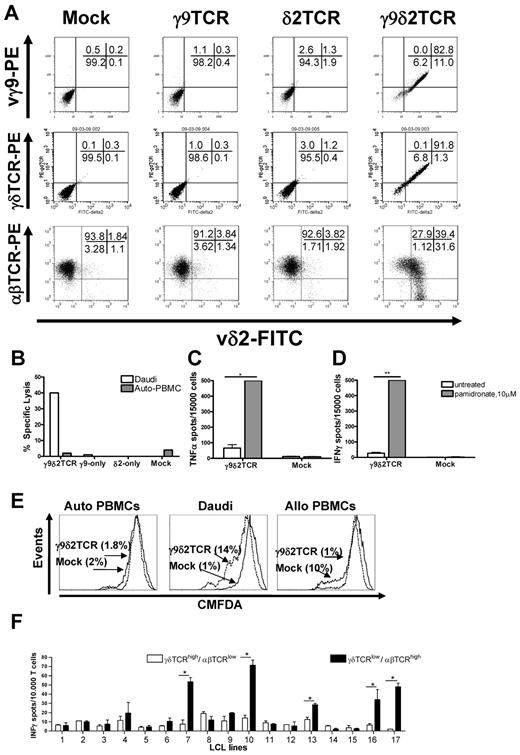
To assess whether peripheral blood αβT cells can be redirected against tumor cells with γ9δ2TCRs, defined γ9δ2TCR chains (clone G115) were transduced into preactivated peripheral blood αβT cells. After selection with antibiotics and further expansion as described in supplemental Methods, γδTCR expression was determined by staining with specific Abs (Figure 1A). Transduction of one of the chains did not result in surface expression of a single chain (∼ 3% for γ9TCR and ∼ 9% for δ2TCR vs ∼ 4% in the mock-transduced). Only when both γ9 and δ2 chains were expressed could they be detected on the cell surface of ∼ 90% of the transduced cells (Figure 1A). This suggested that γ9 and δ2 chains cannot pair with endogenous α and β TCR chains and that the constant domains of γ and δ chains assemble appropriately in αβT cells. To test function, αβT cells transduced with mock, single, or both γ9δ2 chains were tested in a 51Cr-release assay against the classic γδT-cell target Daudi. PBMCs served as negative control. Indeed, only αβT cells that expressed both γ9 and δ2 TCR chains could specifically lyse up to 40% of Daudi cells (Figure 1B).
Functional expression of γ9δ2 TCR in αβT cells. Surface expression of specific staining for Vδ2 together with either staining for Vγ9, for γδTCR, or for αβTCR as indicated in panel A. Presented representative dot plots have been confirmed in at least 3 independent experiments. (B) Specific lysis of the Daudi cells or autologous PBMCs (Auto-PBMC) tested by 51Cr-release assay with T cells that were transduced with γ9, δ2, or both chains. Mock-transduced cells served as a negative control in all the experiments. Shown is a representative experiment at the E:T 10:1. TNFα (C) and IFNγ (D) secretion by the γ9δ2TCR or mock-transduced αβT cells in response to Daudi cells was tested. αβT cells were incubated with target cells at the E:T 0.3:1 for 48 hours in absence or presence of 10μM pamidronate, , respectively. Shown are representative results of at least 3 independent experiments. Statistical analysis was determined by 2-way ANOVA analysis; *P < .01; **P < .001. (E) Proliferation of CMFDA-labeled γ9δ2TCR or mock-transduced αβT cells in response to Daudi cells. Autologous and allogeneic PBMCs were tested following 4 days at an E:T ratio of 3:1. CMFDA labeling is diluted in the proliferating cells. (F) γ9δ2TCR-transduced αβT cells were FACS-sorted based on their δ2TCR and αβTCR expression resulting in 2 distinct populations either expressing γ9δ2TCRhigh/αβTCRlow or γ9δ2TCRlow/αβTCRhigh. The FACS-sorted fractions were cocultured with partially mismatched LCL lines 1-17 for 24 hours, and production of IFNγ was determined via ELISPOT analysis. Data show mean ± SEM of duplicate samples. Comparable results were obtained in 3 independent experiments and by using transduced primary T cells of 2 healthy donors. Statistical analysis was determined by 2-way ANOVA analysis; *P < .001.
, respectively. Shown are representative results of at least 3 independent experiments. Statistical analysis was determined by 2-way ANOVA analysis; *P < .01; **P < .001. (E) Proliferation of CMFDA-labeled γ9δ2TCR or mock-transduced αβT cells in response to Daudi cells. Autologous and allogeneic PBMCs were tested following 4 days at an E:T ratio of 3:1. CMFDA labeling is diluted in the proliferating cells. (F) γ9δ2TCR-transduced αβT cells were FACS-sorted based on their δ2TCR and αβTCR expression resulting in 2 distinct populations either expressing γ9δ2TCRhigh/αβTCRlow or γ9δ2TCRlow/αβTCRhigh. The FACS-sorted fractions were cocultured with partially mismatched LCL lines 1-17 for 24 hours, and production of IFNγ was determined via ELISPOT analysis. Data show mean ± SEM of duplicate samples. Comparable results were obtained in 3 independent experiments and by using transduced primary T cells of 2 healthy donors. Statistical analysis was determined by 2-way ANOVA analysis; *P < .001.
Functional expression of γ9δ2 TCR in αβT cells. Surface expression of specific staining for Vδ2 together with either staining for Vγ9, for γδTCR, or for αβTCR as indicated in panel A. Presented representative dot plots have been confirmed in at least 3 independent experiments. (B) Specific lysis of the Daudi cells or autologous PBMCs (Auto-PBMC) tested by 51Cr-release assay with T cells that were transduced with γ9, δ2, or both chains. Mock-transduced cells served as a negative control in all the experiments. Shown is a representative experiment at the E:T 10:1. TNFα (C) and IFNγ (D) secretion by the γ9δ2TCR or mock-transduced αβT cells in response to Daudi cells was tested. αβT cells were incubated with target cells at the E:T 0.3:1 for 48 hours in absence or presence of 10μM pamidronate, , respectively. Shown are representative results of at least 3 independent experiments. Statistical analysis was determined by 2-way ANOVA analysis; *P < .01; **P < .001. (E) Proliferation of CMFDA-labeled γ9δ2TCR or mock-transduced αβT cells in response to Daudi cells. Autologous and allogeneic PBMCs were tested following 4 days at an E:T ratio of 3:1. CMFDA labeling is diluted in the proliferating cells. (F) γ9δ2TCR-transduced αβT cells were FACS-sorted based on their δ2TCR and αβTCR expression resulting in 2 distinct populations either expressing γ9δ2TCRhigh/αβTCRlow or γ9δ2TCRlow/αβTCRhigh. The FACS-sorted fractions were cocultured with partially mismatched LCL lines 1-17 for 24 hours, and production of IFNγ was determined via ELISPOT analysis. Data show mean ± SEM of duplicate samples. Comparable results were obtained in 3 independent experiments and by using transduced primary T cells of 2 healthy donors. Statistical analysis was determined by 2-way ANOVA analysis; *P < .001.
, respectively. Shown are representative results of at least 3 independent experiments. Statistical analysis was determined by 2-way ANOVA analysis; *P < .01; **P < .001. (E) Proliferation of CMFDA-labeled γ9δ2TCR or mock-transduced αβT cells in response to Daudi cells. Autologous and allogeneic PBMCs were tested following 4 days at an E:T ratio of 3:1. CMFDA labeling is diluted in the proliferating cells. (F) γ9δ2TCR-transduced αβT cells were FACS-sorted based on their δ2TCR and αβTCR expression resulting in 2 distinct populations either expressing γ9δ2TCRhigh/αβTCRlow or γ9δ2TCRlow/αβTCRhigh. The FACS-sorted fractions were cocultured with partially mismatched LCL lines 1-17 for 24 hours, and production of IFNγ was determined via ELISPOT analysis. Data show mean ± SEM of duplicate samples. Comparable results were obtained in 3 independent experiments and by using transduced primary T cells of 2 healthy donors. Statistical analysis was determined by 2-way ANOVA analysis; *P < .001.
Functional reprogramming of a T cell includes not only mediating lysis but also cytokine secretion and proliferation. Therefore, the ability of γ9δ2TCR or mock-transduced αβT cells to secrete cytokines in response to the specific target was analyzed by ELISPOT after incubation with Daudi cells for 48 hours. Cytokine secretion was observed for γ9δ2TCR but not mock-transduced αβT cells and was rather moderate. IPP, an intermediate product of the mevalonate pathway, is dysregulated in multiple tumor cells11 and is suggested to be recognized through the γ9δ2TCR. Blockade of farnesyl-pyrophosphate synthase by synthetic aminobisphosphonate compounds, such as the clinically widely used pamidronate, leads to further accumulation of the intermediate upstream products including IPP. To test whether the application of therapeutic concentrations of pamidronate20 can further enhance cytokine secretion, Daudi cells and PBMCs were preincubated with or without pamidronate overnight. Addition of pamidronate significantly enhanced the amount of TNFα and IFNγ produced by γ9δ2TCR-expressing αβT cells (7- to 18-fold, P < .01, Figure 1C-D). Also IL2 secretion of CD4+ γ9δ2TCR-transduced αβT cells in response to Daudi target cells could be detected after treatment with pamidronate (supplemental Figure 1). To further test the proliferative response to specific targets, CD8+ γ9δ2TCR or mock-transduced αβT cells were labeled with CMFDA and then incubated with irradiated Daudi cells or autologous PBMCs for 4 days. Fourteen percent of γ9δ2TCR-expressing cells but only 1% of mock-transduced αβT cells diluted CMFDA selectively in response to Daudi cells, indicating a specific proliferation in response to Daudi cells (Figure 1E). In summary, a γ9δ2TCR can be efficiently expressed in αβT cells and reprogram non–tumor-reactive αβT cells against the classic γδT-cell tumor target Daudi.
Reduced alloreactivity of γ9δ2TCR-transduced αβT cells
To test whether the original specificity of an αβT cell is affected after introduction of exogenous γ9δ2TCRs, proliferation of transduced αβT cells was measured not only against autologous PBMCs as indicated as indicated in Figure 1E but also allogeneic PBMCs. As expected, 10% of Mock-transduced αβT cells proliferated in line with the assumption that within a T-cell repertoire there is population of αβT cells that mediate alloreactivity.21 In contrast, only 1% of γ9δ2TCR-transduced αβT cells showed proliferation in response to allogeneic PBMCs which was similar to baseline proliferation as measured against autologous PBMCs (1.8% for γ9δ2TCR and 2% for Mock-transduced αβT cells; Figure 1E). To confirm that other functions like cytokine secretion were also reduced in response to alloantigens, mock and γ9δ2TCR-transduced αβT cells were tested against a panel of 17 mismatched LCL lines (supplemental Table 1), and 5 allogeneic LCL lines induced a cytokine response of mock-transduced αβT cells. In contrast, γ9δ2TCR-transduced αβT cells did not reveal any alloreactivity against all tested LCL lines (data not shown). To reveal the possible reason for this reduced allogeneic response by γ9δ2TCR-expressing cells, αβTCR expression was determined on the surface of the γ9δ2TCR-transduced αβT cells. Flow cytometric analysis depicted in Figure 1A showed that γ9δ2TCR-transduced αβT cells had substantially down-regulated expression of the endogenous αβTCR in 40% of δ2-positive cells compared with the level of αβTCR on the mock-transduced αβT cells. Transduction of only γ9 or only δ2 chains of TCR did not affect levels of the endogenous αβTCRs (Figure 1A).
γ9δ2TCR-transduced αβT cells still maintained a low percentage of T cells expressing a higher amount of the αβTCR and a lower amount of γ9δ2TCR and a residual alloreactivity of this minor population might not have been detected. Therefore, γ9δ2TCR-transduced αβT cells were sorted into γ9δ2TCRhigh/αβTCRlow and γ9δ2TCRlow/αβTCRhigh expressing αβT cells and expanded to enrich, in particular, the latter fraction (supplemental Figure 2A). Sorted cells were tested again for their reactivity against 17 mismatched LCL cell lines by IFNγ ELISPOT (Figure 1F). Indeed, selectively, the initially small fraction of γ9δ2TCRlow/αβTCRhigh expressing αβT cells showed now a significant reactivity against 5 allogeneic LCL lines. However, proliferation in response to a pool of 4 mismatched LCL lines indicated that a response to allogeneic LCL lines was restricted to a small subset of αβT cells with very low or virtually no γδTCR expression (supplemental Figure 2B). This suggests that the observed reduced alloreactivity of γ9δ2TCR-transduced αβT cells is a consequence of the reduced surface expression of endogenous αβTCRs, and a residual alloreactivity is restricted to a small fraction of αβT cells expressing very low levels of or no γ9δ2TCRs.
Reprogramming of CD4+ as well as CD8+ αβT cells by a γ9δ2TCR
Naturally occurring γδT cells do not require CD4+ and CD8+ for their target recognition and function. Consequently, we assumed that expression of a γ9δ2TCR should be able to redirect both subsets of αβT cells, CD4+ and CD8+ against cancer cells. Therefore, CD8+ and CD4+ γ9δ2TCR-expressing T cells (Figure 2A) were separated and incubated with Daudi cells in the presence of pamidronate. Both CD8+ and CD4+ γ9δ2TCR-expressing T cells separately secreted IFNγ in response to Daudi cells, with a slight advantage for CD4+ γ9δ2TCR-transduced αβT cells (CD4+: 450, CD8+: 288 IFNγ spots, Figure 2B), either because of their increased TCR expression (CD4+: MFI = 17, CD8+: MFI = 11, Figure 2A) or the fact that CD4+ αβT cells are more prone to produce cytokines. In contrast, when examining lytic activity of both subsets with 51Cr-labeled Daudi cells or autologous PBMCs, CD8+ and total γ9δ2TCR-positive cells lysed ∼ 75% and ∼ 50%, respectively, of Daudi cells at the E:T ratio of 3:1, while CD4+ γ9δ2TCR-transduced cells showed ∼ 35% of specific lysis of Daudi cells at the same E:T ratio (Figure 2C). The reduced lytic activity of CD4+ γ9δ2TCR-transduced αβT cells was most likely because of a lower expression of perforin (P = .02) and granzyme B (P = .03; supplemental Figure 3). In summary, these data suggest that a γ9δ2TCR can efficiently redirect both CD4+ and CD8+ αβT cells against Daudi cells.
CD4+ as well as CD8+ αβT cells are reprogrammed with a γ9δ2TCR. (A) Mock and γ9δ2TCR-transduced αβT cells were stained with γδTCR, CD4- and CD8-specific Abs, and analyzed by flow cytometry. Percentage of positive cells for each quadrant is indicated. (B) CD4+ and CD8+ αβT cells were isolated and incubated with Daudi cells with 10μM pamidronate for 48 hours. IFNγ secretion was measured by ELISPOT. The test was performed in duplicate for every condition in 3 independent experiments. (C) CD4+ (95% purity), CD8+ (90% purity), or mixed–total γ9δ2TCR-transduced αβT cells or mock cells were incubated with 51Cr-loaded Daudi or PBMCs at the indicated E:T ratios for 4 hours and the specific lysis was calculated. Shown are representative experiments of at least 3 independent repetitions. (D) Immature DCs were incubated with mock CD4+ or γ9δ2TCR-transduced CD4+ αβT cells without or with pamidronate (10μM) for 48 hours at ratio of 1:2 (iDC: αβT cells). Shown is a representative FACS analysis of CD80, CD86, HLA-DR, CD83, and CD40.
CD4+ as well as CD8+ αβT cells are reprogrammed with a γ9δ2TCR. (A) Mock and γ9δ2TCR-transduced αβT cells were stained with γδTCR, CD4- and CD8-specific Abs, and analyzed by flow cytometry. Percentage of positive cells for each quadrant is indicated. (B) CD4+ and CD8+ αβT cells were isolated and incubated with Daudi cells with 10μM pamidronate for 48 hours. IFNγ secretion was measured by ELISPOT. The test was performed in duplicate for every condition in 3 independent experiments. (C) CD4+ (95% purity), CD8+ (90% purity), or mixed–total γ9δ2TCR-transduced αβT cells or mock cells were incubated with 51Cr-loaded Daudi or PBMCs at the indicated E:T ratios for 4 hours and the specific lysis was calculated. Shown are representative experiments of at least 3 independent repetitions. (D) Immature DCs were incubated with mock CD4+ or γ9δ2TCR-transduced CD4+ αβT cells without or with pamidronate (10μM) for 48 hours at ratio of 1:2 (iDC: αβT cells). Shown is a representative FACS analysis of CD80, CD86, HLA-DR, CD83, and CD40.
DC maturation can be induced by CD4+ γ9δ2TCR-transduced αβT cells
Several studies reported previously that phosphoantigen-stimulated γ9δ2T cells can induce maturation of dendritic cells (DCs).22 In addition, CD4+ αβT cells also have been reported to induce Ag-specific maturation of DCs.17 To test whether αβT cells transduced with γ9δ2TCR can lead to DC maturation, immature DCs were incubated with CD4+ mock- or CD4+ γ9δ2TCR-transduced αβT cells with or without 10μM pamidronate for 48 hours and then stained for maturation surface markers. Indeed, flow cytometric analysis depicted a higher percentage of CD80/CD86, CD80/HLA-DR, CD80/CD83, and CD80/CD40 double-positive DCs selectively after incubation with CD4+ γ9δ2TCR-transduced αβT cells in presence of pamidronate only (31.7%, 25.2%, 15.3%, and 25.4%, respectively) but not CD4+ mock-transduced αβT cells with or without pamidronate (∼ 2%, ∼ 2%, 1%, and ∼ 4.5%; Figure 2D). Moreover, the level of expression of maturation markers such as CD86, HLA-DR, and CD40 was notably higher after coincubation with CD4+ γ9δ2TCR-transduced αβT cells and pamidronate (1.7-, 2.4-, 1.5-fold, respectively) compared with all other controls. Finally, IL12p70 secretion by DCs was selectively detected after incubation with CD4+ γ9δ2TCR-transduced αβT cells in the presence of pamidronate, but not without pamidronate or with CD4+ mock-transduced αβT cells nor after incubation with conventional γδT cells (4.5 pg/mL, P < .05; data not shown). Altogether, these results suggest that CD4+ γ9δ2TCR-transduced αβT cells can, in contrast to conventional γδT cells, lead to a full maturation of DCs after incubation with pamidronate. In addition, maturation capacity of γ9δ2TCR-transduced αβT cells is comparable with the Ag-specific maturation of DCs by αβTCR-redirected αβT cells as reported by us recently.17
γ9δ2TCR-transduced αβT cells target a broad panel of tumor cells while ignoring the normal tissue
Antitumor activity of peripheral γ9δ2T cells was shown against a variety of malignancies, and therefore we tested whether a γ9δ2TCR can redirect αβT cells to specifically react against a broad panel of tumor cells in a 51Cr-release assay. γ9δ2TCR-transduced αβT cells demonstrated efficient cytolysis of cancer cell lines derived from lymphomas and leukemias such as Burkitt lymphoma (Daudi), chronic myelogenous leukemia (K562, KCL22), acute promyelocytic leukemia (NB4), acute T- (Jurkat) or pre-B-cell (BV173) leukemia, and multiple myeloma (U266), as well as from solid tumors such as osteogenic sarcoma (Saos-2), colon carcinoma (SW480), renal cell carcinoma (MZ1851RC), breast cancer (MDA-MB231), and head and neck tumor cell lines (SCC-9, FaDu, A231, RPMI2650, Cal27, and HNT08). Only the leukemia cell lines HL60, ML1, and the liver carcinoma cell line HepG2 were not lysed (Figure 3A). Lytic activity of γ9δ2TCR-transduced αβT cells against most tumor targets was comparable with the lytic activity of conventional γ9δ2T cells generated from a healthy donor (supplemental Figure 4). Also, cytokine secretion was enhanced against many tumor cell lines (supplemental Table 2). To examine the potential self-reactivity of γ9δ2TCR-transduced αβT cells, their ability to lyse primary hepatocytes, fibroblasts (Psf5, MS1 and MRC5), and PBMCs was tested. Lysis of all listed cells by γ9δ2TCR-expressing αβT cells was not significantly higher than the lysis of the negative control PBMC (Figure 3A). To further verify lack of self-reactivity, we performed series of ELISPOT for IFNγ secretion with various normal primary cells such as CD19+ B cells, αβT cells, primary hepatocytes, and fibroblasts from several healthy donors as targets in the absence or presence of pamidronate. The IFNγ secretion by γ9δ2TCR-transduced αβT cells in response to all of these targets was not higher than background levels of IFNγ secretion in the medium (supplemental Figure 5).
Broad recognition of tumor cells, but not of the normal tissues, is dependent on mevalonate-pathway intermediates. (A) Tumor cell lines and normal tissue-derived cells were loaded with 51Cr and incubated with γ9δ2TCR-transduced αβT cells at E:T ratio 10:1 for 4-5 hours. Percentage of the specific lysis is shown as the mean of at least 3 independent replicates for each target. (B) Various target cells, as indicated, were subjected to the lysis by γ9δ2TCR-transduced αβT cells at E:T ratio of 10:1 after treatment with 25μM mevastatin, 10μM pamidronate, with 15μM IPP or untreated. Shown is a summary of fold change of the specific lysis of treated (□, mevastatin; , pamidronate; and ■, IPP treatment) versus untreated target in at least 3 independent experiments for each cell line. WT1-specific TCR-transduced αβT cells served as a control for mevastatin/pamidronate/IPP-independent specific lysis of T2 cells loaded with wt1 peptide. Significance was determined by 2-way ANOVA analysis; *P < .01; **P < .001.
, pamidronate; and ■, IPP treatment) versus untreated target in at least 3 independent experiments for each cell line. WT1-specific TCR-transduced αβT cells served as a control for mevastatin/pamidronate/IPP-independent specific lysis of T2 cells loaded with wt1 peptide. Significance was determined by 2-way ANOVA analysis; *P < .01; **P < .001.
Broad recognition of tumor cells, but not of the normal tissues, is dependent on mevalonate-pathway intermediates. (A) Tumor cell lines and normal tissue-derived cells were loaded with 51Cr and incubated with γ9δ2TCR-transduced αβT cells at E:T ratio 10:1 for 4-5 hours. Percentage of the specific lysis is shown as the mean of at least 3 independent replicates for each target. (B) Various target cells, as indicated, were subjected to the lysis by γ9δ2TCR-transduced αβT cells at E:T ratio of 10:1 after treatment with 25μM mevastatin, 10μM pamidronate, with 15μM IPP or untreated. Shown is a summary of fold change of the specific lysis of treated (□, mevastatin; , pamidronate; and ■, IPP treatment) versus untreated target in at least 3 independent experiments for each cell line. WT1-specific TCR-transduced αβT cells served as a control for mevastatin/pamidronate/IPP-independent specific lysis of T2 cells loaded with wt1 peptide. Significance was determined by 2-way ANOVA analysis; *P < .01; **P < .001.
, pamidronate; and ■, IPP treatment) versus untreated target in at least 3 independent experiments for each cell line. WT1-specific TCR-transduced αβT cells served as a control for mevastatin/pamidronate/IPP-independent specific lysis of T2 cells loaded with wt1 peptide. Significance was determined by 2-way ANOVA analysis; *P < .01; **P < .001.
The involvement of mevalonate metabolites in triggering a broadly tumor-reactive γ9δ2TCR
To test whether metabolites of the mevalonate pathway are involved in general in the recognition of cancer cells by the here-investigated γ9δ2TCR, tumor cells were coincubated with pamidronate or loaded with IPP to increase mevalonate metabolites or with the inhibitor mevastatin and γ9δ2TCR-transduced αβT cells. Killing was assessed in a 51Cr-release assay. This revealed that the lysis of most targets that are recognized by γ9δ2TCR-transduced αβT cells could be further significantly enhanced by either pretreatment with pamidronate or by direct loading with IPP, or significantly inhibited by mevastatin (25μM; Figure 3B). Notably, neither the outcome against ML1, HL60, nor PBMCs that are not recognized by γ9δ2TCR-expressing cells nor lytic activity against T2 cells loaded with WT1126-134 peptide by WT1126-134–specific TCR-transduced αβT cells4 were affected by the additional application of pamidronate or IPP. The latter one was also not affected by mevastatin (Figure 3B). In addition, 3 cell lines were identified where killing was neither affected by mevastatine or pamidronate: U266, SW480, and MDA-MB231; and IPP loading of these 3 cell lines only enhanced lysis of SW480, significantly (P < .05). Cytokine secretion was enhanced against many tumor cell lines usually after adding pamidronate but not without (data not shown and supplemental Table 2). This suggests that metabolites of the mevalonate pathway considerably contribute to the recognition of most cancer cells by the here-investigated γ9δ2TCR, that mevalonate metabolites are most likely overexpressed at different levels on different tumor cells, and that killing is easier achieved than the induction of cytokines. As IPP loading did not enhance recognition of all tested tumor cell lines which were susceptible to the modulation of the mevalonate pathway, we conclude that a potential IPP-presenting molecule is either still susceptible to additional mevalonate-metabolite loading (eg, K562) or already completely loaded with mevalonate metabolites (eg, Daudi cells). However, 2 tumor cell lines could be identified (U266 and MDA-MB231) where mevalonate-metabolite modulating agents and IPP loading did neither influence killing ability nor cytokine secretion, suggesting that other mechanisms are involved in their recognition.
The involvement of F1-ATPase in triggering T cells transduced with a broadly tumor-reactive γ9δ2TCR
We have chosen to focus on the best-studied γ9δ2TCR, namely G115, to further verify the tumor recognition mechanism for γ9δ2TCR-transduced αβT cells. This TCR has been suggested to bind a complex of Apolipoprotein AI (ApoAI) and F1-ATPase,15 a complex mitochondrial enzyme found on the surface of many malignant cells.16 Therefore, surface expression of F1-ATPase in the target cells was tested by staining the cells with specific Abs for α and β subunits of F1-ATPase. Flow cytometric analysis of the staining reveals that Daudi cells express both α and β subunits of F1-ATPase, while αβT cells were negative for both subunits on their surface (Figure 4A). Then, we tested whether blocking F1-ATPase subunits with specific Abs interferes with the recognition of the target cells by γ9δ2TCR-transduced αβT cells. IFNγ secretion in response to Daudi cells in the presence of pamidronate was significantly diminished by specific Abs for α and β subunits of F1-ATPase compared with IFNγ secretion without Ab interference (P < .0001 and P = .0009, respectively; Figure 4B), but these Abs did not inhibit specific killing of Daudi cells (data not shown). Incubation with Ab against CD8 did not inhibit IFNγ secretion by γ9δ2TCR-transduced αβT cells in response to Daudi, but it dramatically abolished IFNγ secretion by WT1126-134-specific αβT cells after incubation with T2 cells loaded with WT1126-134-peptide (data not shown).
Role of F1-ATPase and NKG2D in the target recognition by γ9δ2TCR-transduced αβT cells. (A) Typical FACS histograms of Daudi cells and PBMCs stained with specific Abs for F1-ATPase α and β subunit followed with secondary goat–anti-mouse–PE. Secondary Ab alone was used as a negative control staining. (B-C) Daudi cells were incubated with blocking Abs either for the α subunit or β subunit of F1-ATPase (B) or for NKG2D (C) and γ9δ2TCR-transduced αβT cells for 48 hours with or without pamidronate (100μM). IFNγ secretion was measured by ELISPOT. The experiment was performed in duplicate and statistical analyses were performed using the Student t test. Similar results were obtained in at least 3 independent experiments; *P < .01; **P < .001. (D-E) γ9δ2TCR-transduced NKG2D− and NKG2D+ γ9δ2TCR- or mock-transduced αβT cells were incubated with Daudi in the absence (□) or presence of 10μM pamidronate ( ) for 48 hours. Then IFNγ and TNFα secretion was measured by ELISPOT. Shown are representative results of 3 independent experiments.
) for 48 hours. Then IFNγ and TNFα secretion was measured by ELISPOT. Shown are representative results of 3 independent experiments.
Role of F1-ATPase and NKG2D in the target recognition by γ9δ2TCR-transduced αβT cells. (A) Typical FACS histograms of Daudi cells and PBMCs stained with specific Abs for F1-ATPase α and β subunit followed with secondary goat–anti-mouse–PE. Secondary Ab alone was used as a negative control staining. (B-C) Daudi cells were incubated with blocking Abs either for the α subunit or β subunit of F1-ATPase (B) or for NKG2D (C) and γ9δ2TCR-transduced αβT cells for 48 hours with or without pamidronate (100μM). IFNγ secretion was measured by ELISPOT. The experiment was performed in duplicate and statistical analyses were performed using the Student t test. Similar results were obtained in at least 3 independent experiments; *P < .01; **P < .001. (D-E) γ9δ2TCR-transduced NKG2D− and NKG2D+ γ9δ2TCR- or mock-transduced αβT cells were incubated with Daudi in the absence (□) or presence of 10μM pamidronate ( ) for 48 hours. Then IFNγ and TNFα secretion was measured by ELISPOT. Shown are representative results of 3 independent experiments.
) for 48 hours. Then IFNγ and TNFα secretion was measured by ELISPOT. Shown are representative results of 3 independent experiments.
Next, it was tested whether serum compounds such as ApoAI are necessary for the target recognition. Therefore, Daudi, T2 cells loaded with p53264-272 peptide and PBMCs were vigorously washed with serum-free medium and then subjected for the specific lysis by γ9δ2TCR-, p53264-272-specific αβTCR17 or mock-transduced αβT cells in a 51Cr-release assay with 5% or 0% FCS. In the absence of serum specific-lysis of Daudi cells by γ9δ2TCR-transduced αβT cells was dramatically reduced to < 10% comparing to 30% of lysis at E:T ratio of 30:1, but the lysis of T2 cells loaded with p53264-272 peptide by p53264-272–specific TCR-transduced αβT cells was not affected in the absence of serum (supplemental Figure 6). However, in contrast to previous reports15 supplementation of ApoAI could not restore killing by γ9δ2TCR-transduced αβT cells (data not shown) suggesting that serum compounds but not necessarily ApoAI are involved in this process.
To test whether recognition of F1-ATPase is a general mechanism by which γ9δ2TCR G115-transduced αβT cells recognize tumor cells, a panel of tumor and normal cells was stained for F1-ATPase surface expression. To quantify the expression level of F1-ATPase, the ratio of fluorescent intensity of the specific staining for every subunit over the control staining was calculated. Because of the less efficient Ab for the α subunit and highly significant correlation between the expressions of the 2 subunits (supplemental Figure 7A), the expression of the β subunit was used as surrogate marker for F1-ATPase expression for further analyses. Most of the normal cells like αβT cells, hepatocytes, and fibroblasts stained negative for F1-ATPase on their surface (mean fluorescence intensity [MFI] ratios ∼ 1), while tumor cells had different levels of F1-ATPase expression (MFI ratios from 1 to 18). Spearmen correlation analysis that was performed did not reveal any significant correlations between F1-ATPase expression and IFNγ secretion in response to the same target with or without pamidronate (supplemental Figure 7B). F1-ATPase–negative cells also did not become positive in flow cytometry after demasking as described recently.23 Notably, tumor cell lines were also recognized in the absence of F1-ATPase expression (NB4, MZ1851RC, and SCC9), and other tumor cells (ML1 and HL60) with high expression of F1-ATPase were not recognized by γ9δ2TCR-transduced αβT cells (supplemental Table 2). These results suggest that the interaction of the γ9δ2TCR G115 with F1-ATPase is additive but not mandatory and alone not sufficient for the activation of γ9δ2TCR-transduced αβT cells.
Role of NKG2D and their ligands in the tumor recognition by γ9δ2TCR-expressing αβT cells
NKG2D is expressed on most γδT cells and considered to contribute substantially to the activation of γδT cells. Subsets of αβT cells also express NKG2D. Therefore, the involvement of NKG2D in the recognition of targets by γ9δ2TCR-transduced αβT cells was tested. Indeed, the amount of IFNγ spots produced by NKG2D+ γ9δ2TCR-transduced αβT cells against untreated Daudi cells or in presence of pamidronate was reduced significantly in an IFNγ-ELISPOT when anti-NKG2D Ab was added (P = .025 and P = .031, respectively). Presence of mevastatin in the assay reduced the amount of IFNγ spots to almost negligible numbers and therefore additional blocking of NKG2D was not significant, but showed an inhibitory trend (Figure 4C). Notably, blocking Ab for NKG2D did not affect killing of Daudi cells as well as F1-ATPase blocking (data not shown) indicating again that for cytokine secretion a more complex cell-cell interaction is necessary than for killing.
Next, the expression of NKG2D ligands MICA, MICB, ULBP1-4 on tumor cells was tested by flow cytometry. Quantification of the staining was calculated as a ratio of MFI of the specific staining over the control staining. Among all tested tumor cells at least one or more NKG2D ligands were expressed (supplemental Table 2), however, no correlation was found between the expression of NKG2D ligands and the recognition of targets (data not shown).
It has been previously suggested that NKG2D is important for TNFα, but not for IFNγ secretion in γδT cells.24 Therefore, NKG2D− and NKG2D+-γ9δ2TCR–transduced αβT cells were sorted (data not shown) and tested in their ability to specifically produce both cytokines in response to Daudi cells. The amount of TNFα spots measured after 48 hours of incubation with Daudi cells and pamidronate that was produced by NKG2D− was equal to that produced by total (∼ 500 spots) and higher than TNFα spots made by NKG2D+ γ9δ2TCR-transduced αβT cells (∼ 400 spots; Figure 4D). IFNγ secretion by NKG2D− cells was even slightly higher than by total or NKG2D+ γ9δ2TCR-transduced αβT cells in response to Daudi with pamidronate (450, 326, and 288 IFNγ spots, respectively; Figure 4E). Furthermore, NKG2D− γ9δ2TCR-transduced αβT cells efficiently lysed Daudi cells (data not shown). Altogether, these data suggest that involvement of NKG2D does not enhance the secretion of TNFα compared with IFNγ, and that NKG2D is additive but not mandatory for the activation of γ9δ2TCR-transduced αβT cells.
Specific recognition of the primary AML blasts cells by γ9δ2TCR-transduced αβT cells
Next, we analyzed whether γ9δ2TCR-transduced αβT cells can specifically react against primary acute myeloid leukemic (AML) blasts (supplemental Table 3). Therefore, ELISPOT analysis for IFNγ secretion was performed after 48 hours of incubation of primary AML blasts with γ9δ2TCR-transduced αβT cells in the presence or absence of pamidronate. Daudi and αβT cells served as positive and negative control targets. Three AML samples from p4, p5, and p7 could induce IFNγ secretion in the presence of pamidronate that was significantly higher compared with IFNγ secretion that was induced by negative control αβT cells (Figure 5A). AML blasts from patients 1, 2, 3, and 6 induced lower amounts of IFNγ which did not differ significantly from the negative control including CD34+ cells from healthy donors (Figure 5B).
Specific recognition of the primary AML blasts by γ9δ2TCR-transduced αβT cells. (A) γ9δ2TCR-transduced αβT cells of healthy donor were incubated with AML blasts without (□) or with 10μM pamidronate ( ) for 48 hours at E:T ratio of 0.3:1. IFNγ secretion was measured by ELISPOT. Daudi and αβT cells were used as positive or negative target control, respectively. Shown is the result of the mean of 2 experiments performed in duplicates for the same donor. Similar results were observed for 2 other healthy donor-derived γ9δ2TCR-transduced αβT cells. *P = .05, **P < .01. (C) γ9δ2TCR or mock-transduced αβT cells were incubated with AML blasts or PBMCs of healthy donors enriched with CD34+ cells for 4 hours at E:T ratio 4:1. Then cells were plated in methylcellulose and, after 10 days, colony formation was quantified using an inverted microscope. Shown is the percentage of CFU formed after the incubation with γ9δ2TCR-transduced cells, while CFU formed after the incubation with mock-transduced αβT cells is used as 100%. Shown is 1 of 3 representative experiments, which have been performed with different donors. (D) Microscopic photographs of the representative field of methylcellulose colony formation cultures of AML and healthy donor taken on the same day of the assay. (E-F) αβT cells of AML patient 5 were isolated with CD3-specific beads and transduced with γ9δ2-TCR with standard procedures. After selection and expansion, αβT cells were stained with γδTCR-specific Ab and the expression was assessed by FACS analysis. IFNγ secretion in response to AML cells and the control targets was analyzed by ELISPOT as described in panel B.
) for 48 hours at E:T ratio of 0.3:1. IFNγ secretion was measured by ELISPOT. Daudi and αβT cells were used as positive or negative target control, respectively. Shown is the result of the mean of 2 experiments performed in duplicates for the same donor. Similar results were observed for 2 other healthy donor-derived γ9δ2TCR-transduced αβT cells. *P = .05, **P < .01. (C) γ9δ2TCR or mock-transduced αβT cells were incubated with AML blasts or PBMCs of healthy donors enriched with CD34+ cells for 4 hours at E:T ratio 4:1. Then cells were plated in methylcellulose and, after 10 days, colony formation was quantified using an inverted microscope. Shown is the percentage of CFU formed after the incubation with γ9δ2TCR-transduced cells, while CFU formed after the incubation with mock-transduced αβT cells is used as 100%. Shown is 1 of 3 representative experiments, which have been performed with different donors. (D) Microscopic photographs of the representative field of methylcellulose colony formation cultures of AML and healthy donor taken on the same day of the assay. (E-F) αβT cells of AML patient 5 were isolated with CD3-specific beads and transduced with γ9δ2-TCR with standard procedures. After selection and expansion, αβT cells were stained with γδTCR-specific Ab and the expression was assessed by FACS analysis. IFNγ secretion in response to AML cells and the control targets was analyzed by ELISPOT as described in panel B.
Specific recognition of the primary AML blasts by γ9δ2TCR-transduced αβT cells. (A) γ9δ2TCR-transduced αβT cells of healthy donor were incubated with AML blasts without (□) or with 10μM pamidronate ( ) for 48 hours at E:T ratio of 0.3:1. IFNγ secretion was measured by ELISPOT. Daudi and αβT cells were used as positive or negative target control, respectively. Shown is the result of the mean of 2 experiments performed in duplicates for the same donor. Similar results were observed for 2 other healthy donor-derived γ9δ2TCR-transduced αβT cells. *P = .05, **P < .01. (C) γ9δ2TCR or mock-transduced αβT cells were incubated with AML blasts or PBMCs of healthy donors enriched with CD34+ cells for 4 hours at E:T ratio 4:1. Then cells were plated in methylcellulose and, after 10 days, colony formation was quantified using an inverted microscope. Shown is the percentage of CFU formed after the incubation with γ9δ2TCR-transduced cells, while CFU formed after the incubation with mock-transduced αβT cells is used as 100%. Shown is 1 of 3 representative experiments, which have been performed with different donors. (D) Microscopic photographs of the representative field of methylcellulose colony formation cultures of AML and healthy donor taken on the same day of the assay. (E-F) αβT cells of AML patient 5 were isolated with CD3-specific beads and transduced with γ9δ2-TCR with standard procedures. After selection and expansion, αβT cells were stained with γδTCR-specific Ab and the expression was assessed by FACS analysis. IFNγ secretion in response to AML cells and the control targets was analyzed by ELISPOT as described in panel B.
) for 48 hours at E:T ratio of 0.3:1. IFNγ secretion was measured by ELISPOT. Daudi and αβT cells were used as positive or negative target control, respectively. Shown is the result of the mean of 2 experiments performed in duplicates for the same donor. Similar results were observed for 2 other healthy donor-derived γ9δ2TCR-transduced αβT cells. *P = .05, **P < .01. (C) γ9δ2TCR or mock-transduced αβT cells were incubated with AML blasts or PBMCs of healthy donors enriched with CD34+ cells for 4 hours at E:T ratio 4:1. Then cells were plated in methylcellulose and, after 10 days, colony formation was quantified using an inverted microscope. Shown is the percentage of CFU formed after the incubation with γ9δ2TCR-transduced cells, while CFU formed after the incubation with mock-transduced αβT cells is used as 100%. Shown is 1 of 3 representative experiments, which have been performed with different donors. (D) Microscopic photographs of the representative field of methylcellulose colony formation cultures of AML and healthy donor taken on the same day of the assay. (E-F) αβT cells of AML patient 5 were isolated with CD3-specific beads and transduced with γ9δ2-TCR with standard procedures. After selection and expansion, αβT cells were stained with γδTCR-specific Ab and the expression was assessed by FACS analysis. IFNγ secretion in response to AML cells and the control targets was analyzed by ELISPOT as described in panel B.
To test whether γ9δ2TCR-expressing αβT cells can target the colony-forming progenitors within AML blasts or CD34+ cells from healthy donors, AML blasts or healthy hematopoietic-progenitor cells were incubated with γ9δ2TCR- or mock-transduced αβT cells on the methylcellulose matrix for the colony formation assay. Colonies were counted 8-10 days later and CFU were normalized to the number of colonies that grew after incubation with mock-transduced αβT cells for every sample. Mock-transduced αβT cells did not affect colony formation of AML blasts or healthy donor samples compared with CFU that formed after incubation with medium only (data not shown). γ9δ2TCR-transduced αβT cells significantly inhibited (P = .005) CFU of AML samples to 40% but not of CD34+ cells (Figure 5C). Colony formation of CD34+ cells from the healthy donors was not inhibited by γ9δ2TCR-positive αβT cells (93% of mock-transduced αβT cell–treated group). In addition to its number, colonies that formed by AML blasts after incubation with γ9δ2TCR-transduced αβT cells were much smaller compared with the colonies formed by AML blasts after incubation with mock-transduced αβT cells (Figure 5D). The sizes of colonies grown from the healthy donor samples after γ9δ2TCR and mock-transduced αβT-cell incubation were comparable (Figure 5D).
Finally, we tested whether αβT cells from AML patients can be transduced with γ9δ2TCR and be redirected against autologous AML blasts. αβT cells were isolated from the blood sample of AML patient 5, activated and retrovirally transduced with γ9δ2. After selection with antibiotics and expansion, αβT cells were ∼ 82% positive for the transduced γ9δ2 TCR (Figure 5E). Then, the functionality of transduced αβT cells from the patient was examined for IFNγ secretion. γ9δ2TCR-transduced patient αβT cells secreted IFNγ in response to autologous AML blasts and blasts of patients p4 and p7 as well as in response to Daudi cells in the presence of pamidronate (P < .0001 for p4, p7, and Daudi and P = .04 for p5 [autologous], Figure 5F).
Altogether, γ9δ2 TCR can redirect αβT cells from healthy individual as well as from the AML patients to react against primary AML blasts and to target specifically colony-forming progenitors within AML-population but not of the healthy donors.
γ9δ2TCR-transduced αβT cells inhibit leukemia growth in vivo
The efficacy of γ9δ2TCR-transduced αβT cells against leukemia cells in vivo was tested in the Rag2−/−γc−/− double knockout (KO) mouse. The leukemia cell line Daudi engineered to express luciferase (Daudi-Luc) was injected IV with or without pamidronate to sublethally preirradiated mice. On the same day and on day 3, mice were treated IV with either γ9δ2TCR or MDM281-88–specific25 TCR-transduced αβT cells. IL2 was given to mice subcutaneously every 3 weeks starting on day 1 (Figure 6A). Mice follow-up was conducted with bioluminescent imaging (BLI) to determine the tumor load once a week in addition to general examination. All the mice developed tumors, but notably the mice that were treated with γ9δ2TCR-transduced αβT cells and pamidronate showed a significantly lower tumor load at all time points compared with 3 other groups (P < .01; Figure 6B). To summarize, γ9δ2TCR-transduced αβT cells could only inhibit tumor development when applied together with pamidronate in this very aggressive leukemia model.
In vivo effect of γ9δ2TCR-transduced αβT cells on Daudi tumor growth. (A) Schematic overview of the experiment. RAG2−/−γc−/− double knockout mice received a sublethal total body irradiation with 2.0 Gy x-rays on day 0. The next day, mice were intravenously injected with 0.5 × 106 Daudi-Luc cells and 107 γ9δ2TCR or MDM281-88–specific TCR-transduced αβT cells. The same dose of T cells was injected on day 3. Mice in the indicated groups received an injection of pamidronate on day 1 IV and on days 21 and 42 IP. All mice received 6 × 105 IU of IL2 with IFA subcutaneously on days 1 and 21. (B) Tumor load was measured by quantifying the luciferase activity produced by the transduced Daudi tumor cells using the Biospace In Vivo Imaging System once a week. Shown are the results of 1 representative experiment of 2 independent repetitions (n = 5).
In vivo effect of γ9δ2TCR-transduced αβT cells on Daudi tumor growth. (A) Schematic overview of the experiment. RAG2−/−γc−/− double knockout mice received a sublethal total body irradiation with 2.0 Gy x-rays on day 0. The next day, mice were intravenously injected with 0.5 × 106 Daudi-Luc cells and 107 γ9δ2TCR or MDM281-88–specific TCR-transduced αβT cells. The same dose of T cells was injected on day 3. Mice in the indicated groups received an injection of pamidronate on day 1 IV and on days 21 and 42 IP. All mice received 6 × 105 IU of IL2 with IFA subcutaneously on days 1 and 21. (B) Tumor load was measured by quantifying the luciferase activity produced by the transduced Daudi tumor cells using the Biospace In Vivo Imaging System once a week. Shown are the results of 1 representative experiment of 2 independent repetitions (n = 5).
Discussion
Our studies demonstrate that introducing a γ9δ2TCR into αβT cells can efficiently redirect human αβT cells against a broad range of tumor cells and thereby overcomes major hurdles of current immunotherapeutic concepts: the classic HLA restriction of αβT cells1 as well as unwanted specificities because of pairing of introduced with endogenous αβTCR chains.3,4 In addition, it also tackles the major limitation of current efforts which take advantage of γδT cells: exhaustion because of massive ex vivo expansion prior to application26 and general proliferation deficiency in most cancer patients.27 Furthermore, it allows for transfer of a defined highly active anticancer receptor instead of a polyclonal γδT-cell population with a diverse function and most likely less efficacy.1,7
The here-used γ9δ2TCR G115 had a sufficient affinity to its ligand to reprogram both CD8+ and CD4+ and αβT cells. CD4+ αβT cells can thereby provide important help to the maintenance of a robust immune response28 as also suggested by our observation that γδTCR-transduced CD4+ αβT cells promote the maturation of DCs. Thus, these rather easily accessible γδTCRs have the potential to sufficiently reprogram the immune system against cancer cells. This property has so far only been reported for unique high-affinity αβTCRs, which are usually not found in the human T-cell repertoire and need to be engineered.17,25,29,30 However, although γδTCR-transduced αβT cells lysed tumor cells in the absence of phosphoantigen-stimulating agents, cytokine secretion of γ9δ2TCR reprogrammed αβT cells usually required therapeutic concentrations of the clinically widely used mevalonate-modulating agent pamidronate.20 Also, in the in vivo model, inhibition of tumor growth was selectively observed after additional application of pamidronate. This suggests that the induction of cytokine secretion of γ9δ2TCR-transduced αβT cells requires a higher density of mevalonate metabolites, and thus a more profound receptor ligand interaction, and is crucial for in vivo control of leukemia. This discrepancy between lytic activity and cytokine secretion is usually not observed with αβT cells reprogrammed with αβTCRs.17,29,31 This could be either a consequence of differences in the transmembrane domain of the γδ and αβTCR32 or differences in the glycosylation of their CD3 complex.33
A crucial question for the usage of γδT cells and their TCRs remains their target definition. Recognition of tumor cells was in our model mainly dependent on intermediate products of the mevalonate-metabolic pathway, presumably IPP.11 Thus, redirected αβT cells sense a dysregulated mevalonate pathway, a ubiquitous and essential pathway for cell survival.11,34-36 Yet, it is important to mention that not all investigated tumor cells were recognized by γ9δ2TCR-transduced αβT cells. This is in line with the observation that either some tumor cells do not have a dysregulated mevalonate pathway11 or that they are not able to present mevalonate metabolites to the TCR. The latter hypothesis is supported by our finding that direct loading of IPP did not induce killing of these “inert” tumor cell lines. Thus, one problem remains: there is no obvious marker of a dysregulated mevalonate pathway in cancer, and how can we identify patients who benefit from such a therapy? However, our proposed concept of targeting leukemic blasts easily allows testing the sensitivity of blasts isolated from patients ex vivo with γ9δ2TCR-transduced αβT cells before therapeutic application.
The hypothesis of a phosphoantigen-presenting molecule is supported by the observation that soluble IPP is unable to stimulate γ9δ2T cells and direct cell-to-cell contact is required to induce activation of a γ9δ2TCR.37,38 F1-ATPase in complex with Apolipoprotein AI has been shown to interact with γ9δ2TCR directly15 and F1-ATPase itself has been proposed to serve as a phosphoantigen-presenting molecule.39 Although no direct association of F1-ATPase with IPP could be demonstrated, the ATP analog ApppI can bind to F1-ATPase. ApppI is then cleaved by exogenous nucleotide pyrophosphatase (NPP) into IPP + AMP.40 Indeed, ApppI-loaded F1-ATPase–immobilized beads induced γ9δ2TCR aggregation and cytokine production in the presence of NPP.39 Although we observed that F1-ATPase can be involved in the activation of γ9δ2TCR-transduced αβT cells, we did not find any correlation between the recognition of tumor cells by γ9δ2TCR-transduced αβT cells and the expression of F1-ATPase on a broad panel of tumor and normal cells. Even F1-ATPase–negative cell lines were recognized by γ9δ2TCR-transduced cell lines and vice versa; tumor cell lines that strongly expressed F1-ATPase were not recognized by γ9δ2TCR-positive cells even after intensive pamidronate treatment or direct loading with IPP. Altogether our observations make it unlikely that F1-ATPase is a general phosphoantigen-presenting molecule, and that it is essential for γ9δ2TCR binding and activation.
Although the interaction between the costimulatory receptor NKG2D and its ligands is considered to play a major role in the activation of γ9δ2T cells,24 NKG2D− γ9δ2TCR-transduced αβT cells were highly functional. However, it cannot be excluded that NKG2D ligands directly bind to the γ9δ2TCR as reported for ULBP4.41 Also, other ULBP-family members such as ULBP1 were found to determine lymphoma susceptibility to γδT cells; however, lysis was in these studies independent of the γδTCR.42-44 In line with these observations, no association between the pattern of NKG2D ligand expression and responsiveness of target cells to γ9δ2TCR-transduced αβT cells was detected. Thus, our data indicate that NKG2D functions as a coreceptor with additive contribution to tumor cytotoxicity or cytokine secretion of γ9δ2TCR-transduced αβT cells but is not mandatory.
Taken together, our data support the concept that γ9δ2TCR-transduced αβT cells target a dysregulated mevalonate pathway, and thus should not attack normal cells. In line with this assumption, healthy tissue-derived cells such as primary hepatocytes, fibroblast, and T and B cells did not induce any activation of γ9δ2TCR-transduced αβT cells. However, as not all normal tissues can be formally tested for recognition and as mouse models are devoid of γ9δ2T cells,45 it will not be possible to preclinically address this issue further. Only the controlled clinical administration γ9δ2T cells or γ9δ2TCR-transduced αβT cells to patients will finally prove safety and efficacy of this approach. At least so far, no toxicity has been observed of in vivo–activated or adoptively transferred γ9δ2T cells.46,47
In summary, we propose a new concept in the field of adoptive immunotherapy, where easily accessible γ9δ2TCR can be used to reprogram CD4+ and CD8+ αβT cells against a broad panel of tumor cells. Thereby, metabolites of a dysregulated mevalonate pathway are targeted by the γ9δ2TCR, and the additional application of widely used biphosphonates seems to be crucial for in vivo efficacy most likely because of its modulating effect on cytokine secretion of γ9δ2TCR-transduced αβT cells. Expression of NKG2D on the T cell as well as expression of F1-ATPase on the target cell can be further involved in recognition of the tumor cell but are not mandatory. γ9δ2TCR-redirected αβT cells did not only efficiently kill cancer cell lines in vitro but inhibited, in an autologous system, colony formation of primary leukemic blasts and reduced leukemia growth in vivo. Thus, γ9δ2TCR-redirected αβT cells are an attractive concept for further clinical evaluation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We appreciate material support by T. Mutis (UMC Utrecht, The Netherlands), M. Theobald (Mainz, Germany), and P. D. Greenberg (Seattle, WA) as indicated in supplemental Methods.
This work has been supported by the ZonMW 40-40300-98-07003, ZonMW VIDI 917.11.337, LSBR 0902, AICR 10-0736, and UU 2010-4669.
Authorship
Contribution: V.M.-M., S.H., M.v.B., L.H., Z.S., K.S., and J.K. designed, performed, and analyzed experiments; S.S. and A.M. provided unique material and expertise; J.K. supervised all experiments; V.M.-M. and J.K. wrote the manuscript; and all authors agreed on the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for V.M.-M. is Laboratory for Immunoregulation, The Sheba Cancer Center, Chaim Sheba Medical Center, Tel Hashomer, Israel.
Correspondence: Jürgen Kuball, Department of Hematology and Department of Immunology, (Huispostnr.: KC02.085.2), UMC Utrecht, Lundlaan 6, 3584 EA Utrecht, The Netherlands; e-mail: j.h.e.kuball@umcutrecht.nl.