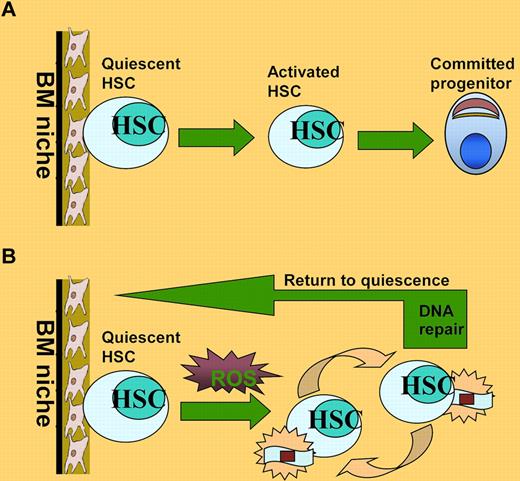
In this issue of Blood, Yahata and colleagues demonstrate that reactive oxygen species (ROS)–induced DNA damage impairs the self-renewal capacity of human HSCs, and that oxidative DNA damage repair is less efficient in quiescent stem cells than in progenitor cells.1
HSCs are a rare population of pluripotent cells that can self-renew and produce various types of cells of the blood lineage. Under steady physiologic conditions, the most primitive HSCs are in a quiescent state and reside in the BM niche where they preserve the capacity to self-renew and to continue to produce all types of blood cells throughout a prolonged lifespan.2 In response to stress or stimulation, the HSCs can move out of the BM niche, entering cell cycle and undergoing division (see figure panel A). In addition, the cycling HSCs may return to the BM niche and regain their quiescent state.3 Disruption of HSC quiescence prematurely exhausts the HSC pool and causes hematologic failure under various stresses, such as oxidative, replicative, and metabolic, and DNA damage.4
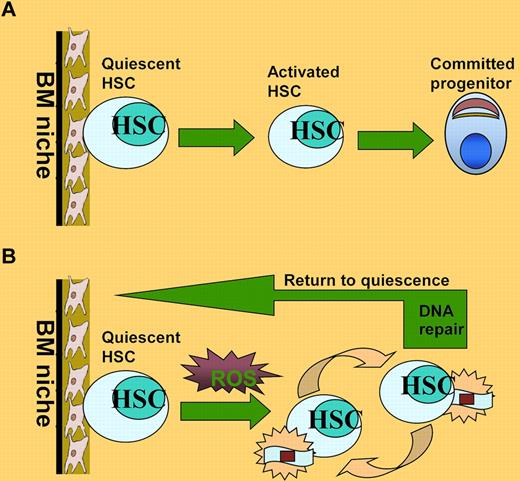
HSC maintenance under oxidative stress. (A) Under steady-state conditions, HSCs are maintained in a quiescent state and reside in the BM niche where they preserve the capacity to self-renew and to continue to produce all types of blood cells throughout a prolonged lifespan. (B) ROS generated by replicative stress during serial transplantation can induce DNA damage and drive HSCs into cell division, which could cause progressive loss of HSC functions. After successful repair of the oxidative DNA damage, HSCs can return to quiescent state.
HSC maintenance under oxidative stress. (A) Under steady-state conditions, HSCs are maintained in a quiescent state and reside in the BM niche where they preserve the capacity to self-renew and to continue to produce all types of blood cells throughout a prolonged lifespan. (B) ROS generated by replicative stress during serial transplantation can induce DNA damage and drive HSCs into cell division, which could cause progressive loss of HSC functions. After successful repair of the oxidative DNA damage, HSCs can return to quiescent state.
HSCs are exposed to various ROS, which are routinely generated during metabolic or inflammatory process (reviewed in Naka and Hirao5 ). ROS induce a variety of responses in HSCs, including cellular proliferation and apoptosis. ROS can also cause DNA damage and drive HSCs into cell division, which appears to be essential for DNA repair processes.6 Recent studies in mice suggest that mechanisms involving antioxidant defense and DNA repair appear to be essential for the maintenance of HSC self-renewal capacity and the suppression of malignant transformation. Indeed, mice with mutations in several oxidative stress response (Atm, Fancc, Fancd2, FoxO) and DNA damage repair (Lig4, Dna-pk, Ku80, Xpd, mTR) genes exhibit premature exhaustion of HSCs because of accumulation of ROS or DNA damage.4,5,7
Less is known about how oxidative DNA damage affects the function and lifespan of human HSCs. Yahata and colleagues use a strategy of detecting the in vivo repopulating dynamics of individual human HSCs to test the hypothesis that the continuous production of ROS during long-term repopulation induces an accumulation of DNA damage that leads to exhaustion of human HSCs. The authors have previously reported that the repopulating potential of human HSCs progressively deteriorated as they went through extensive repopulation process.8 In the present study, Yahata et al demonstrate that the decreased repopulating capacity of human HSCs during serial transplantation is accompanied by increased ROS and DNA damage in the repopulating donor HSCs (see figure panel B). Interestingly, the authors observed that higher DNA damage remained unrepaired in quiescent HSCs than in cycling progenitors. In addition, the oxidative DNA damage increases expression of cell-cycle inhibitors, causing HSCs to undergo premature senescence and consequently leading to the functional impairment of HSCs in vivo. Finally, treatment with the antioxidant NAC can abrogate the effect of oxidative DNA damage on HSC function.1
These results underscore the importance of oxidative DNA damage repair to maintaining the function of HSCs. One intriguing aspect of the findings in the study by Yahata et al is that actively cycling progenitors (Lin−CD34+CD38+) accumulate much less ROS or DNA damage than relatively quiescent HSCs (Lin−CD34+CD38−) do. In explaining why and how the HSCs and the progenitors respond differently to the same DNA damage, the authors argue that progenitor cells may either possess more efficient DNA repair mechanisms or simply purge the severely damaged cells. This would be consistent with a notion that compared with quiescent HSCs, proliferating progenitors are more resistant to DNA damage.4 Nevertheless, this finding is contrary to 2 recent reports showing that common myeloid progenitors in mice and flies produce significantly increased levels of ROS compared with HSCs.9,10 One possible explanation for this discrepancy is that the repopulated progenitors may have arisen from HSCs containing low ROS.
Another intriguing finding by Yahata et al is the observation that the cell-cycle status of human HSCs can reversibly change from quiescence to oxidative stress–induced activation during repopulation (see figure panel B). Analysis of BrdU incorporation shows that at 2 weeks after transplantation, Lin−CD34+CD38− cells exited quiescence and underwent activation and expansion. However, these proliferating Lin−CD34+CD38− cells were able to regain quiescence at the later phase of primary transplantation as well as in serial transplantation. This finding raises important questions as to whether activation is required for efficient repair of oxidative DNA damage in stressed HSCs and whether elimination of the DNA damage is sufficient for the activated HSCs to return to quiescent state. Although further evidence needs to be provided, it is tempting to speculate that reversibility between quiescence and activation may be a physiologic function of activated HSCs at the interface between damage repair and re-establishment of homeostasis. In this context, it is noteworthy that a recent study in mice shows that HSCs reversibly switch between dormancy and self-renewal at the interface between homeostasis and repair.6
One major caveat associated with these findings is that the study used Lin−CD34+CD38− population, which may be not homogenous but contain both stem cells and progenitors. Recent studies from the Dick group demonstrate that sorting based on CD49f identifies highly purified human HSCs.11 Nevertheless, the findings by Yahata and colleagues shed new light on oxidative DNA damage response in human HSCs and extend our understanding of HSC maintenance under stress. Moreover, the model of combined human xeno-transplant with antioxidant supports the view that antioxidants could be of considerable therapeutic value in the clinical setting of HSC transplantation.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■
REFERENCES
National Institutes of Health