Abstract
Standard myeloma treatment response criteria are determined principally by changes in the monoclonal protein. Reduction in the size of the proliferative component of malignant plasma cells may be an additional metric of assessing response to therapy. We retrospectively analyzed 176 patients with newly diagnosed myeloma with a measurable plasma cell labeling index (PCLI) at diagnosis and repeat measurement 4 months after initiation of therapy. PCLI response was defined as a ≥ 60% reduction. Baseline PCLI is an independent prognostic factor; therefore, we categorized patients into 3 groups: PCLI ≥ 3% (high), ≥ 1% (intermediate), and < 1% (low). Patients achieving a greater PCLI response had improved median overall survival of 54 months compared with 29 months in nonresponders (P = .02). Improved median overall survival with PCLI response occurred in the high initial PCLI group (28 vs 7 months; P = .003) and intermediate group (64 vs 24 months; P = .002). The application of PCLI response and serum M-spike response together provided further prognostic information. On multivariate analysis, the prognostic value of PCLI response was independent of β2-microglobulin, elevated creatinine, serum M-spike response, and baseline PCLI. We conclude that a significant reduction in plasma cell proliferation in patients with newly diagnosed myeloma is an important predictor of survival.
Introduction
Survival in myeloma varies widely and includes rapidly progressive disease as well as indolent disease with prolonged survival. The International Staging System (ISS) is a simple and reliable tool that uses serum albumin and serum β2-microglobulin (β2M) for patient stratification at time of diagnosis.1 Newly diagnosed patients with multiple myeloma also may be stratified into high-risk versus standard-risk disease by risk factors such as proliferative rate, deletion of chromosome 13 or hypodiploidy on metaphase cytogenetic studies, deletion 17p, and certain IgH translocations.2 Immunophenotyping via multiparameter flow cytometry and gene expression profiling are additional available methods that provide further prognostic information. However, one of the most important factors governing long-term outcome is response to therapy.3,4 Response to therapy is determined principally by changes in the monoclonal protein, and an accurate measurement is critical for patient care, as well as the assessment of various treatment strategies and new drugs in myeloma in clinical trials.
Although a reduction in the M protein is an excellent surrogate for a reduction in overall myeloma cell burden, it may be less sensitive in detecting the effects of treatments that primarily target the proliferative fraction of plasma cells. Furthermore, some investigators have demonstrated the magnitude of M-protein response is not always correlated with improved survival.5 We hypothesized that a reduction in the size of the proliferative component of malignant plasma cells in myeloma may be an additional metric of assessing response to therapy. Because the size of the proliferative compartment in myeloma is usually small, a reduction may not necessarily produce a significant decrease in M protein. The purpose of this study was to determine whether a significant reduction in the plasma cell proliferative rate after therapy is associated with improved survival, validating its potential role as a metric of assessing treatment efficacy in myeloma.
Methods
We assessed plasma cell proliferation rate by using the plasma cell labeling index (PCLI). The PCLI is a slide-based immunofluorescence technique that uses antibodies against BrdU, which are incorporated into actively dividing plasma cells, thereby providing the percentage of plasma cells in the S-phase of the cell cycle.6-8 Multiple authors have confirmed the independent prognostic value of an elevated PCLI of > 1%, which is predictive of shorter time to progression9 and decreased survival.10-12 To test our hypothesis, we studied 176 patients seen at Mayo Clinic between 1985 and 2005 with newly diagnosed myeloma who had a measurable PCLI of ≥ 0.5%, which was obtained at time of diagnosis and again 4 months after initiation of therapy. Approval for the study was obtained from the Mayo Clinic Institutional Review Board according to federal regulations and patients provided informed consent in accordance with the Declaration of Helsinki.
Because baseline PCLI is an independent prognostic factor for outcome and greater degrees of response, we first categorized patients into 3 risk groups: initial PCLI ≥ 3% (high), ≥ 1% (intermediate), and < 1% (low). Baseline and subsequent serum and urine M protein, immunofixation, initial and subsequent therapy, stem cell transplantation status, progression date, and date of death or last follow-up were recorded. Laboratory parameters, including heavy chain type, hemoglobin, serum creatinine, β2M, serum albumin, and ISS stage, also were noted. Myeloma was diagnosed with the use of standard criteria.13,14
Clinical responses were classified by the International Myeloma Working Group uniform response criteria.13,15 We tested different cut-off values for defining PCLI response and used a cutoff of 60% reduction or greater, which was the closest approximation of the median PCLI reduction (53% and 47% patients in the resultant 2 groups) after therapy. Induction therapy was separated into optimal and suboptimal therapy to ensure that differences in therapy did not confound interpretation of the study results. Optimal therapy for the purposes of this analysis was defined as novel agents, including thalidomide, lenalidomide, or bortezomib. Suboptimal therapy was defined as dexamethasone alone; melphalan-prednisone; vincristine, doxorubicin, dexamethasone; or vincristine, carmustine, melphalan, cyclophosphamide, prednisone. To avoid confounding because of longer follow-up times in patients diagnosed earlier in the study period, median overall survival beyond 72 months was censored.
Statistical analysis
Calculations were performed with JMP Version 8.0 (SAS Institute Inc). Median overall survival was the primary end point and defined as the time from initial date of diagnosis to time of death from any cause or last contact. Survival analysis was estimated by the use of the Kaplan-Meier method.16 Curves were compared with the log-rank test.17 The following prognostic factors for overall survival were tested in a univariate analysis: age > 65 years, albumin < 3.5 g/dL, β2M > 3.5 mg/dL, creatinine > 1.4 mg/dL, hemoglobin < 10 g/dL, baseline PCLI ≥ 1% and ≥ 3%, absence of serum M-protein response to therapy (< 50% reduction), and absence of PCLI response (< 60% reduction). P values < .05 were considered to be significant.
Results
Patient characteristics
A total of 176 patients were available for analysis. Patient characteristics, including age, sex, baseline laboratory characteristics, PCLI and M spike at diagnosis, and induction therapy were stratified by response to PCLI and are shown in Table 1. There were 114 men (64%); median age at diagnosis was 59 years (range, 27-78 years), and the median follow-up was 54 months (range, 3.5-297 months). Patients were treated according to the standard of care at time of diagnosis. One-hundred twenty patients (68%) underwent stem cell transplantation after induction therapy. Overall median survival was 46 months (95% confidence interval [CI] 36-54 months; range, 4-165 months). The median baseline PCLI was 1.2% (0.5%-14.4%). Median initial serum M spike was 3.6 g/dL (range, 0-9.4 g/dL); 30 patients (17%) had no measurable serum M spike at time of diagnosis, and only 7 (4%) patients were nonsecretory, without measurable serum or urine M protein. The free light chain assay was available and used to assess treatment response in 4 of these patients; serial BM plasma cell percentage was used to assess response to therapy in the remaining 3 patients.
Assessment for confounding variables was performed between patients who achieved a PCLI response alone versus an M-spike response only and demonstrated no differences between ISS stage (P = .66), initial PCLI risk group (P = .21), serum creatinine (P = .13), hemoglobin (P = .98), serum albumin (P = .74), β2M (P = .43), or optimal versus suboptimal treatment (P = .46).
Impact of reduction in plasma cell proliferation on median overall survival
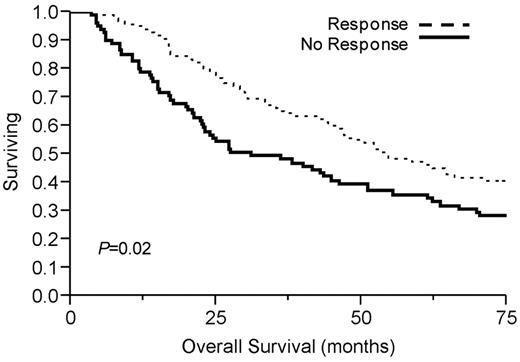
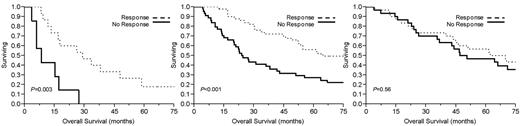
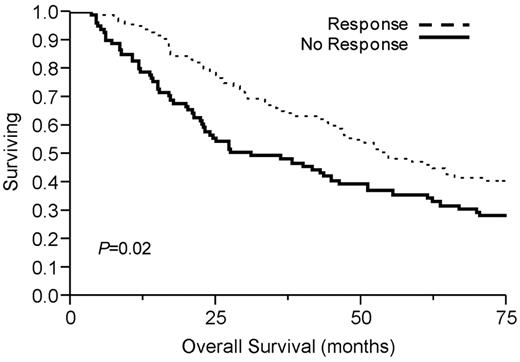
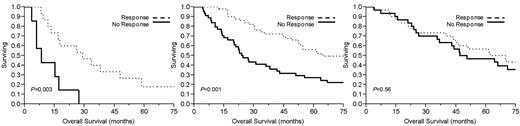
Table 2 demonstrates median overall survival rates for the group after initial therapy. Ninety-four patients (53%) demonstrated a PCLI response, defined as a ≥ 60% reduction in PCLI after initial therapy. This conferred a significant median overall survival advantage of 54 months compared with 29 months in those who achieved < 60% PCLI reduction (P = .02; Figure 1). Overall survival was significantly longer in the high and intermediate PCLI groups if they achieved a PCLI response to therapy (Figure 2). Patients achieving a PCLI response in the high PCLI group had median overall survival of 28 months versus 7 months in nonresponders (P = .003). PCLI responders in the intermediate PCLI group had median overall survival of 64 months versus 24 months in nonresponders (P = .002). In the low baseline PCLI group (< 1%), median overall survival was comparable in both responders and nonresponders (66 months vs 49 months; P = .56).
Kaplan-Meier survival analysis by PCLI response. Median overall survival was significantly longer in PCLI responders (54 months, 95% CI 45-297; P = .02) versus 29 months (95% CI 22-44) in nonresponders (P = .02).
Kaplan-Meier survival analysis by PCLI response. Median overall survival was significantly longer in PCLI responders (54 months, 95% CI 45-297; P = .02) versus 29 months (95% CI 22-44) in nonresponders (P = .02).
Kaplan-Meier survival analysis by baseline proliferative rate. Comparison of median overall survival for patients with multiple myeloma who achieved a PCLI response versus PCLI nonresponders who had (left) high baseline PCLI (≥ 3%), (middle) intermediate PCLI (> 1% but < 3%), and (right) low PCLI (< 1%).
Kaplan-Meier survival analysis by baseline proliferative rate. Comparison of median overall survival for patients with multiple myeloma who achieved a PCLI response versus PCLI nonresponders who had (left) high baseline PCLI (≥ 3%), (middle) intermediate PCLI (> 1% but < 3%), and (right) low PCLI (< 1%).
Impact of M-spike response on median overall survival
One-hundred forty-six patients had a measurable serum M protein at time of diagnosis. Thirty patients had no measurable serum M spike. Ninety-two (63%) patients achieved at least a 50% reduction in the initial M spike after 4 months of therapy. Median overall survival in patients with ≥ 50% reduction in the serum M spike was 55 months versus 29 months in patients not reaching this level of response (P = .004). The median overall survival for complete response was not reached. No significant difference in median overall survival was present between very good partial response (VGPR) and partial response (PR; P = .57); however, the combined VGPR + PR group achieved a survival advantage of 55 months versus 32 months in the stable disease group and 8 months in the progressive disease group (P = .006).
Univariate and multivariate survival analysis
Table 3 allows comparison in a univariate model of several common prognostic markers, including advanced age, albumin, β2M, elevated creatinine, anemia, baseline PCLI at diagnosis, and serum M-spike response. Initial PCLI ≥ 3%, serum M-spike reduction < 50%, PCLI reduction < 60%, serum β2M > 3.5 mg/dL, and creatinine > 1.4 mg/dL were determined to be poor prognostic indicators on univariate analysis. In multivariate analysis, these prognostic factors were ranked by proportional hazard ratio (Table 4). The prognostic value of PCLI nonresponse was independent of initial PCLI ≥ 3% and M-spike nonresponse. The addition of β2M > 3.5 mg/dL and creatinine > 1.4 was not significant on multivariate analysis.
Concordance between PCLI and M-spike response
Of the 92 patients with M-spike response, 36% did not meet criteria for PCLI response. Conversely, of the 81 PCLI responders with a measurable serum M protein, 27% did not achieve a > 50% reduction in the M protein. In patients who did not achieve an M-spike response, if a PCLI response was attained (n = 22), median overall survival was 49 months compared with 21 months in patients who failed to respond by either M spike or PCLI after induction therapy (P = .03). If both a M spike and PCLI response occurred (n = 59), there was a nonstatistically significant trend toward improved overall survival of 62 months versus 47 months (P = .32) in patients who achieved a M spike but not PCLI response (n = 33).
Discussion
In this study, we evaluated reduction in plasma cell proliferative rate as an additional metric for assessing response to therapy. Because the proliferative compartment in myeloma is usually small, a reduction may not necessarily produce a significant decrease in M protein. The baseline proliferative rate is a known independent predictor of survival, and because it is easier biologically and mathematically to meet criteria for response when the starting values are greater, we stratified patients in to 3 groups on the basis of the PCLI level before therapy. Our findings show that in patients with intermediate- and high-risk initial plasma cell proliferation values, a reduction in the proliferative rate after initial therapy identifies patient subsets with significantly different survival rates. Similar median overall survival was demonstrated between PCLI responders and M-spike responders. The improved median overall survival in patients achieving reduction in the plasma cell proliferation after initial therapy measured by PCLI was independent of changes in M-protein levels as determined by multivariate analysis. With the combined application of M-spike and PCLI response, a distinct subset of patients with improved survival was identified. Interestingly, more than one-third of patients meeting M-spike response criteria did not achieve a PCLI response; conversely, nearly 30% of patients with a PCLI response did not achieve a greater than 50% reduction in the M protein. A trend toward improved median overall survival was present in patients who responded by both PCLI and M spike; however, our study was underpowered for this analysis and was unable to demonstrate statistical significance. These findings argue for a need to further study possible integration of these 2 biomarkers (M protein and proliferative rate) to comprehensively assess response to therapy.
Our results, if confirmed, may be important for clinical practice for several reasons. The aforementioned summarized data provide support for the concept that reduction in plasma cell proliferative rate after therapy is clinically beneficial and is independent of M-component response. Assessment of the malignant proliferative component could be complementary to the currently used M-component response criteria and may provide the clinician a more comprehensive approach when considering response to therapy. For example, patients who do not achieve an adequate response as defined by a significant reduction in plasma cell proliferative rate may require change in therapy, even though their M protein levels indicate achievement of an objective response. The PCLI was used in this study because it has repeatedly been shown to be a powerful prognostic tool in newly diagnosed myeloma patients and provided an accurate measure of the malignant plasma cell proliferative rate.10,18-20
In addition, in the current setting of numerous effective myeloma treatment options, assessment of plasma cell proliferation in phase 1/2 clinical trials may be a valuable method of identifying novel agents.21 Novel agents that do not reduce M-protein levels significantly because their effect may be primarily on the slow-growing but nevertheless critical small proliferative compartment of plasma cells in myeloma may be discovered with use of the PCLI or another measure of change in proliferation. In such trials, a reduction in the measured plasma cell proliferative rate may be a better marker for success of therapy and hence needs to be explored further.
Clearly, a more widely available and reproducible tool to assess proliferative capacity would offer significant clinical benefit compared with the PCLI for incorporation into practice and in clinical trials. Flow cytometry has become widely available in most clinical centers and is a promising method for assessment of the proliferative component of malignant plasma cells. A recent study by Minarik et al22 demonstrated that the combined assessment of proliferation in conjunction with apoptosis is a strong prognostic factor that is able to identify proliferation subtype and outcome. Multiparameter flow cytometry also allows the detection of small numbers of plasma cells, which may be missed by less-sensitive morphologic and immunohistochemical evaluation, improving the evaluation of minimal residual disease after treatment.23-25 Use of the PCLI has not gained widespread acceptance largely because of its labor-intensive fluorescent microscope slide-based method. At our institution, the labeling index is commonly obtained at diagnosis and applied clinically as part of a risk-adapted therapy strategy.2
Our data do confirm that reduction of M protein remains an important surrogate end point in myeloma because patients who achieved PR, VGPR, or complete response had improved median overall survival. Median overall survival was similar between the VGPR and PR groups, a finding that is in contrast to previous large, prospective trials involving induction therapy.26-30 Of note, Martinez-Lopez et al31 found no difference in survival between the VGPR and PR group after long-term follow-up in patients after stem cell transplantation. The patient population in our study was not representative of the full spectrum of myeloma patients at initial diagnosis as those with a PCLI of < 0.5% were excluded.
Limitations of our study include the retrospective nature of the design and the heterogeneous therapy administered during a 2-decade period. To avoid bias resulting from different available follow-up time, overall survival was censored after 72 months. Only one-third of patients in this study were treated with the novel biologic agents currently in use, such as thalidomide, lenalidomide, and bortezomib as front-line therapy. The limited availability of the PCLI will restrict its widespread use. However, the emphasis of this study is not on the use of a specific test but rather the concept of the importance of proliferation of myeloma plasma cells in assessing prognosis. Proliferation may be better measured by the use of multiparametric flow cytometric methods, which can be more widely used; such studies need to be performed. We conclude that a significant reduction in plasma cell proliferative rate in patients with newly diagnosed multiple myeloma is an important predictor of survival and is therefore an additional marker of response to therapy. On the basis of our results, we recommend that further studies be undertaken to evaluate reduction in myeloma cell proliferative rate as an additional marker of response assessment.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by the Jabbs Foundation (Birmingham, United Kingdom); National Cancer Institute grants CA107476, CA 62242, CA100707, and CA83724; and the Predolin Foundation.
National Institutes of Health
Authorship
Contribution: J.T.L. collected and analyzed data and wrote the manuscript; C.E.C. analyzed the data; S.V.R. designed and conducted research and analyzed data; and J.A.L. and P.R.G. designed and conducted research.
Conflict-of-interest disclosure: P.R.G. holds a patent and has collected royalties for the plasma cell labeling index. All other authors declare no competing financial interests.
Correspondence: S. Vincent Rajkumar, MD, Division of Hematology, Mayo Clinic, 200 First St SW, Rochester, MN 55905; e-mail: rajks@mayo.edu.