Abstract
CD3+CD56+ cytokine-induced killer (CIK) cells display a potent cytolytic activity. The adhesion molecule lymphocyte function-associated antigen-1 plays a crucial role in binding as well as in cytolytic activity of CIK cells against tumor target cells expressing the corresponding ligands. CIK cells express activating natural killer (NK) receptors, including NKG2D, DNAX accessory molecule-1 (DNAM-1), and low levels of NKp30. Cell signaling not only through TCR/CD3 but also through NKG2D, DNAM-1, and NKp30 leads to CIK cell activation resulting in granule exocytosis, cytokine secretion, and cytotoxicity. Antibody blocking experiments showed that DNAM-1, NKG2D, and NKp30 are involved in the TCR-independent tumor cell recognition and killing. Anti–CMV-specific CIK cells could be expanded in standard CIK cultures and mediate both specific, MHC-restricted recognition and TCR-independent NK-like cytolytic activity against leukemic cell lines or fresh leukemic blasts. Antibody blocking of lymphocyte function-associated antigen-1 and DNAM-1 led to significant reduction of both CTL and NK-cell functions, whereas blocking of NKG2D and NKp30 only inhibited NK-like cytotoxicity. Their dual-effector function suggests that CIK cells, when used in a clinical setting, may control both neoplastic relapses and viral infections, 2 frequently associated complications in patients who received a transplant.
Introduction
Cytokine-induced killer (CIK) cells are ex vivo–activated lymphocytes that can be obtained in large numbers within 3 weeks of culture from either human peripheral blood or BM, or cord blood mononuclear cells by the sequential addition of IFN-γ, anti-CD3 Ab (OKT3), and high doses of recombinant human IL-2 (rhIL-2).1-4 CIK cells represent an heterogeneous cell population, including a large majority of CD3+CD56+ cells and minor fractions of typical T cells (CD3+CD56−) and NK cells (CD3−CD56+).5,6 CIK cells can lyse a broad array of tumor targets, including acute myeloid leukemia, chronic myelogenous leukemia, and chronic lymphocytic leukemia by a non–MHC-restricted, natural killer (NK)–like mechanism.3,6 Interestingly, in mice, CIK cells display negligible alloreactivity and cause minimal GVHD compared with allogeneic splenocytes, when infused after allogeneic BM transplantation in murine models.7,8 CIK cells express several chemokine receptors and can migrate to the site of tumors after intravenous administration, as shown by models in vivo.9-13 Because CIK cells could be produced by a simple approach and displayed antitumor activity in vitro, they appeared to be suitable candidates for cell therapy in solid and hematopoietic tumor treatment. Indeed, both autologous and allogeneic CIK cells have been used in phase 1 and 2 clinical trials for the treatment of different tumor types. In these trials, they displayed limited toxicity, whereas evidence has been obtained that they exert antitumor activity.14-20
The molecular structures that account for tumor recognition and killing by CIK cells are only partially understood. Previous observations suggested a possible involvement of the NKG2D and lymphocyte function-associated antigen-1 (LFA-1) molecules21-23 ; however, little is known on the role of other activating NK receptors, including DNAX accessory molecule-1 (DNAM-1), NKp30, NKp44, NKp46, as well as on the involvement of TCR/CD3 complex in the cytolytic activity of CIK cells.
Our previous studies clarified that CD3+CD56+ CIK cells have phenotypic characteristics typical of terminally differentiated CD8+ effector memory T cells (TEMRA; CCR7−, CD45RA+, CD62Llow, CD11a+, CD27+, CD28−). We also showed that CIK cells originate in vitro from CD56−CD8+ T-cell progenitors that strongly expand on culture in the presence of IL-2 and acquire CD56 antigen.5 In addition, CIK cells share several characteristics with NK cells, such as the large granular lymphocyte structure, the capacity to kill the HLA class I–negative cell line K562, and the surface expression of high densities of CD56 and NKG2D. Different from NK cells, however, CIK cells express low densities of NKp30, whereas they do not express NKp44 and NKp46, and the inhibitory killer immunoglobulin-like receptors, NKG2A and CD94.5
In the present study, we investigated in detail the receptors involved in the NK-like cytolytic activity of CIK cells and analyzed whether they have retained also their ability to elicit specific cytolytic effector T-cell function.
Methods
Cells
The human cell lines BJAB (B lymphoma), KARPAS422 (B lymphoma), MOLT4 (T-cell leukemia), RAJI (B-cell leukemia), and K562 (erythroleukemia) were maintained in RPMI-1640 medium (Cambrex Bio Science) supplemented with 10% FBS (Euroclone), 2mM l-glutamine (Euroclone), and 110μM gentamycin (PHT Pharma), hereafter called complete medium.
Leukemic cells, obtained by Ficoll-Hypaque density centrifugation of peripheral blood samples, were used for cytolytic assays after thawing.
PHA-induced blasts were obtained by stimulating peripheral blood lymphocytes with 1 μg/mL PHA (Sigma-Aldrich) and 120 U/mL rhIL-2 (Proleukin; Chiron).
Generation of CIK cells
CIK cells were prepared as previously described.5 Briefly, the CD56-depleted fractions separated from PBMCs by negative selection with the use of MACS (Miltenyi Biotec) with anti-CD56 microbeads (Miltenyi Biotec) were cultured in serum-free X-VIVO-15 medium (BioWhittaker) with 1000 U/mL IFN-γ (Gammakine; Boehringer Ingelheim) added on day 0, 50 ng/mL anti-CD3 (OKT-3; Janssen-Cilag S.p.a.) added on day 1, and 500 U/mL rhIL-2 included in the medium from day 1 onward. Expansion was performed for 21-28 days. At the end of the expansion, CD56+ CIK cells were immunoselected with anti-CD56 microbeads according to the manufacturer's instructions.
Anti–CMV-specific CIK cells were generated, as described earlier, starting from peripheral blood lymphocytes obtained from selected HLA-A*0201 CMV-seropositive healthy donors. Anti–CMV-specific lymphocytes were identified by flow cytometry after staining with PE-conjugated HLA-A*0201/pp65495-503 tetramer (Immunotech Laboratories, Beckman Coulter) and FITC-conjugated anti-CD8 Ab (Becton Dickinson). Anti–CMV-specific cells were isolated at the end of the expansion, using cognate PE-conjugated tetramers followed by separation with anti-PE Ab-coated microbeads (Miltenyi Biotec) and MACS (Miltenyi Biotec). After sorting, tetramer-positive cells were resuspended in X-VIVO-15 medium containing 500 U/mL rhIL-2 and irradiated allogeneic feeder cells and were cultured for 2 weeks. At the end of culture, CD56+ CMV-specific CIK cells were immunoselected.
Anti–EBV-specific CIK cells were generated from peripheral blood lymphocytes from HLA-A*0201-positive persons with recent onset of acute infectious mononucleosis. Anti–EBV-specific lymphocytes were identified by staining with PE-conjugated HLA-A*0201 EBV (GLCTLVAML) tetramer (Beckman Coulter).
mAbs and flow cytometric analysis
To characterize the surface expression of the activating receptor ligands on the tumor cell lines, the following panel of mAbs was used: anti–ULBP-1 (UL16 binding protein-1; 170818 clone), anti–ULBP-2 (165903 clone), anti–ULBP-3 (166510 clone), and anti-MICA/B (MHC class I–related chain molecules A/B; 159207 clone; R&D Systems). Anti–Nectin-2 (L14 clone) and anti-PVR (poliovirus receptor; L95 clone) were previously described.24 The adhesion molecules CD54 (ICAM-1), CD102 (ICAM-2), and CD50 (ICAM-3) were detected with PE or FITC-conjugated Ab clones HA58, CBR-1C2/2, and TU41, respectively (BD Biosciences PharMingen).
CIK cells were characterized with FITC-conjugated anti-CD3 (SK7 clone) and PE-conjugated anti-CD56 (NCAM16.2 clone) mAbs (Becton Dickinson). To analyze the expression of activating receptors on CIK cells, the following mAbs were used: anti-NKG2D (BAT221 clone), anti-NKp30 (A76 clone), anti-NKp46 (BAB281 clone), anti-NKp44 (KS38 clone), and anti–DNAM-1 (F22 clone).24,25 CD11a (LFA-1) was detected with FITC-conjugated Ab clone G-25.2 (BD Biosciences). mAb24 that recognizes LFA-1 in high-affinity conformation was also used (Abcam).
For indirect immunofluorescence staining, cells were stained with the primary mAb, followed by PE-conjugated goat anti–mouse IgG1 (Invitrogen) or FITC-conjugated goat anti–mouse Ig second reagent (BD Biosciences). A FACScan flow cytometer device (BD Biosciences) was used to analyze the samples.
CFSE and PKH26 staining
Target cells were washed and resuspended in 1 mL of PBS with 1% BSA at a final concentration of 5 × 106/mL, then labeled for 10 minutes at 37°C with green fluorescent dye CFSE (2μM; Sigma-Aldrich). Quench-staining was performed on ice for 5 minutes by adding 5 volumes of ice-cold complete RPMI. Cells were then washed 3 times with ice-cold PBS with 1% BSA and cultured under appropriate conditions.
For PKH-26 staining of CIK cells, 10 × 106 cells were incubated with lipophilic red fluorescent dye PKH-26 (Sigma-Aldrich) according to the manufacturer's instructions.
Cell conjugation assay
A total of 5 × 105 PKH26-labeled CIK cells and 5 × 105 CFSE-labeled target cells were resuspended in 200 μL of complete medium containing or lacking 1mM EDTA (Sigma-Aldrich) and mixed in a 12 × 75-mm polystyrene tube (BD Biosciences Labware). The effector-target cell mixture was centrifuged at 1000 rpm for 1 minute and incubated at 37°C for the indicated time points. The cells were then gently resuspended and analyzed by flow cytometry. The conjugation ratio was calculated as the portion of FITC/PE double-positive events within the PE-positive events. For Ab-blocking experiments, 5 × 105 PKH26-labeled CIK cells were preincubated with 10 μg/mL blocking anti–LFA-1 mAb for 30 minutes, and then the conjugation ratio was detected by flow cytometry as described.
Calcein release cytotoxicity assay
Cell lysis was evaluated with the calcein release assay as previously described.2 In some experiment, the cytotoxicity assay was performed in the presence of 1mM EDTA.
In some experiments, autologous or recipient PHA-induced blasts pulsed with pp65495-503 or a HLA-A*0201–binding control peptide (Primm) were used as target cells. Peptide-pulsed cells were incubated with 10 μg/mL peptide for 16 hours at 37°C before labeling with calcein-AM.
For masking experiments, anti-NKp30 (F252 clone), anti–DNAM-1 (F5 clone), anti-NKG2D (BAT221 clone), and anti–HLA-I (W6/32 clone) were used.26 Masking of LFA-1 was performed with TS1-22 hybridoma supernatant (a kind gift of Dr P. Allavena, Istituto Clinico Humanitas). CIK cells were preincubated 15 minutes at room temperature either in the absence or in the presence of 10 μg/mL of specific or isotype-matched control mAbs or supernatant and, after washing, used in the cytolytic assay tested.
Redirected cytotoxicity assay
For redirected killing assay, FcγR-positive cell line P815 (murine mastocytoma) was labeled with calcein-AM as described earlier. Anti-CD3 (OKT3 clone), anti-NKG2D (BAT221 clone), anti-NKp30 (A76 clone), anti–DNAM-1 (F22 clone), anti-NKp46 (BAB281 clone), isotype-matched control or no mAbs were added at saturating concentration and incubated for 15 minutes. The P815 target cells were then used as target in the calcein release cytotoxicity assay as described.
Degranulation assay
Degranulation assay was performed as previously described.27,28 Briefly, CIK and P815 cells loaded with anti-CD3, anti-NKG2D, anti-NKp30, anti–DNAM-1, anti-NKp46, isotype-matched control or no mAbs were plated at E/T ratio of 1:1 and incubated for 2 hours at 37°C in the presence of CD107a-PE mAb (BD Pharmingen). CIK cell degranulation was assessed by cell surface staining for the lysosomal markers CD107a by flow cytometry.
Statistical analysis
The data were analyzed with paired or unpaired Student t tests, as appropriate (*P < .05, **P < .01, and ***P < .001).
Results
Natural cytotoxicity of CD3+CD56+ CIK cells correlates with the binding rate to tumor targets and is LFA dependent
In the standard protocol for CIK cell expansion, cultures are initiated starting from unselected PBMCs and performed as previously described by us5 and others.1 In the resulting bulk cultures 3 subpopulation can be distinguished: CD3+CD56+ terminally differentiated CIK cells, responsible for the HLA-unrestricted antitumor activity, CD3+CD56− early effector T cells, and a minor fraction of CD3−CD56+ NK cells.5 To directly assess the functional activity of CD3+CD56+ CIK cells derived from purified CD3+CD56− cells (ie, the putative peripheral blood precursors of CD3+CD56+ CIK cells), PBMCs were depleted of CD56+ cells and then cultured by applying the standard expansion protocol (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Final yields and expansion folds were not changed compared with CIK cultures started from unmanipulated PBMCs (data not shown). At the end of the culture, CD3+CD56+ CIK cells were purified by CD56+ selection (mean purity, 95%; range, 88%-99% in 5 independent experiments; supplemental Figure 1A; data not shown). The surface phenotype of such CIK cells did not differ from that previously described for CIK cells obtained from unselected PBMCs and is consistent with αβ CD8+ TEMRA cells (supplemental Figure 1B).5 All the following experiments were performed with the use of such purified CD3+CD56+ CIK cells to directly test their antitumor function.
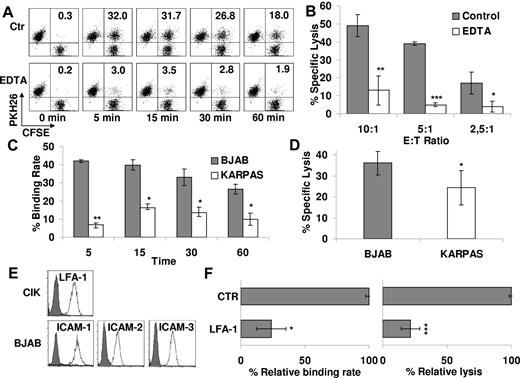
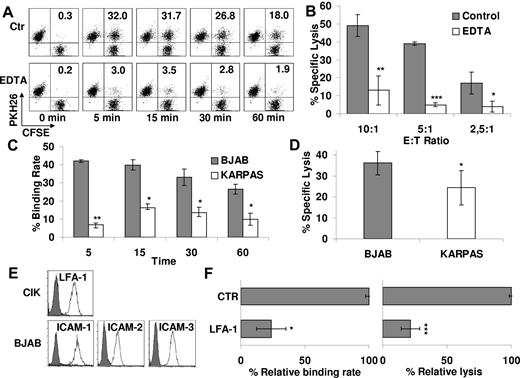
To investigate whether cytotoxicity of CIK cells requires effector/target cell contact and involves conjugate formation, PKH26-labeled CIK and CFSE-labeled B lymphoma cell line BJAB was mixed and incubated at 37°C for different time intervals. The cells were then analyzed by flow cytometry. Figure 1A shows a representative experiment of 3 experiments performed. CIK cells (34% ± 3%) were able to bind target after 5 minutes whereas a slow progressive decline was observed at later intervals (24% ± 2% after 1 hour; Figure 1A). To investigate whether binding to target cells is necessary to CIK-mediated cytotoxicity, experiments were performed in which conjugate formation was blocked by adding EDTA, a calcium chelator that prevents cell adhesion. Binding of CIK to BJAB cells was significantly inhibited by EDTA at all time points (average reduction of binding rate, 77% ± 3%; Figure 1A). Similarly, the cytolytic activity of CIK cells pretreated with EDTA against BJAB cells was significantly reduced by 79% ± 7% at all E/T ratios tested (P < .05; Figure 1B).
Natural cytotoxicity of CD3+CD56+ CIK correlates with binding to tumor targets and is LFA-1 dependent. (A) PKH26-labeled CIK and CFSE-labeled BJAB cells were mixed and incubated at 37°C for the indicated times with or without 1μM EDTA and analyzed by flow cytometry. Percentage of binding (values indicated in the top right quadrants) is calculated as the portion of CFSE/PKH-26 double-positive events within the PKH-26–positive events. One representative example is shown. (B) Cytotoxicity of CIK cells against BJAB in the presence (white bars) or absence (gray bars) of 1μM EDTA was evaluated at the indicated E/T ratios. Data were mean ± SD collected from 3 independent experiments and were analyzed by Student t test; *P < .05, **P < .01, and ***P < .005. (C) CIK cells binding to BJAB or KARPAS 422 at different times was analyzed by flow cytometry. Data were obtained from ≥ 3 independent experiments and analyzed by Student t test; *P < .05, **P < .01. (D) Cytotoxicity of CIK cells against BJAB or KARPAS 422 was evaluated by calcein-release assay. Data were mean ± SD obtained from ≥ 3 independent experiments and were analyzed by Student t test; *P < .05. (E) Expression of LFA-1 and their ligands (ICAM-1, -2, and -3) was determined, respectively, on CIK cells and BJAB target by flow cytometry. Background staining with the use of an isotype control Ab is shown in each histogram (gray curve). (F) The functional role of LFA-1 in CIK binding to BJAB (left) and in cytotoxicity (right) was evaluated. For binding assays, PKH-26–labeled CIK cells were exposed to saturating concentrations of blocking Ab anti–LFA-1 or medium alone (CTR) for 30 minutes and then incubated with CFSE-labeled BJAB for 15 minutes. For cytotoxicity assays, CIK cells were preincubated with saturating concentration of anti–LFA-1 or medium alone (CTR) for 15 minutes and then tested in a calcein-release assay against BJAB. Data are the mean percentage of lysis obtained with respect to untreated controls. Data were mean ± SD obtained from ≥ 3 independent experiments and were analyzed by Student t test; *P < .05, **P < .01, and ***P < .005 compared with control in the absence of mAbs.
Natural cytotoxicity of CD3+CD56+ CIK correlates with binding to tumor targets and is LFA-1 dependent. (A) PKH26-labeled CIK and CFSE-labeled BJAB cells were mixed and incubated at 37°C for the indicated times with or without 1μM EDTA and analyzed by flow cytometry. Percentage of binding (values indicated in the top right quadrants) is calculated as the portion of CFSE/PKH-26 double-positive events within the PKH-26–positive events. One representative example is shown. (B) Cytotoxicity of CIK cells against BJAB in the presence (white bars) or absence (gray bars) of 1μM EDTA was evaluated at the indicated E/T ratios. Data were mean ± SD collected from 3 independent experiments and were analyzed by Student t test; *P < .05, **P < .01, and ***P < .005. (C) CIK cells binding to BJAB or KARPAS 422 at different times was analyzed by flow cytometry. Data were obtained from ≥ 3 independent experiments and analyzed by Student t test; *P < .05, **P < .01. (D) Cytotoxicity of CIK cells against BJAB or KARPAS 422 was evaluated by calcein-release assay. Data were mean ± SD obtained from ≥ 3 independent experiments and were analyzed by Student t test; *P < .05. (E) Expression of LFA-1 and their ligands (ICAM-1, -2, and -3) was determined, respectively, on CIK cells and BJAB target by flow cytometry. Background staining with the use of an isotype control Ab is shown in each histogram (gray curve). (F) The functional role of LFA-1 in CIK binding to BJAB (left) and in cytotoxicity (right) was evaluated. For binding assays, PKH-26–labeled CIK cells were exposed to saturating concentrations of blocking Ab anti–LFA-1 or medium alone (CTR) for 30 minutes and then incubated with CFSE-labeled BJAB for 15 minutes. For cytotoxicity assays, CIK cells were preincubated with saturating concentration of anti–LFA-1 or medium alone (CTR) for 15 minutes and then tested in a calcein-release assay against BJAB. Data are the mean percentage of lysis obtained with respect to untreated controls. Data were mean ± SD obtained from ≥ 3 independent experiments and were analyzed by Student t test; *P < .05, **P < .01, and ***P < .005 compared with control in the absence of mAbs.
The BJAB and KARPAS422 human B lymphoma cell lines displayed different sensitivities to CIK-mediated cytotoxicity, of 36% ± 6% and 23% ± 6% specific lysis at 10:1 E/T ratio, respectively (n = 3; P < .05; Figure 1D). Notably, CIK-BJAB conjugates were significantly more abundant than those formed with KARPAS422 cells during the 60-minute incubation period (n = 3; P < .05; Figure 1C). These results suggest that CIK cell binding to target and formation of the cellular conjugates are prerequisite to and may correlate with CIK-mediated cytotoxicity against at least 2 cell lines.
Cell surface adhesion molecules, such as LFA-1, are known to participate in effector/target recognition and stable conjugate formation. CIK cells expressed LFA-1 (Figure 1E), and in particular a significant percentage constitutively express LFA-1 in its high-affinity conformation, as identified by mAb24 (67.5% ± 12.4%; n = 3; data not shown). Moreover, the most susceptible target BJAB expressed all the major LFA-1 ligands (ICAM-1, -2, and -3; Figure 1E). In contrast, KARPAS422 did not express ICAM-1 and only low levels of ICAM-2 and -3 (supplemental Figure 2A). In addition, although CIK-target cell conjugate formation and cytotoxicity against BJAB were strongly inhibited by anti–LFA-1 mAb (respectively, 76% ± 11% and 78% ± 7%; n = 3; P < .05; Figure 1F), binding and cytotoxicity against KARPAS422 were only marginally affected (supplemental Figure 2B-C). These results confirm that LFA-1 has a crucial role in binding as well as in cytotoxicity of CIK cells and that additional molecules may also be involved in target cell recognition by CIK cells.
Functional role of CD3 and activating NK receptors in CIK cells
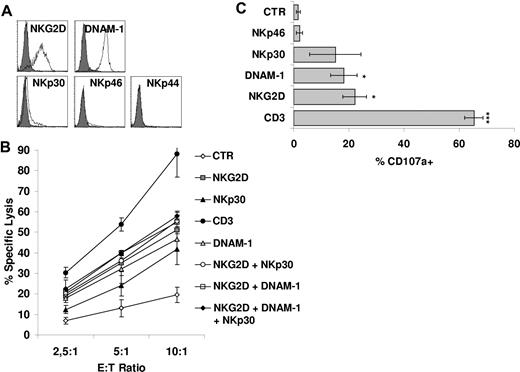
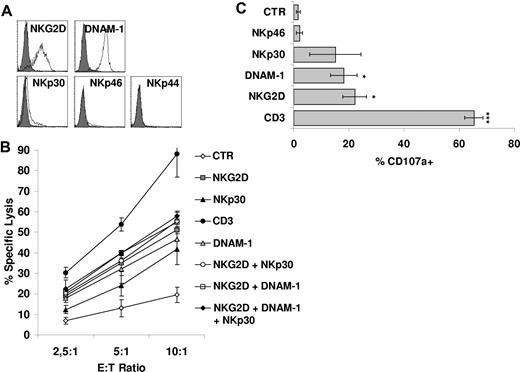
We next attempted to identify the receptors mediating non–HLA-restricted tumor cell killing by CIK cells. To investigate this point, we analyzed the main receptors involved in NK-cell triggering in the process of tumor cell lysis. These include NKp46, NKp30, NKp44, NKG2D, and DNAM-1. As previously reported by our group5 and others,21 CIK cells express NKG2D at high density. The NCRs NKp44 and NKp46 were not or were very poorly expressed, whereas NKp30 was present at low densities5 (Figure 2A). However, DNAM-1 was found to be highly expressed (Figure 2A).
Functional role of CD3/TCR and NK cytotoxic activating receptors on CIK cells. (A) Expression of activating receptors NKG2D, DNAM-1, NKp30, NKp46, and NKp44 on CIK cells was analyzed by flow cytometry. Gray profiles represent isotype controls. (B) Redirected killing assay was performed with the use of CIK cells treated with indicated agonist mAbs or without mAbs and calcein-labeled P815 cells. The data were mean ± SD obtained from 3 independent experiments. (C) CIK cells were triggered with P815 cells loaded with different mAbs and then assayed for degranulation by cell surface staining for the lysosomal markers CD107a. The data were mean ± SD obtained from ≥ 3 independent experiments and were analyzed by Student t test; *P < .05, ***P < .005 compared with control in the absence of mAbs.
Functional role of CD3/TCR and NK cytotoxic activating receptors on CIK cells. (A) Expression of activating receptors NKG2D, DNAM-1, NKp30, NKp46, and NKp44 on CIK cells was analyzed by flow cytometry. Gray profiles represent isotype controls. (B) Redirected killing assay was performed with the use of CIK cells treated with indicated agonist mAbs or without mAbs and calcein-labeled P815 cells. The data were mean ± SD obtained from 3 independent experiments. (C) CIK cells were triggered with P815 cells loaded with different mAbs and then assayed for degranulation by cell surface staining for the lysosomal markers CD107a. The data were mean ± SD obtained from ≥ 3 independent experiments and were analyzed by Student t test; *P < .05, ***P < .005 compared with control in the absence of mAbs.
We investigated the functional status of the various NK receptors expressed on CIK cells by a reverse Ab-dependent cellular cytotoxicity assay. As shown in Figure 2B, robust redirected lysis was obtained by crosslinking of NKG2D and DNAM-1, although less robust than one observed by anti-CD3 mAb. NKp30 crosslinking also led to redirected cytolysis, albeit to a lesser extent. On the contrary, an isotype control Ab or an anti-NKp46 mAb did not induce target cell lysis (Figure 2B; data not shown). The concomitant presence of anti-NKp30, anti–DNAM-1, or both of them with anti-NKG2D did not significantly change the activity of anti-NKG2D alone (Figure 2B). We also tested cytotoxic granule release after receptor engagement, using a mAb directed against the lysosomal-associated membrane protein-1 (CD107a).27,28 Consistently with cytotoxicity data, CD3 ligation resulted in an increase in the percentage of cells undergoing degranulation (P < .005; Figure 2C). Ligation of DNAM-1, NKG2D, and partially NKp30 also resulted in some degranulation (Figure 2C). Finally, also the production of IFN-γ and TNF-α was investigated after receptor engagement. Crosslinking of CD3 resulted in IFN-γ and TNF-α production (P < .05). A weaker cytokine secretion was detected also after stimulation of NKp30 and NKG2D (supplemental Figure 3A-B).
Altogether, these results show that signals delivered not only through TCR but also through activating NK receptors can lead to CIK cell activation, resulting in granule exocytosis, cytokine secretion, and cytotoxicity.
Effect of mAb-mediated blocking of NKG2D, DNAM-1, and NKp30 on CIK-mediated target cell lysis
To identify the triggering molecules that are actually involved in the induction of CIK-mediated lysis of tumor targets, we first analyzed the ligands of activating NK receptors expressed on target cells.
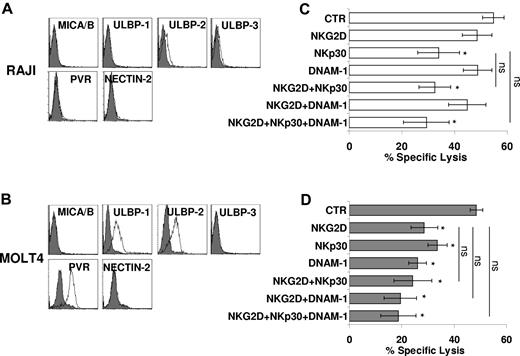
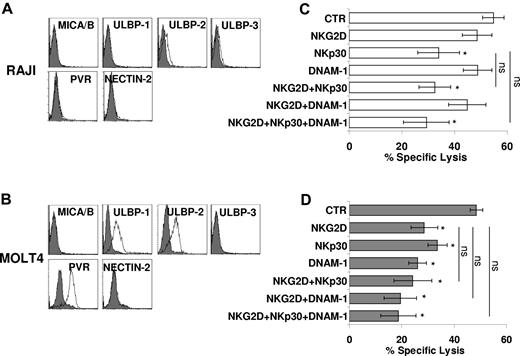
Thus, we assessed the expression of ligands of NKG2D (MICA/B and ULBPs) and DNAM-1 (Nectin-2 and PVR) by the use of specific mAb and FACS analysis. Figure 3 shows 2 targets similarly susceptible to CIK-mediated lysis that differ for the expression of these ligands. Although RAJI cells were virtually negative for all ligands tested, MOLT4 cells were positive for PVR and ULBP-1 and -2 (Figure 3A-B).
Direct role of activating receptors in CIK-mediated lysis of tumor targets. (A-B) RAJI (A) and MOLT4 (B) cells were analyzed for expression of MICA/B and ULBP-1, -2, and -3 (NKG2D ligands), and PVR and Nectin-2 (DNAM-1 ligands) by flow cytometry. Gray profiles represent isotype control. (C-D) Blocking of activating receptors NKG2D, NKp30, and DNAM-1 in CIK cells cytotoxicity against RAJI (C) and MOLT4 (D) targets. CIK cells were preincubated with saturating concentrations of anti-NKG2D, anti-NKp30, anti–DNAM-1, and cytotoxic activity was measured in calcein-release assays. The data were mean ± SD obtained from 3 independent experiments and were analyzed by Student t test, *P < .05, compared with control in the absence of mAbs.
Direct role of activating receptors in CIK-mediated lysis of tumor targets. (A-B) RAJI (A) and MOLT4 (B) cells were analyzed for expression of MICA/B and ULBP-1, -2, and -3 (NKG2D ligands), and PVR and Nectin-2 (DNAM-1 ligands) by flow cytometry. Gray profiles represent isotype control. (C-D) Blocking of activating receptors NKG2D, NKp30, and DNAM-1 in CIK cells cytotoxicity against RAJI (C) and MOLT4 (D) targets. CIK cells were preincubated with saturating concentrations of anti-NKG2D, anti-NKp30, anti–DNAM-1, and cytotoxic activity was measured in calcein-release assays. The data were mean ± SD obtained from 3 independent experiments and were analyzed by Student t test, *P < .05, compared with control in the absence of mAbs.
Analysis of the effect of Ab blocking of the activating receptors expressed by CIK cells was consistent with data on ligand expression. Indeed, masking of DNAM-1 had no effect on CIK-mediated lysis of RAJI cells, whereas lysis of MOLT4 cells was partially inhibited. Similarly, NKG2D-masking resulted in a sharp inhibition of lysis of MOLT4 cells only. Interestingly, mAb-mediated blocking of NKp30 consistently resulted in a significant inhibition of killing of both targets implying that RAJI and MOLT4 cells express NKp30 ligand(s).29 An isotype control Ab has no effect on cytotoxicity (data not shown). The concomitant addition of more than 1 Ab does not significantly increase the inhibitory effect detected by NKG2D, DNAM-1, or NKp30 alone (Figure 3C-D).
Taken together, these data indicate that NKG2D, DNAM-1, and NKp30 may be involved in CIK-mediated killing of target cells upon recognition of their specific ligands.
CIK cells retain TCR-mediated Ag specificity
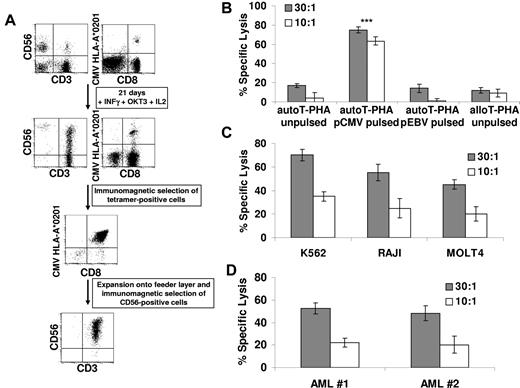
The data described in the previous subsection suggested that CIK cells may maintain a functional TCR/CD3 complex and acquire NK-like cytolytic functions through the expression of activating NK receptors, including NKG2D, NKp30, and DNAM-1. Therefore, we asked whether TCR expressed by CIK cells could mediate Ag-specific binding and function. To this end, CIK expansion was induced in PBMCs from CMV-seropositive healthy donors. As exemplified in the case of the HLA-A*0201 donor shown in Figure 4A, the presence of tetramer+ CD8+ T cells was maintained after the expansion of CIK cultures. In 5 separate experiments the percentage of CMV tetramer+ T cells did not significantly change from the beginning (average percentage of tetramer+ CD8+ cells, 1.7%; range, 0.1%-3.5%) to the end of the culture (average percentage of tetramer+ CD8+ cells, 1.8%; range, 0.3%-4.2%; data not shown). More importantly, in these experiments, CMV tetramer+ T cells expanded by 36.7-fold (range, 5.4- to 59.9-fold), with similar efficiency for the total CD8+ T cells (mean, 34.5; range, 26.9-44.9; Table 1). This allowed us to obtain a mean absolute number of 42.4 × 106 CMV tetramer+ T cells starting from PBMCs obtained from 15 mL of peripheral blood.
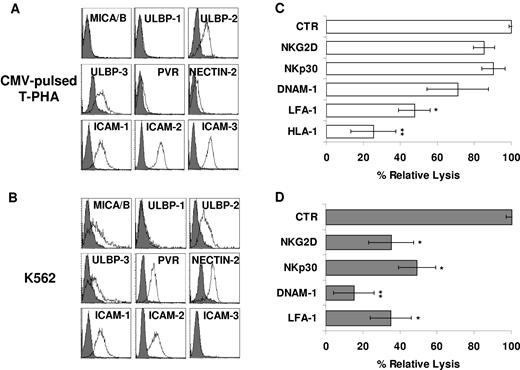
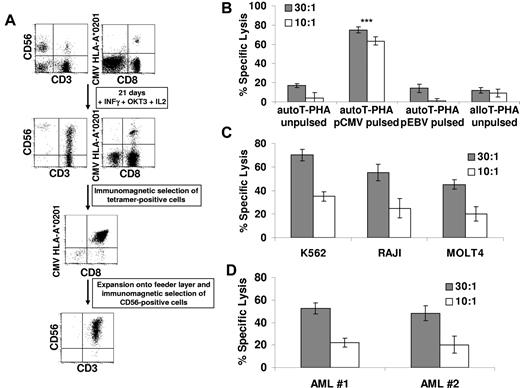
CMV-specific CIK cells maintain their Ag specificity and acquire HLA-unrestricted cytotoxicity. (A) Peripheral blood lymphocytes from HLA-A*0201 seropositive donors were expanded for 21 days in the presence of IFN-γ, OKT3, and IL-2, and the percentage of anti–CMV-specific cells was measured at the end of culture by tetramer binding. CMV-specific CIK cells were isolated by immunomagnetic selection and expanded onto irradiated allogeneic feeder layer for further 2 weeks. At the end of the culture, CD56+ CMV-specific CIK cells were immunoselected. The dot plots of CD3-CD56 and HLA-A*0201/pp65495-503 tetramer-CD8 staining, obtained during a representative experiment, are shown. (B) Cytotoxic activity of expanded CMV-specific CIK cells was determined on unpulsed, CMV-pulsed, EBV-pulsed autologous T–PHA-induced blasts and on unpulsed allogeneic T–PHA-induced blasts in calcein-release assays. The data were obtained from 3 independent experiments and were analyzed by Student t test; ***P < .005. (C-D) MHC-unrestricted cytotoxic activity of expanded CMV-specific CIK cells against a panel of malignant cell lines (C) or against freshly isolated myeloid leukemic cells (D) was determined in calcein-release assays. The results show the mean ± SD values of 3 independent experiments.
CMV-specific CIK cells maintain their Ag specificity and acquire HLA-unrestricted cytotoxicity. (A) Peripheral blood lymphocytes from HLA-A*0201 seropositive donors were expanded for 21 days in the presence of IFN-γ, OKT3, and IL-2, and the percentage of anti–CMV-specific cells was measured at the end of culture by tetramer binding. CMV-specific CIK cells were isolated by immunomagnetic selection and expanded onto irradiated allogeneic feeder layer for further 2 weeks. At the end of the culture, CD56+ CMV-specific CIK cells were immunoselected. The dot plots of CD3-CD56 and HLA-A*0201/pp65495-503 tetramer-CD8 staining, obtained during a representative experiment, are shown. (B) Cytotoxic activity of expanded CMV-specific CIK cells was determined on unpulsed, CMV-pulsed, EBV-pulsed autologous T–PHA-induced blasts and on unpulsed allogeneic T–PHA-induced blasts in calcein-release assays. The data were obtained from 3 independent experiments and were analyzed by Student t test; ***P < .005. (C-D) MHC-unrestricted cytotoxic activity of expanded CMV-specific CIK cells against a panel of malignant cell lines (C) or against freshly isolated myeloid leukemic cells (D) was determined in calcein-release assays. The results show the mean ± SD values of 3 independent experiments.
At the end of cultures, the CMV tetramer+ T cells were mostly CD3+CD56+ CIK cells (mean, 65%; range, 40%-88%). Most of them showed an effector memory (CD62L−CD45RA−; mean, 40%; range, 17%-68%) or a TEMRA (CD62L−CD45RA+; mean, 54%; range, 29%-87%) phenotype (data not shown).
CIK expansions were also induced starting from peripheral blood lymphocytes obtained from selected HLA-A*0201-positive persons with recent onset of acute infectious mononucleosis. In agreement with what is observed for CMV-positive CD8+ T cells, also EBV-positive CD8+ T cells were maintained after the expansion of CIK cultures. In 4 separate experiments, the percentage of EBV tetramer+ T cells did not significantly change from the beginning (average percentage of tetramer+ CD8+ cells, 0.14%; range, 0.07%-0.23%) to the end of culture (average percentage of tetramer+ CD8+ cells, 0.19%; range, 0.11%-0.27%; data not shown). EBV tetramer+ T cells had expanded by 38.8-fold (range, 15.3- to 59.5-fold) and with equivalent efficiency for total CD8+ T cells that showed a mean fold increase of 34.5 (range, 11.7- to 67.2-fold increase; supplemental Table 1).
To directly test if indeed Ag-specific CD8+ T cells expanded in CIK culture conditions had maintained TCR-dependent cytolytic activity, we analyzed purified, Ag-specific CD3+CD56+ CIK cells. In some experiments, as exemplified in Figure 4A, after 21 days of culture, CMV tetramer+ T cells were positively selected with the use of anti-PE paramagnetic beads (average percentage of purity, 85%; range, 75%-94%; n = 3; data not shown) and then cultured for 2 weeks in the presence of irradiated allogeneic feeder and rhIL-2. At the end of the culture, CD3+CD56+ CIK cells were purified by immunomagnetic selection (average percentage, 85%; range, 78%-91%; n = 3; data not shown) and used for functional experiments.
As shown in Figure 4B, CMV-specific CIK cells showed a strong cytolytic activity against CMV-pulsed autologous T–PHA-induced blasts (average lysis, 63% ± 3%; n = 3) at E/T 10:1. No lysis was observed against PHA-induced blasts pulsed with EBV peptides (average lysis, 1% ± 4%). No killing was detected against autologous unpulsed (average lysis, 4% ± 2%) or allogeneic T–PHA-induced blasts (average lysis, 8% ± 3%).
We next analyzed whether CMV tetramer+ CIK cells could mediate NK-like MHC-unrestricted cytotoxicity against a variety of malignant cell lines, including K562 (eritroleukemia), RAJI (B-cell leukemia), and MOLT4 (T-cell leukemia). As shown in Figure 4C, CMV tetramer+ CIK cells efficiently killed all malignant cell lines tested with 58%-82% specific lysis at 30:1 E/T ratio. Perhaps more importantly, CMV tetramer+ CIK cells were able to lyse freshly isolated (allogeneic) leukemic blasts (Figure 4D).
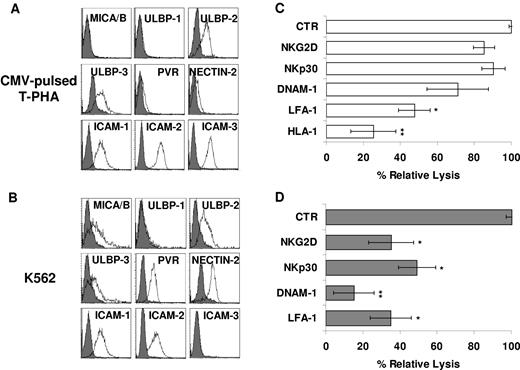
Along this line, CMV tetramer+ CIK cells displayed an expression profile of NKG2D, NKp30, DNAM-1, and LFA-1 similar to that of unselected CIK cells (data not shown). Therefore, we further tested the functional capability of all these receptors in the context of both TCR-dependent (Ag-specific) and TCR-independent cytotoxicity. K562 and CMV-pulsed T–PHA-induced blasts were assessed for the expression of ligands recognized by NKG2D, DNAM-1, and LFA-1. As shown in Figure 5, the K562 cells expressed MICA/B, ULBP-2, ULBP-3, PVR, Nectin-2, ICAM-1, and ICAM-2 but not ICAM-3 (Figure 5B), whereas CMV-pulsed autologous T–PHA-induced blasts expressed ULBP-2 and -3 and low levels of PVR and Nectin-2 (Figure 5A). Calcein-release assays were performed in the presence and absence of anti–LFA-1, anti–DNAM-1, anti-NKG2D, and anti-NKp30 blocking mAbs. Importantly, the addition of blocking mAb directed against LFA-1 and DNAM-1 induced a strong inhibition of lysis of both targets, possibly interfering with binding of the effector to the target cells (Figure 5B,D). More importantly, anti-NKG2D and anti-NKp30 blocking mAbs affected the non–MHC-restricted cytotoxicity but had no effect on TCR-dependent killing of CMV-pulsed autologous T–PHA-induced blasts (Figure 5B,D), suggesting a role in NK-like cytotoxicity only. It is of note that mAb-mediated masking of HLA-class I molecules inhibited lysis of CMV-pulsed autologous T–PHA-induced blasts but not K562 (Figure 5C).
NKp30, NKG2D, LFA-1, and DNAM-1 are differently involved in Ag-specific and HLA-unrestricted cytotoxicity exerted by CMV-specific CIK cells. (A-B) CMV-pulsed autologous T–PHA-induced blasts (A) and K562 cell line (B) were analyzed for expression of MICA/B and ULBP-1, -2, and -3 (NKG2D ligands) and PVR and Nectin-2 (DNAM-1 ligands) by flow cytometry. Gray profiles represent isotype control. (C-D) Blocking of activating receptors NKG2D, NKp30, and DNAM-1 in CIK cells cytotoxicity against CMV-pulsed autologous T–PHA-induced blasts (C) and K562 cell line (D) targets. CIK cells preincubated with saturating concentrations of the indicated mAbs were used in calcein-release assays. The results shown are the mean percentage of cytotoxic activity of treated compared with untreated CIK cells. The data were ± SD obtained from 3 independent experiments and were analyzed by Student t test. *P < .05, **P < .01 compared with control in the absence of mAbs.
NKp30, NKG2D, LFA-1, and DNAM-1 are differently involved in Ag-specific and HLA-unrestricted cytotoxicity exerted by CMV-specific CIK cells. (A-B) CMV-pulsed autologous T–PHA-induced blasts (A) and K562 cell line (B) were analyzed for expression of MICA/B and ULBP-1, -2, and -3 (NKG2D ligands) and PVR and Nectin-2 (DNAM-1 ligands) by flow cytometry. Gray profiles represent isotype control. (C-D) Blocking of activating receptors NKG2D, NKp30, and DNAM-1 in CIK cells cytotoxicity against CMV-pulsed autologous T–PHA-induced blasts (C) and K562 cell line (D) targets. CIK cells preincubated with saturating concentrations of the indicated mAbs were used in calcein-release assays. The results shown are the mean percentage of cytotoxic activity of treated compared with untreated CIK cells. The data were ± SD obtained from 3 independent experiments and were analyzed by Student t test. *P < .05, **P < .01 compared with control in the absence of mAbs.
Discussion
In this report we analyzed CD3+CD56+ CIK cells obtained from PBMCs after in vitro expansion for 3 weeks by the sequential addition of IFN-γ, OKT3, and IL-2. We show that CIK cells are characterized by a dual function, acting both as CD8+-specific effector T and NK-like cells. Thus, they represent a particularly useful tool, in adoptive immunotherapy, to treat not only cancer but also associated life-threatening viral infections.
A number of reports on CIK cells, including clinical trials of adoptively transferred CIK-cell infusion, used bulk-expanded CIK cultures. Within bulk cultures 2 main subpopulations can be distinguished, one coexpressing the CD3 and CD56 molecules, characterized by a phenotype typical of terminally differentiated effector cells, a potent non–MHC-restricted cytotoxicity and low proliferative capacity. The other cell fraction, displaying a CD3+CD56− phenotype, is composed of effector T cells with reduced cytotoxicity but higher capability of proliferating. The bulk culture also comprises a small CD3−CD56+ classic NK-cell subset.5 In the present study, we characterized in detail the CD3+CD56+ CIK cell fraction, in terms of their molecular mechanism of activation and cytolytic function.
CD3+CD56+ CIK cells are capable of rapid binding to lymphoma target cells, such as BJAB. Preventing cell adhesion with EDTA sharply reduces cytolytic activity of CIK cells against BJAB. Nonetheless, we cannot rule out also a direct role of calcium chelation induced by EDTA on the cytolytic activity of CIK cells. A direct correlation seems to exist between degree of binding and sensitivity to CIK-mediated cytotoxicity in different targets, even if this is, at the moment, just a hypothesis based only on 2 cell lines. In this context, a significant inhibition of CIK-mediated cytotoxicity was obtained by blocking the cell surface adhesion molecule LFA-1.23,30,31 Although it is clear the crucial role of LFA-1 in CIK cells for functional binding and cytotoxicity against target cells such as BJAB, which express high amounts of ICAM-1, -2, and -3 (the main LFA-1 ligands) in the case of other target cells such as KARPAS422, it is probable that other surface molecules are involved in binding. The surface expression of high-affinity conformation of LFA-1 does not correlate with the cytotoxic activity of the different subpopulations of CIK cultures, being that this integrin is equally present on CD3+CD56+ and on the less cytotoxic CD3+CD56− cells (data not shown). This confirms that LFA-1 may not be sufficient for target cell recognition and killing mediated by CD3+CD56+ CIK cells.
CIK cells express high levels of CD3 and respond to TCR/CD3-mediated stimulation by degranulating, killing P815 cells loaded with anti-CD3 mAb and producing cytokines such as TNF-α and IFN-γ. This is in agreement with our previous observations that CIK cells are characterized by a phenotypic profile typical of terminally differentiated effector CD8+ T cells that originate in vitro from CD56−CD8+ T-cell progenitors. In addition, CIK cells express NKG2D, DNAM-1, and, at lower surface densities, NKp30. In contrast, NKp44 and NKp46 are virtually absent. With the use of redirected killing, degranulation assays, and cytokine secretion we could show that NKG2D, DNAM-1, and NKp30 expressed by CIK cells are functional. Remarkably, CD3 triggering induced higher cytotoxicity in redirected killing and higher degranulation and cytokine production after receptor triggering by activating mAb, compared with NKG2D, DNAM-1, and NKp30, even when used in combination. These results suggest that NKp30 and DNAM-1 do not provide costimulation to NKG2D signaling.
Despite various studies on CIK cells, the molecular mechanism by which they kill tumor cells was not clearly identified. Although the sensitivity of MOLT4 and RAJI cell lines to the cytolytic activity of CIK cells did not appear to correlate with the surface expression of the ligands of DNAM-1 and NKG2D, the inhibitory effect exerted by anti–DNAM-1 or -NKG2D blocking antibodies in CIK-mediated lysis of MOLT4 cells suggested a role of these receptors at least in the case of targets (such as MOLT4) expressing the specific ligands. A partial inhibition of lysis of both targets could be achieved by mAb-mediated blocking of NKp30, which was not significantly increased by the addition of a combination of antibodies.
NKG2D has a significant role in triggering IL-2–activated NK cells, and its ligation induces calcium flux, cytokine release, and cytotoxicity. NKG2D is also expressed on γδ T cells and CD8+ TCR αβ T cells.32,33 In contrast to NK cells, crosslinking of NKG2D in Ag-specific CTL clones did not induce calcium flux or cytokine production or cytotoxicity. However, NKG2D signaling has been shown to augment cytotoxic and proliferative responses of T cells to Ag stimulation, thus qualifying NKG2D as a T-cell costimulatory molecule.33-35 A main role for NKG2D molecule in CIK-cell function has already been proposed.21,22 Studies of Abs blocking NKG2D molecules, RNA interfering, and redirected cytolysis indicated that the antitumor cytotoxic activity of CIK cells is exerted through NKG2D rather than TCR engagement.21,22
In the present study we provide evidence that also DNAM-1 and NKp30 play a role in CIK cell–mediated antitumor cytotoxicity. We show that NKp30, although expressed at relatively low density, is involved in the recognition and killing of lymphoma targets. Notably, a functional role of NKp30 in T cells has been described so far only in the case of IL-15 long-term cultured cord blood lymphocytes.36 The expression of NKp30 in CIK cells is intriguing also in light of the reciprocal activation between CIK and dendritic cells37,38 and of the crucial role of NKp30 in the interaction between NK cells and dendritic cells.39-41
Other relevant information provided by our study is the demonstration that Ag-specific CD8+ T cells (displaying the CD3+CD56+ CIK phenotype) can be expanded in CIK cultures, as shown here for both CMV- and EBV-specific memory cells. We show that these cells are characterized by a dual function. Indeed, they can both specifically kill autologous cells loaded with CMV peptides and lyse tumor cell lines and freshly isolated leukemic blasts. Cytotoxicity mediated by CIK cells is non-MHC restricted, and the addition of anti-MHC class I Ab to target cells had no inhibitory effect.31 Moreover, CIK cells killed with similar efficiency allogeneic and autologous leukemic cells.4 Killer inhibitory receptors have not been detected in CIK cells.5 Interestingly, anti-NKG2D and anti-NKp30 blocking mAbs inhibit the CIK-mediated killing of the HLA class I–negative K562, whereas no effect was detected on TCR-dependent killing of CMV-pulsed autologous T–PHA-induced blasts. These data suggest that these receptors are only involved in NK-like cytotoxicity. Notably, previous reports suggested that, in T cells, NKG2D plays a role as costimulating molecule rather than as a true receptor. In contrast blocking mAbs against LFA-1 and DNAM-1 induced a significant inhibition of lysis of both target types, most probable by interfering with binding between effector and target cells.
On the basis of the data presented, we can hypothesize that Ag-specific T cells may, in particular inflammatory conditions (here mimicked by IFN-γ and IL-2 stimulation), acquire additional NK-like functions, similar to those already suggested for a population of T cells expanded in the presence of IL-15.42-44 Indeed, if this was the case, Ag-specific T “CIK” cells may home to Ag sites (such as the lung in case of CMV infection) and there exploit also their NK-like potential by contributing to kill infected cells and release cytokines.
The information that CIK cells retain the CTL function (here tested only for anti–CMV-specific memory) adds further interest to their potential clinical application. In patients who received a BM transplant the recovery of functional specific T cells is particularly delayed, and most patients experience severe infections.45,46 In this respect, CIK cells may thus represent a “one coin-2 sides” chances of treating leukemia relapses and transferring the donor T-cell memory to cope with most frequent and possibly fatal infections (ie, CMV, EBV, Aspergillus, etc). Indeed, the absolute numbers of CMV-specific CIK cells, which can be obtained from a small amount of peripheral blood, are fully compatible with the idea of obtaining a clinically effective anti-CMV CTL (< 1 × 105 in most reports47,48 ) in standard CIK cultures approved with good manufacturing practices.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr C. Passerini Tosi (Microbiology and Virology Division, Ospedali Riuniti di Bergamo) for providing samples.
This work was supported in part by research funding from the Italian Association for Cancer Research (AIRC; regional grant Lombardia and Special Program in Molecular Clinical Oncology 5x1000 “Innate Immunity in Cancer”), by the Italian Ministry of Health (progetto ordinario, Provincia autonoma di Bolzano), and the Associazione Italiana contro le Leucemie, Linfomi e Mieloma (AIL), Bergamo-Sezione Paolo Belli (Bergamo, Italy).
Authorship
Contribution: A.P. designed research, performed experiments, analyzed data, and wrote the paper; G.B. performed experiments and analyzed data; D.P. and L.M. provided reagents, analyzed data, and edited the paper; A.R. and L.M. edited the paper; J.G. designed research, analyzed data, and wrote the paper; and M.I. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Martino Introna, Laboratory of Cellular Therapy “G. Lanzani,” Ospedali Riuniti, c/o Presidio Matteo Rota, Via Garibaldi 11-13, 24128 Bergamo, Italy; e-mail: mintrona@ospedaliriuniti.bergamo.it.