Abstract
Allogeneic hematopoietic cell transplantation led to the discovery of the allogeneic GVL effect, which remains the most convincing evidence that immune cells can cure cancer in humans. However, despite its great paradigmatic and clinical relevance, induction of GVL by conventional allogeneic hematopoietic cell transplantation remains a quite rudimentary form of leukemia immunotherapy. It is toxic and its efficacy is far from optimal. It is therefore sobering that since the discovery of the GVL effect 3 decades ago, the way GVL is induced and manipulated has practically not changed. Preclinical and clinical studies suggest that injection of T cells primed against a single Ag present on neoplastic cells could enhance the GVL effect without causing any GVHD. We therefore contend that Ag-targeted adoptive T-cell immunotherapy represents the future of leukemia immunotherapy, and we discuss the specific strategies that ought to be evaluated to reach this goal. Differences between these strategies hinge on 2 key elements: the nature of the target Ag and the type of Ag receptor expressed on T cells.
Introduction
The GVL reaction refers to the ability of donor immune cells to eliminate host leukemic cells after allogeneic hematopoietic cell transplantation (AHCT). In 1956, Barnes et al1 were the first to report cure of leukemia in mice after total body irradiation and AHCT. Further studies of GVL in animal models were pioneered by Bortin et al2 in the 1970s.3 The relevance of the GVL reaction in humans was established by the Seattle group in 1979, and key insights into its mechanisms were reported in a landmark study from the International Bone Marrow Transplant Registry in 1990.4,5 Strikingly, the latter study that was based on data from 2254 subjects treated by 142 teams showed that GVL was abrogated if T cells were depleted from the graft or if the AHCT donor was an identical twin.5 On the basis of these data, it was therefore inferred that GVL depended on donor T cells and on the existence of histocompatibility differences between the donor and its recipient (absent among identical twins). Because the International Bone Marrow Transplant Registry study involved HLA-identical siblings, the sole histocompatibility differences between donors and recipients were minor histocompatibility Ags (MiHAs).6 The molecular nature of MiHAs was elucidated in 1990, and the first human MiHA was sequenced in 1995.7,8 Increasing recognition that cure after AHCT for leukemia is largely because of the GVL effect led to the introduction of nonmyeloablative conditioning regimens and donor lymphocyte infusions (DLIs).9,10 Remarkably, DLI can eradicate ≤ 1012 neoplastic cells in patients with chronic myelogenous leukemia,11,12 and the allogeneic GVL effect represents the most convincing evidence that immunotherapy can cure human neoplasias.13,14 Nowadays, AHCT can be viewed primarily as a quest for the GVL effect.12 In AHC transplant recipients, the effect of GVL on leukemia-free survival is so important that it supersedes the deleterious effect of GVHD and protracted immunodeficiency.
After conventional AHCT, GVL results from alloreactivity
After conventional AHCT (unmanipulated graft), GVL is T-cell dependent: it is abrogated after T-cell depletion of the graft, or when the leukemic cells are MHC-deficient and therefore cannot interact with donor T cells.5,15 Studies of numerous experimental models have firmly established that GVL specifically depends on recognition of host histocompatibility Ags on leukemic cells. Indeed, (1) GVL is not observed after syngeneic AHCT5 ; (2) when parental strain rats (A and B) were used as donors for F1 hybrid recipients (AB) bearing leukemic cells derived from parental strain A, both A and B donors induced GVHD, but only B-strain donors induced GVL16 ; (3) in H2b and H2bm1 recipients bearing H2b leukemic cells, GVHD follows H2b→H2bm1 and H2bm1→H2b AHCT, but GVL is elicited only in H2bm1→H2b AHCT17 ; (4) GVL can be enhanced by pre-AHCT donor immunization against host MHC or MiHAs2,3 ; and (5) GVHD, which results from alloreactivity, correlates with low leukemic relapse rates after AHCT.4,18 Two conclusions can be inferred from these studies. First, in MHC-identical donor-recipient pairs, the most common situation in human AHCT, GVL depends on donor T cells that recognize host MiHAs. Second, that GVL does not occur in the presence of GVHD targeted to Ags absent on leukemic cells16,17 means that GVL requires direct interactions between donor T cells and leukemic cells and cannot be ascribed solely to paracrine (eg, cytokine-mediated) effects of GVHD.
Natural killer (NK) cells, which rapidly recover to normal blood levels after AHCT, may also contribute to the GVL effect.19,20 However, for reasons that remain unclear, NK cells display antileukemic activity predominantly if not exclusively against myeloid leukemias. Depending on the context, NK cell–mediated GVL may involve MHC I–dependent and –independent mechanisms. NK cells express inhibitory killer immunoglobulin-like receptors (KIRs) that prevent NK-cell killing on binding to their cognate MHC I molecule on target cells. Benjamin et al20 and Ruggeri et al21 have shown in patients with acute myeloid leukemia (AML) treated by T cell–depleted haploidentical AHCT, that NK cells can kill AML cells that do not express the ligands for donor inhibitory KIRs. After HLA-matched AHCT (where inhibitory KIRs are irrelevant), several clinical studies suggest that expression of specific activating NK-cell receptors on donor cells is associated with a decreased risk of AML relapse.19,20,22 The nature of the (non-MHC) ligands recognized by activating NK receptors on myeloid cells remains unknown and is a subject of great interest. A more in-depth evaluation of the role of NK cells in GVL can be found in recent review articles.
Nature of MiHAs
MHC molecules present peptides at the cell surface. Under normal circumstances (in the absence of infection), all of these peptides originate from proteolytic degradation of cell proteins.23 Some MHC-associated peptides (MAPs) are polymorphic; they are present in some persons, but in other MHC-matched subjects they are absent or present a slightly different amino acid sequence.7,24 For historical reasons, polymorphic MAPs are referred to as MiHAs. They are a consequence of any form of accumulated genetic variation that hinders MAP generation (eg, gene deletion) or the structure of a MAP (eg, single nucleotide polymorphisms).6,24,25 MiHAs are essentially genetic polymorphisms viewed from a T-cell perspective.
Do leukemia-associated Ags contribute to the GVL effect?
Hundreds of tumor Ags have been found on various types of neoplastic cells, and leukemia cells are no exception to this rule.26 A comprehensive and up-to-date database on human T cell–defined tumor Ags can be found at http://www.cancerimmunity.org/peptidedatabase/Tcellepitopes.htm. Although some leukemia-associated Ags (LAAs) are only overexpressed on leukemic cells (relative to normal cells), other LAAs are truly specific to leukemic cells. Such Ags should therefore be seen as neo-Ags by donor T cells. Therefore, donor T cells would be expected to respond at least to some host LAAs. However, the lack of GVL in the absence of alloreactivity (eg, identical twins) means that LAAs are not sufficient to elicit curative antileukemic response after conventional AHCT. This conclusion must be tempered however, by the fact that the amount of data on GVL after syngeneic AHCT in humans remains limited. Further studies of anti-LAA immune responses in the syngeneic setting would be of great interest. Furthermore, despite the lack of direct evidence that LAAs play a role in GVL, evidence suggests that LAAs are immunogenic and might contribute to GVL initiated by MiHA-responsive T cells. Expansion of donor T cells specific for the PR1 LAA (an HLA-A2–associated peptide derived from proteinase 3) was found after AHCT in subjects treated for chronic myelogenous leukemia.27 Likewise, expansion of CD8 T cells specific for the WT1 LAA correlated with the occurrence of GVL in 10 subjects treated for acute lymphoblastic leukemia.28 Moreover, recent studies provide compelling evidence that MiHA-triggered GVL initiates Ag spreading that leads to T-cell responses against LAAs and B-cell responses against differentiation Ags. Thus, after AHCT in patients with chronic lymphocytic leukemia, achievement of remission correlated with the development LAA-specific T cells and antibodies against B-cell lineage Ags.29,30 Furthermore, in a human subject with melanoma, vaccination with MAGE-A Ags led to expansion of CD8 T cells specific for a tumor-specific peptide that was not present in the vaccine, and the latter T cells contributed to regression of melanoma metastases.31 Finally, in an MiHA-mismatched mouse model of nonmyeloablative AHCT for treatment of prostate cancer, post-AHCT vaccination of recipients against tumor Ags enhanced the graft-versus-tumor effect, thereby suggesting that MiHA-specific T cells may collaborate with tumor-specific T cells.32 Together, these studies suggest that, although alloreactivity is necessary, it may not be sufficient for effective GVL which may also require immune responses against LAAs.
Why are T cells specific for host MiHAs more effective than LAA-specific T cells for leukemia immunotherapy? The potential role of immunoediting
The immune system acts as an extrinsic tumor suppressor that neoplastic cells must evade to survive. One key component of immunoevasion is immunoediting whereby neoplastic cells that express highly immunogenic tumor Ags are eliminated.33 Down-regulation of immunogenic tumor Ags is used by tumor cells to evade immune surveillance.34-36 According to this paradigm, leukemic cells have undergone negative selection pressure by recipient's LAA-specific T cells, and the sole leukemic cells that were “successful” are those that did not express highly immunogenic LAAs. However, before AHCT, leukemic cells have never encountered MiHA-specific T cells (which are present only in allogeneic subjects) and have not “evolved” an evasive strategy toward MiHA-specific T cells; even leukemia cells with highly immunogenic MiHAs were under no negative selection pressure. We therefore surmise that because of immunoediting, leukemic cells are prepared to evade LAA-specific but not MiHA-specific T cells: leukemic cells do not express highly immunogenic LAAs but can express highly immunogenic MiHAs. Furthermore, with rare exceptions, donor T cells have never encountered host MiHAs and have not been tolerized against them. We therefore propose that because of immunoediting, LAAs are less immunogenic than MiHAs, explaining the overwhelming importance of MiHAs to elicit a strong GVL. This hypothesis is testable and would require comparing the immunogenicity of human MiHAs and LAAs. Nonetheless, the putative advantage of MiHAs over LAAs is not permanent: after AHCT, immunoediting may affect immune responses against leukemic cells in many ways and may lead to reduced expression of all Ags expressed on leukemic cells.37
Relation between GVHD and GVL
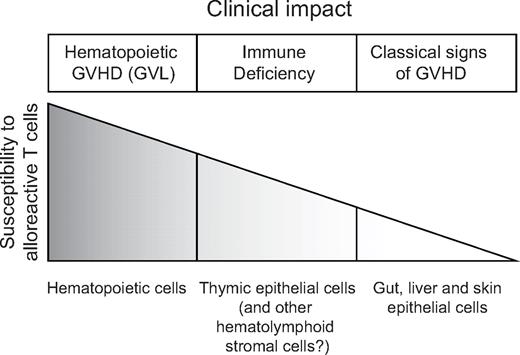
The most commonly asked question in the field of AHCT is whether GVHD and GVL can be separated. It is generally accepted that GVHD and GVL are linked but can be separated in several clinical and experimental models. We do not want to provide a comprehensive discussion of all relevant experimental models because this fundamental issue has been addressed in excellent review articles.38-41 Nonetheless, we would like to address the GVHD-GVL conundrum from a different perspective by asking the following question: if both GVHD and GVL result from alloreactivity (directed specifically against MiHAs in the case of MHC-matched persons), how can alloreactive T cells spare normal cells (no GVHD) and kill leukemic cells (GVL)? We propose a simple answer that integrates data from numerous reports. We posit that the susceptibility to alloreactive T cells is different for various cell types and can be ranked as follows: hematolymphoid cells > thymic epithelial cells > epithelial cells from the gut, liver, and skin > other organs that are spared by GVHD (eg, heart, kidney) (Figure 1). Implicit to this model, the clinical correlates of hematolymphoid GVHD are complete donor chimerism and GVL, whereas destruction of thymic epithelial cells causes immune deficiency, and damage to other epithelial cells leads to typical clinical signs of GVHD.
Schematic working model for the relation between susceptibility to alloreactive T cells and GVHD and GVL. Of note, organs such as the heart and kidney are spared by GVHD and are therefore not shown in this figure.
Schematic working model for the relation between susceptibility to alloreactive T cells and GVHD and GVL. Of note, organs such as the heart and kidney are spared by GVHD and are therefore not shown in this figure.
The idea that hematolymphoid cells are more sensitive than other cell types to alloreactive T cells is supported by several observations: (1) in patients who do not receive any myeloablative regimen and have GVHD induced by blood transfusion, pancytopenia (because of BM aplasia) is the main cause of death42 ; (2) likewise, in mouse models of AHCT where nonmyeloablated recipients receive allogeneic lymphoid cells (but not hematopoietic progenitors), pancytopenia is the first sign of GVHD43,44 ; (3) after conventional AHCT, the rate of clearance of recipients' hematolymphoid cells in the early posttransplantation period predicts the occurrence of GVHD in humans45 ; and (4) after DLI, conversion from mixed chimerism to full donor chimerism (elimination of host hematolymphoid cells) is an absolute prerequisite for the occurrence GVL and is often but not always followed by “epithelial GVHD.”12
Proliferation of alloreactive T cells is initiated in secondary lymphoid organs.46 Activated T cells must then extravasate from secondary lymphoid organs and infiltrate epithelia to cause GVHD.47 The thymic epithelium is excessively sensitive to damage even by very small numbers of alloreactive cells that can be detected in the thymus as early as days 2 or 3 after AHCT.48,49 Thymic GVHD of course hampers immune reconstitution.50,51 In addition, thymic failure is a watershed event that enhances GVHD in 2 ways: by preventing generation of natural CD4 regulatory T cells that mitigate GVHD52-54 and by allowing thymic export of host-reactive T cells that have not undergone proper negative selection.55 Alike thymic epithelial cells, stromal cells in other hematolymphoid organs are damaged by alloreactive T cells. Thus, after MHC-mismatched AHCT, alloreactive CD4 T cells were found to rapidly eliminate BM osteoblasts.56 Destruction of osteoblasts and of other constituents of the hematopoietic stem cell niche (eg, Nestin+ mesenchymal stem cells57 ) could contribute to GVHD-associated BM hypoplasia. In addition, evidence suggests that GVHD-induced damage to the stroma of secondary lymphoid organs hampers reconstitution of peripheral T-cell pools: T cells from lymphopenic GVHD+ mice are able to expand normally when transferred into GVHD− mice, but injection of T cells from healthy donors does not correct the T-cell deficit of GVHD+ mice.50 Finally, host-reactive T cells infiltrate many target organs such as the skin, liver, and gastrointestinal tract; recruit other cell types; and orchestrate complex immunopathologic events.58-60
In the proposed model, GVL induced by conventional AHCT simply results from hematolymphoid GVHD. Accordingly, the question of whether GVL can be separated from GVHD might be formulated differently: can GVHD be limited to the hematolymphoid compartment? We believe that models reporting on preservation of GVL without GVHD essentially represent systems in which GVHD occurs but remains limited to hematolymphoid cells. An attractive way to dampen alloreactivity and thereby limit GVHD to the hematolymphoid compartment is to manipulate the numbers of regulatory T cells.61,62 However, as long as we are unable to separate alloreactive T cells that mediate GVHD and GVL, transforming a graded phenomenon (the strength of alloreactivity) into a binary output (hematolymphoid GVHD but no systemic GVHD) may remain difficult.
From conventional AHCT to Ag-specific adoptive T-cell immunotherapy
The term adoptive T-cell immunotherapy (ATCI) refers to transfusion of T lymphocytes that may come from different types of donors: the patient (autologous), a genetically identical donor (syngeneic), or a nonidentical donor (allogeneic). Conventional AHCT represents a simple form of ATCI because it involves transfer of unselected T cells. Despite its great paradigmatic and clinical relevance, the GVL effect induced under these conditions is still a quite rudimentary form of leukemia immunotherapy. First, it lacks specificity and is therefore highly toxic; unselected allogeneic T cells react against a multitude of host MiHAs and thereby induce GVHD in 60% of recipients. Second, it induces only an attenuated form of GVL reaction because donor T cells are not being primed (preactivated) against specific Ags expressed on leukemic cells before injection into the patient. Although primed T cells are resistant to tolerance induction, naive T cells can be tolerized by tumor cells.63-66
More recently, ATCI with Ag-specific “activated” T cells has been used, mainly in 3 distinct settings. First, autologous T cells harvested from tumors (and presumably specific for tumor Ags) and expanded in vitro in the presence of IL-2 are being used for treatment of metastatic human solid tumors.67,68 Second, ATCI with allogeneic CD8 T cells directed against viral Ags (expanded in vitro in the presence of their cognate Ag) is used with great success for treatment of human CMV, EBV, and adenovirus infection.69,70 Finally, the use of allogeneic MiHA-specific T cells for cancer treatment has been evaluated in animal models and a landmark phase 1 clinical study.71-73 It must be stressed that the goal in ATCI is not to discover a “one size fits all” off the shelf blockbuster. It would be impossible to treat all hematologic malignancies by targeting the same Ag in all patients. ATCI should rather be considered as a form of personalized medicine in which the target epitope is determined by the HLA genotype of the subject and the proteome of the leukemic cells. For example, the PR-1 LAA is present uniquely on myeloid leukemic cells from HLA-A2+ subjects, and the HA-3 MiHA is expressed only on cells from HLA-A1 subjects bearing the R allele of the KIAA0020 gene.24,27 Because of these constraints, ATCI has been an academic endeavor led by a few centers with no input from large companies. We hope that things will change in the future, but, for the time being, cellular immunotherapy in general and ATCI in particular remain mostly academic enterprises. We therefore deem it important to present a tentative frame for translational research in ATCI of leukemia. To this end, we summarize herein the key insights gained from preclinical and clinical studies and present the different Ag-specific ATCI approaches that need to be explored for next-generation leukemia immunotherapy. The key features of these various approaches are summarized in Table 1. For the sake of brevity, we limit our discussion to ATCI with Ag-specific T cells and refer the reader to seminal articles for discussion of 2 other promising strategies for leukemia immunotherapy: injection of allogeneic NK cells and manipulation of regulatory T cells.21,61
Ex vivo generation of fit MiHA- and LAA-specific T cells
In healthy preimmune subjects, T cells that can recognize MiHAs and LAAs are mostly if not exclusively in the naive T-cell compartment. The frequency of Ag-specific T cells in naive persons is ∼ 10−5, and ∼ 109 T cells are needed for ATCI of leukemia.72,74 This means that Ag-specific ATCI requires massive expansion of Ag-specific T cells, which has to be performed ex vivo in humans. Unfortunately, most methods for ex vivo expansion lead to an exhaustion of Ag-primed T cells.72,75 Exhausted T cells have shortened telomeres and lose functional attributes in a hierarchical manner, beginning with the ability to produce IL-2 and to proliferate in vivo.76 That is a big drawback because a major study of ATCI in patients with metastatic melanoma has shown that objective antitumor response correlated with longer telomeres of the infused cells and their long-term persistence in vivo.77 After Ag stimulation, the fittest T cells in terms of self-renewal potential are memory stem cells (TSCM, CD44loCD62LhiSca-1hiCD122hiBcl-2hi) and, to a lesser extent, central memory T cells (TCM, CD44hiCD62Lhi).78,79 The challenge here is therefore to develop ex vivo culture conditions that promote expansion of TSCM and TCM as opposed to effector memory T cells (TEM, CD44hiCD62Llo). Recent reports suggest that 2 strategies may help achieve this goal. First, Gattinoni et al80 have found that induction of Wnt-β-catenin signaling blocked differentiation of CD8 T cells into TEM and promoted the generation of TSCM with substantial proliferative and antitumor properties. Second, 2 teams have reported that provision of specific metabolic conditions during Ag stimulation (eg, inhibition of the mammalian target of rapamycin pathway or modulation of fatty acid metabolism) may promote the generation of memory as opposed to effector T cells.81,82 Further studies are needed to evaluate the merits of these various approaches for ex vivo generation of clinically relevant numbers of fit Ag-specific TCM and TSCM.
Another attractive strategy for ex vivo production of MiHA- or LAA-specific T cells is to transfect Ag-specific TCRs in polyclonal T-cell populations. This approach has 2 main advantages: it minimizes the need for T-cell expansion and it is flexible because TCR transgenes can be inserted in a variety of autologous or allogeneic T-cell subsets.79,83 Transfecting MiHA-specific TCRs into autologous T cells would obviate the need for an allogeneic donor. Care must be taken, however, to avoid host reactivity that might result from mispairing of introduced TCR chains with endogenous TCR chains.84 In addition, it is noteworthy that the T-cell population reactive to any given Ag is usually diversified and contains polyclonal TCRs. It is therefore theoretically possible that ATCI with monoclonal TCRs might be less effective than with polyclonal TCRs. Furthermore, the specific T-cell subset in which TCR should be inserted remains controversial. A report from Hinrichs et al85 suggests that when autologous cells are used for ATCI, naive T cells offer the best potential. Relative to TCM and TEM, naive T cells retained longer telomeres and displayed minimal signs of exhaustion. However, injection of (TCR-transfected) allogeneic naive T cells might be dangerous because GVHD is induced primarily by naive (MiHA-responsive) T cells.86 For allogeneic cells, TCR transfection into TCM might therefore represent a safer alternative.79,87
MiHA-targeted ATCI of leukemia
GVL mediated by T cells specific for a single MiHA
Estimates based on mouse data suggest that the number of MiHA differences between 2 persons may be ∼ 30-50.6,88,89 This raises the question, how many MiHAs must be recognized or targeted to induce GVHD and GVL? When T cells reactive to a single host MiHA were injected into irradiated recipient mice, no GVHD has ever been observed. This test has been done with 25 different MiHAs, using naive donors or donors specifically primed against the target MiHA.71,90,91 Hence, at least in mice, it is impossible to induce GVHD by injecting T cells targeted to a single MiHA, even when the MiHA is ubiquitously expressed. The number of MiHAs that need to be recognized to elicit GVHD is unknown. By contrast, T cells primed against a single MiHA can eradicate leukemic cells as long as the target MiHA is immunodominant (highly immunogenic).71,92 Of note, the innocuity of T cells targeted to a single minor was recently challenged when Warren et al72 documented lung injury in 3 recipients of anti-MiHA CD8 T-cell clones. The T-cell clones had been expanded in vitro in the presence of anti-CD3 Ab and IL-2. Whether lung toxicity in these subjects resulted from cognate interactions of the T-cell clones with the lung parenchyma as opposed to some non–Ag-specific toxicity of the activated T cells remains a matter of conjecture. Indeed, T cells expanded in vitro in the presence of IL-2 can cause lung toxicity by TCR-independent mechanisms, by launching an inflammatory reaction in the first organ where they are trapped.93,94 Nevertheless, on the basis of the report by Warren et al72 it would appear hazardous to treat patients with T cells whose target Ag is expressed in the lungs.
H7a (formerly B6dom1), the first immunodominant MiHA discovered in mice, has been studied extensively.95,96 H7 allelic products originate from a single nucleotide polymorphism in the Stt3b gene. The sequence of the H7 peptide is KAPDNRETL in H7a+ mice (eg, C57BL/10) and KAPDNRDTL in H7b+ mice (eg, C3H.SW).96 Injection of anti-H7a T cells cured not only leukemia but also established melanoma.71,73 Eradication of melanoma cells depended on 2 T-cell effector mechanisms: direct killing of neoplastic cells by granule exocytosis and inhibition of angiogenesis by IFN-γ. Importantly, even though H7a is ubiquitously expressed, injection of anti-H7a T cells caused neither GVHD nor any untoward toxicity (eg, vitiligo) to recipients. The efficacy and innocuity of anti-H7a T cells are explained, at least in part, by the fact that anti-H7a effector T cells express high levels of Vla-4,73 and that the ligand of Vla-4 (Vcam-1) is expressed almost exclusively on BM microvessels and tumor neovessels. Therefore, anti-H7a effector T cells extravasate primarily in the BM and the tumor bed, where, in the presence of their target MiHA, they proliferate rapidly and extensively and kill neoplastic cells.73,97,98 It should be of interest to determine whether up-regulation of Vla-4 is a general feature of MiHA-specific effector cells and whether Vcam-1–dependent homing of MiHA-reactive T cells to the BM and solid tumors might explain that hematopoietic cells (Figure 1) and solid tumors are particularly susceptible to MiHA-specific T cells.73 The notion that solid tumors can be more susceptible to T-cell attack than nonhematopoietic cells has been supported in a study in which mice with intracranial melanoma were immunized against a melanoma-associated Ag, dopachrome tautomerase, which normal melanocytes and glial cells also express. Quite remarkably, immunotherapy led to rejection of intracranial tumor cells without damaging the brain despite sharing the target Ag.99 Although the mechanisms regulating the susceptibility of nonhematopoietic tissues and tumors to T-cell attack have yet to be worked out, studies on H7a and dopachrome tautomerase suggest that, at least in certain contexts, solid tumors may be unduly sensitive to T-cell attack.
In a phase 1 study reported in 2010, 7 patients with recurrent leukemia after AHCT were treated with donor-derived ex vivo–expanded MiHA-specific CD8 T-cell clones. Two salient findings emerged from that study.72 First, injected T cells migrated to the BM, and 5 of 7 patients achieved complete remission. However, adoptively transferred T cells failed to persist in vivo and remissions were transient. This important study shows that T cells targeted to a single MiHA can induce a potent GVL reaction in humans. However, it also shows that current methods for ex vivo expansion of T cells specific for noninfectious epitopes are inadequate.
Discovery of human MiHAs
Studies of leukemia immunotherapy with anti-H7a CD8 T cells suggest that it may be possible to obtain a strong GVL without GVHD by targeting MiHAs that have a wide tissue distribution. However, we believe that MiHAs with a wide tissue distribution are not ideal targets; “Ag excess” promotes functional exhaustion and physical demise of MiHA-reactive T cells.98,100 Thus, ideal targets would be MiHAs expressed on hematopoietic cells.101 At the present time, only 30% of patients with leukemia would be eligible for hematopoietic MiHA-based ATCI because of the limited number of human MiHAs that have been molecularly characterized.102 Hence, it is imperative to develop and exploit high-throughput methods for MiHA discovery.103,104
Is a single Ag target sufficient for successful ATCI of leukemia?
Irrespective of the nature of the Ag (MiHA, LAA, cell-surface molecule), it may seem surprising that targeting a single Ag would be sufficient to cure leukemia or other types of cancer. A priori, injection of monospecific T cells should increase the risk of immune escape by selection of Ag loss variants. However, we contend that infiltration of the BM or solid tumors by activated T cells specific for a single Ag launches 2 series of events that facilitate eradication of all neoplastic cells, including Ag loss variants: angiostasis and epitope spreading. First and foremost, although all neoplasias (including leukemias) depend on generation of neovessels, IFN-γ and TNF-α secreted by activated T cells are perhaps the most potent antiangiogenic molecules available. As a consequence, secretion of these 2 cytokines in the tumor bed leads to ischemic death of neoplastic cells and bystander eradication of Ag loss variants.73,105,106 Notably, the antiangiogenic effect of IFN-γ was essential for eradication of melanoma cells by anti-H7a T cells.73 Second, in several models killing of tumor cells by cytotoxic T lymphocytes initiates Ag spreading, that is, priming and recruitment and of T-cell clones specific for Ags (MiHAs and LAAs) different from the initial Ag target.29,31,32 Ag spreading can even extend to B-cell responses against cell-surface molecules.30
Nevertheless, an alternative approach to leukemia immunotherapy pioneered by Bordignon et al107,108 has also yielded promising results: injection of polyclonal allogeneic lymphocytes transduced to express a suicide gene. Expression of a suicide gene confers to cells sensitivity to a molecule (prodrug) that is nontoxic to nonexpressing cells. Thus, when recipients of genetically modified lymphocytes present signs of GVHD, the prodrug is administered to terminate the antihost alloreaction. In essence, this strategy exploits the fact that hematopoietic cells are more susceptible than epithelial cells to attack by alloreactive T cells and that brisk termination of GVHD may not totally abrogate the GVL reaction (Figure 1). In a landmark study, 23 patients received donor lymphocyte infusions with lymphocytes transduced to express the HSV-TK suicide gene for relapse of hematologic malignancies occurring after AHCT.107 Suicide gene transfer is safe and allows at least for some level of GVL activity, but it is limited by the development of transgene-specific CD8 T-cell immune responses that limit the in vivo persistence of genetically modified cells.107,109 Therefore, several new cell and gene transfer approaches are being evaluated in phase 1-2 clinical studies.108
LAA-targeted ATCI of leukemia
The lack of evidence that LAAs contribute to the GVL after conventional AHCT suggest that naive LAA-specific T cells are not sufficient to elicit curative antileukemic responses. Therefore, ATCI of leukemia with LAA-specific T cells remains largely unexplored. However, it remains possible that activated LAA-specific T cells may have more potent antileukemic activity than naive LAA-reactive T cells. Consistent with this, impressive results in patients with solid tumors treated by tumor Ag-targeted ATCI83,110,111 suggest that leukemia immunotherapy with LAA-primed T cells might have significant therapeutic potential. TCRs recognize MHC-associated peptides. Whether the peptide is an MiHA or an LAA, recognition by TCRs is MHC restricted. Accordingly, ATCI that is based on TCR recognition of LAA peptides is fraught with caveats similar to those encountered with MiHA-based ATCI (Table 1): (1) the need for individualized Ag targeting; LAA targeting must be tailored according to the HLA genotype and the proteome of leukemic cells; and (2) irrespective of whether endogenous TCRs or transfected TCRs are used, generation of large numbers of fit LAA-specific T cells will be demanding. However, if high-frequency immunogenic LAAs are found, donor selection for LAA-targeted ATCI would be easier than for MiHA-targeted ATCI. In the case of LAA, any HLA-matched subject is a potential donor, whereas when targeting MiHAs, the donor must be HLA-matched and negative for the target MiHA. Furthermore, in the case of ATCI with non–TCR-transfected T cells, LAAs present another advantage over MiHAs: LAA-specific T cells can be generated from autologous T cells and do not require an allogeneic donor. To this end, it might be advantageous to expand LAA-specific T cells from ex vivo–generated naive autologous T cells that have not been subjected to immunoediting in vivo.112 However, T cells from a healthy allogeneic donor may have an advantage over autologous T cells: healthy subjects that have not been subjected to chemotherapy may have a superior thymic function and a more diversified T-cell repertoire.113
Targeting cell-surface molecules with chimeric Ag receptors
To circumvent the problem of MHC restriction inherent to targeting TCR epitopes, an alternative strategy is now being explored by several groups: targeting cell-surface molecules present on leukemic cells with T cells transfected with chimeric Ag receptors (CARs). CARs are typically composed of fusion proteins between single-chain variable fragments from monoclonal antibodies and intracellular signaling domains such as the CD3ζ-chain.114,115 Thus, CAR-expressing T cells see the world like B cells but react like T cells. Adoptive transfer of autologous anti–CD19-CAR-expressing T cells recently led to regression of a B-cell lymphoma (and of normal CD19+ cells) in a patient, providing a proof of principle that CARs represent a promising approach.116 Accordingly, anti–CD19-CARs are generating substantial enthusiasm, and their value will probably be evaluated in a multiple institutional trial.115 MHC-independent Ag recognition makes CARs broadly applicable. However, there is a significant caveat in seeing the world with a CAR (ie, with an Ab): you can only see cell-surface Ags. This is problematic because cell-surface Ags that are truly leukemia specific and can be recognized by Abs are exceedingly rare. In contrast, because TCRs recognize peptides derived from all cell compartments,117,118 all leukemic cells presumably express MHC-restricted targets (MiHAs and LAAs).
Perspective
The division of labor between B cells and T cells was recently found to be conserved in jawless and jawed vertebrates, in a remarkable example of convergent evolution.119 Over the past 500 million years, survival of all vertebrates on this planet has depended on a combination TCR- and Ab-mediated recognition. We anticipate that both recognition systems will prove valuable in ATCI of leukemia. Nonetheless, development of next-generation leukemia immunotherapy is fraught with 2 main obstacles. The first is “philosophical”: the vestigial (and soon obsolete) reluctance to move from blockbuster medicine to personalized medicine. The second is methodologic: we need to develop reliable methods for ex vivo generation of large numbers of fit Ag-specific T cells. We can envision many strategies that differ with regard to the nature of the target Ag and the type of Ag receptor expressed on T cells (Table 1). However, it would be impossible to evaluate in vivo the fitness of T cells generated with all Ag/receptor combinations. We therefore need to establish reliable criteria for in vitro prediction of in vivo T-cell fitness. We propose as a working hypothesis that after in vitro priming and expansion, it might be possible to predict the fitness of Ag-primed T cells by estimating their telomere length and polyfunctionality index (eg, expression of CD107, CCL4, and TNFα; production of IFN-γ and IL-2).77,120
Acknowledgments
K.V. is supported by a studentship from the Cole Foundation. Work on leukemia immunotherapy in the authors' laboratory has been supported by grants from the Canadian Cancer Society (C.P.) and the Fonds de la Recherche en Santé du Québec (D.-C.R.). C.P. holds a Canada Research Chair in Immunobiology.
Authorship
Contribution: K.V. and C.P. wrote the first draft of the paper. All authors wrote the final version of the article.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Claude Perreault, Institute for Research in Immunology and Cancer, PO Box 6128, Station Centre-Ville, Montreal, QC, Canada H3C 3J7; e-mail: claude.perreault@umontreal.ca.