Abstract
Nelarabine, a purine analog with T-cell specific action, has been approved for relapsed/refractory T-cell acute lymphoblastic leukemia/lymphoma (ALL/LBL). This is a report of a single-arm phase 2 study conducted in adults (18-81 years of age) with relapsed/refractory T-ALL/LBL. After 1 or 2 cycles, 45 of 126 evaluable patients (36%) achieved complete remission (CR), 12 partial remission (10%), and 66 (52%) were refractory. One treatment-related death was observed, and 2 patients were withdrawn before evaluation. A total of 80% of the CR patients were transferred to stem cell transplantation (SCT). Overall survival was 24% at 1 year (11% at 6 years). After subsequent SCT in CR, survival was 31% and relapse-free survival 37% at 3 years. Transplantation-related mortality was 11%. Neurologic toxicities of grade I-IV/grade III-IV were observed in 13%/4% of the cycles and 16%/7% of the patients. This largest study so far with nelarabine in adults showed impressive single-drug activity in relapsed T-ALL/T-LBL. The drug was well tolerated, even in heavily pretreated patients. A high proportion of CR patients were transferred to SCT with low mortality but a high relapse rate. Exploration of nelarabine in earlier stages of relapse (eg, increasing minimal residual disease), in front-line therapy, and in combination is warranted.
Introduction
Treatment results in adult acute lymphoblastic leukemia (ALL) have improved in the past decade with an increase of complete remission (CR) rates to 85%-90% and overall survival rates to 40%-50% with intensified and targeted chemotherapies and stem cell transplantation (SCT).1 The survival of T-ALL improved to 48%-56%,2,3 and T-ALL represents now one of the most favorable subgroups of adult ALL. Despite all improvements of first-line therapy, the outcome of adult ALL after relapse and in patients with failure to standard induction therapy is extremely poor, with < 30% of the patients responding to first salvage therapy and long-term survival of < 10% independent of subgroup and relapse localization.3-6 Therefore, it is essential to optimize salvage therapy of adult ALL, if possible, with targeted, non–cross-resistant drugs and to transfer as many patients as possible in second CR to SCT as the only chance of cure.4
In this context, nelarabine (Compound 506U78, Arranon, Atriance) is of interest as a targeted T cell–directed drug. Nelarabine is a watersoluble pro-drug of 9-β-D-arabinofuranoyslguanine (ara-G), a deoxyguanosine analog.7 In contrast to deoxyguanosine, ara-G is resistant to degradation by purine nucleoside phosphorylase.8 The mechanism of action is based on the phosphorylation of ara-G by deoxycytidine kinase and deoxyguanosine kinase to its triphosphate (ara-GTP) that is required for cytotoxic action. It has been demonstrated that T-lymphatic cells compared with B cells have a decreased catabolism of ara-GTP. A higher initial ara-GTP accumulation in T cells compared with B cells has also been reported.9 The accumulation of ara-GTP leads to inhibition of ribonucleotide reductase, inhibition of DNA synthesis, and subsequent cell death.
The cytotoxic activity of nelarabine has been confirmed in several phase 1 or 2 trials.10 Whereas in early phase 2 trials neurotoxicity was the dose-limiting toxicity, treatment was better tolerated with a modified schedule at a dose of 1.5 g/m2 on alternating days (1, 3, and 5) for adults and 650 mg/m2 daily for 5 days in children. The CR rates ranged from 14%-55% in pediatric patients depending on stage and type of involvement11 and reached 31% in adult patients.12
The German Multicenter Study Group for Adult ALL (GMALL) conducted an exploratory prospective phase 2 study with nelarabine in relapsed or refractory T-ALL or T-lymphoblastic lymphoma (T-LBL). The aim was to evaluate efficacy and tolerability of nelarabine in adult patients and the feasibility of subsequent SCT, with the ultimate goal to improve outcome of this subset of ALL patients. This is so far, to our knowledge, the largest series of adult patients with T-ALL or T-LBL treated with nelarabine.
Methods
The study was started in 2001 as a single-center study at the Goethe University Hospital of Frankfurt. Nelarabine was provided by the Cancer Therapy Evaluation Program (National Cancer Institute) through agreement with GlaxoSmithKline. In February 2006, the protocol was amended. Additional centers in Germany participated, and GlaxoSmithKline, Germany provided the study drug. The Institutional Review Board of the University of Frankfurt had approved the protocol. All patients gave informed consent before start of study treatment in accordance with the Declaration of Helsinki. The study is registered at www.clinicaltrials.gov as #NCT00684619.
Entry criteria
Patients 15 years or older with relapsed or refractory T-ALL or T-LBL could be included, but analysis was restricted to patients 18 years and older. T-ALL was characterized by standard immunophenotyping, and subgroups early, thymic, and mature T-ALL were identified as described earlier.13 T-ALL or T-LBL was defined according to the presentation at first diagnosis, with T-ALL defined by bone marrow (BM) infiltration > 25%. Relapse was defined as the presence of > 5% lymphoblasts in the BM or unequivocal demonstration of extranodal involvement. Primary refractory disease was stated in patients never achieving a CR after standard 2-phase induction. After a protocol amendment, patients with complete hematologic remission but molecular relapse (minimal residual disease [MRD] level > 10−4) could be entered but are not part of this analysis. Patients with evidence of central nervous system (CNS) involvement that required either intrathecal therapy or CNS irradiation, history of seizures, or grade III-IV history of neurotoxicity and other standard exclusion criteria could not be included. No chemotherapy within 7-10 days before study entry was allowed. Initially, patients in first relapse could only be entered after failure of the first relapse therapy with exception of relapse after SCT. After an amendment, nelarabine was also given as first treatment of first relapse. Patients who achieved CR after nelarabine treatment and later relapsed were allowed to enter the study again but were analyzed separately.
Study treatment
Nelarabine was administered in patients as a 2-hour infusion of 1.5 g/m2 per day on days 1, 3, and 5. Use of antiemetic prophylaxis and growth factors according to guidelines was allowed but not part of the protocol. Response evaluation was scheduled after hematologic regeneration with the aim to administer the next cycle on day 21.
In the initial phase of the study, SCT was recommended as soon as a CR was achieved to realize SCT before next relapse. Later the recommendation was to administer, if possible, a second cycle to improve response rate and further reduce the MRD level before transplantation. Study treatment was stopped in patients without CR after 2 cycles. Patients with CR eligible for SCT and available donor were removed from the protocol and transferred to SCT according to standard procedures.
Evaluation of response
Response criteria were defined according to standards. CR was defined as no evidence of leukemic blasts in the BM (< 5%). Recovery to > 1500/μL neutrophil granulocytes in the peripheral blood was not always awaited before subsequent SCT, and platelet recovery > 100 000/μL was not mandatory. If present, all extramedullary manifestations had to be resolved. Partial remission (PR) was diagnosed in case of BM blast count < 25% or in patients with extramedullary involvement a reduction of manifestations by at least 50%. Stable disease was defined as persistence of extramedullary manifestations with < 50% reduction and no appearance of new lesions. Relapse was defined as reappearance of disease either as unequivocal blasts in the BM (> 5%), in the CNS, or at extramedullary sites after prior achievement of CR. MRD was measured by standard technology based on real-time quantitative PCR of leukemia-specific T-cell receptor and immunoglobulin gene rearrangements.14
Data analysis
The major parameters for efficacy evaluation were achievement of CR after 1 cycle and the overall CR rate after the maximum number of cycles administered in each individual patient. Patients with CR after the first cycle and progression after the last cycle were considered as failure. Efficacy evaluation was performed separately for patients who entered the study a second time (N = 13). Toxicity was analyzed as proportion of patients or cycles with adverse events according to Common Toxicity Criteria Version 3.0.
Response and correlation of response to patient, disease, or treatment parameters were analyzed with the χ2 test. Survival was calculated from the date of first study drug application or date of SCT to death or to the date of the last follow-up. Relapse-free survival after transplantation was defined as the time between transplantation and diagnosis of relapse or date of last follow-up. Survival analysis was performed according to the Kaplan-Meier method, and comparison of survival curves was performed with the log-rank test. The statistical analysis was performed in the GMALL Study Center with the SAS program (SAS-PC Version 8.02; SAS Institute). For all analyses, a P value ≤ .05 was considered as statistically significant.
Results
Patient characteristics
Between May 2001 and September 2008, 133 evaluable patients were enrolled from 15 centers. Seven patients were excluded from the analysis (3 patients younger than 18 years and treated according to a pediatric schedule, 4 patients with MRD only, including 1 patient younger than 18 years). Baseline characteristics of 126 evaluable patients are summarized in Table 1. The median age was 33 years (range, 18-81 years). Most patients were 26-55 years of age (59%), but 13% were older than 55 years. At first diagnosis, 107 patients had been classified as T-ALL (85%) and 19 patients as T-LBL (15%). Immunologic subclassification was documented in 93 T-ALL patients. A total of 39% had early, 48% thymic, 11% mature, and 2% biphenotypic T-ALL.
BM involvement > 5% was detected in 104 patients (83%). A total of 66% had marrow involvement only, 17% extramedullary involvement only, and 17% both. The most frequent extramedullary location was mediastinal tumor with or without pleural effusion (19%). Other locations were pleura, skin, lymph nodes, bone, and lung or thorax wall. The majority of patients had advanced disease. A total of 68% (N = 86) were included at relapse during or after chemotherapy (58% at first relapse and 10% at second relapse). A total of 10% (N = 13) had primary refractory disease. A total of 21% had relapsed after SCT (N = 27; 7 sibling donor, 17 matched unrelated donor, and 3 autologous SCT).
Most patients were heavily pretreated. A total of 93 patients (74%) had been refractory to their most recent treatment approach. Overall, 56 patients (44%) were refractory to prior high-dose cytarabine-based cycles (N = 44 CLAEG, N = 12 FLAG-IDA), other intensive chemotherapies (N = 18), or nonintensive chemotherapy (N = 19) as the most recent treatment approach. Patients classified as primary refractory disease were refractory at least to standard 2-phase induction. Ten of 13 patients were also refractory to at least 1 additional salvage therapy based on high-dose methotrexate or high-dose cytarabine.
Response evaluation
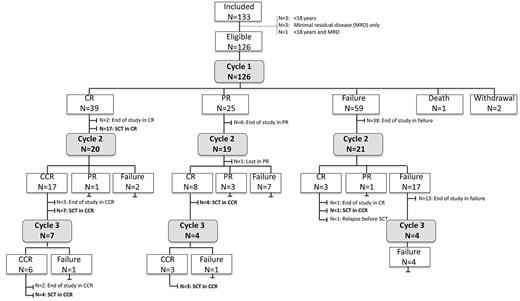
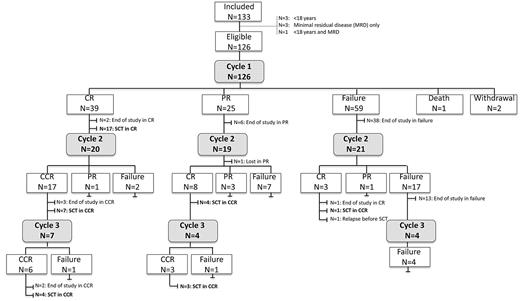
All patients received at least 1 cycle of nelarabine. Two cycles were given in 60 (48%) patients, and 15 (12%) patients had 3 cycles (Figure 1). The median interval from first to second cycle was 22 days (range, 18-50 days) and 21 days (range, 20-36 days) from second to third cycle.
Flow chart with the course of treatment and response in all included patients.
After the first cycle, 40 patients (32%) achieved a CR, 24 (19%) PR, and 59 (47%) had stable disease or progression (Table 2). The overall response rate (CR, PR) was 51%. Three patients did not complete the first cycle. Reasons for early withdrawal were neurotoxicity (N = 1), progression and deterioration of general condition (N = 1). One patient died on therapy because of progression (Figure 1).
A second cycle was administered in 18 patients in PR after 1 cycle, and 7 of these patients achieved a CR (39%). In patients with failure after the first cycle (N = 21), a second cycle yielded CR in 3 patients (14%). On the other hand, only 4 of 21 patients who were treated with additional cycles after initial CR developed progression during subsequent cycles. No additional CRs were achieved after a third cycle (Figure 1). The overall result after 1-3 cycles was 46% in terms of response rate, with 36% CR (N = 45), 10% PR (N = 12), 52% failure (N = 66), 2% withdrawal before evaluation (N = 2), and 1% death on treatment (N = 1).
Thirteen patients entered the study a second time in relapse (8 after matched unrelated SCT, 1 after autologous SCT). Five of these patients achieved a CR after 1 or 2 cycles (38%). Four patients showed nonresponse or relapse after nelarabine in association with the presence of myeloid blast cells. One patient with early T-ALL and 1 patient with biphenotypic leukemia showed after 1 cycle of nelarabine residual infiltration with myeloid blast cells only. Two patients with thymic T-ALL achieved a CR after nelarabine. Both relapsed after transplantation with biphenotypic or bilineage leukemia, respectively. After retreatment with nelarabine, the lymphoid blast population disappeared in both patients whereas the myeloid population persisted.
Eleven patients achieving CR after clinical relapse were also tested for MRD at response evaluation. Six patients reached a MRD level < 10−4 after nelarabine, and 1 relapsed after subsequent SCT (16%). Five patients had MRD > 10−4, 3 relapsed (1 without SCT), and 2 remained in remission. Both of them had achieved a negative MRD status after SCT.
SCT
A total of 36 of 45 patients (80%) who achieved a CR after nelarabine subsequently received a SCT in continuous CR. The realization of transplantation was higher (89%) in CR patients without prior SCT compared with those with a history of SCT (55%; P = .02) and higher in patients younger than 45 years (34 of 37) compared with older patients (2 of 8; P < .0001). The median time from confirmation of CR to transplantation was 21 days (range, 7-104 days). Matched unrelated transplantation was performed in 26 patients, matched sibling in 7, haploidentical in 1, and autologous SCT in 1 patient. The conditioning regimen was 12 Gy total body irradiation with cyclophosphamide in 21 patients (58%). Other conditioning regimens were total body irradiation with etoposide (N = 2), other total body irradiation-based regimens (N = 2), busulfan-based regimens (N = 5), melphalan and fludarabine (N = 5), and treosulfan (N = 1).
Eight patients did not receive a SCT: 3 because of age older than 65 years, 4 because of previous SCT, and 1 patient had no donor. Five patients received a second SCT in CR after nelarabine. One patient died in CR, 2 relapsed, and 2 remained in continuous complete remission.
Detailed data on engraftment were available in subsets of patients. A 2-week period without requiring transfusion of red blood cells at any time up to 100 days after transplantation or until time of death was achieved in 14 of 17 evaluable patients. The time to engraftment of red cells was median 26 days. A platelet count of at least 50 000/μL for 7 days without requiring platelet transfusions was achieved in 15 of 16 evaluable patients. The time to engraftment for platelets was median 22 days. An absolute neutrophil count of at least 500/μL for 3 consecutive days was achieved in 14 of 14 evaluable patients. The time to neutrophil engraftment was median 18 days.
Overall outcome
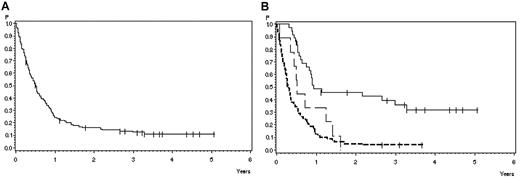
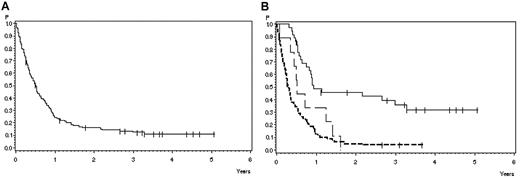
At the time of analysis, 13 of 126 patients treated with the adult protocol were alive (10%), 111 died (88%), and 2 are lost to follow-up (2%). The probability of survival after 1 year is 24% (SE ± 3%) and after 3 years 12% (SE ± 3%; Figure 2A) with a median survival of 6 months.
Overall survival in 126 adult patients with BM relapse. (A) All patients. Survival probability after 1 year, 24% ± 4%; after 3 years, 12% ± 3%. (B) According to response and subsequent treatment. CR with subsequent SCT (solid line), 49% ± 8% after 1 year, 36% ± 8% after 3 years (N = 36); CR without SCT (broad dashed line), 33% ± 16% after 1 year, 0% after 3 years (N = 9); and failure/PR (narrow dashed line), 13% ± 4% after 1 year, 4% ± 2% after 3 years. P = .0001.
Overall survival in 126 adult patients with BM relapse. (A) All patients. Survival probability after 1 year, 24% ± 4%; after 3 years, 12% ± 3%. (B) According to response and subsequent treatment. CR with subsequent SCT (solid line), 49% ± 8% after 1 year, 36% ± 8% after 3 years (N = 36); CR without SCT (broad dashed line), 33% ± 16% after 1 year, 0% after 3 years (N = 9); and failure/PR (narrow dashed line), 13% ± 4% after 1 year, 4% ± 2% after 3 years. P = .0001.
The 3-year survival of patients with failure or PR after nelarabine (N = 81) compared with CR patients (N = 45) was 4% (SE ± 2%) versus 28% (SE ± 7%), respectively (P < .0001). No difference in terms of survival was observed in PR patients compared with patients with failure. Patients with CR after 1 cycle (N = 36) tended to have a better 3-year survival (32% ± 8%) compared with those with later achievement of CR (N = 9; 11% ± 10%; P = .06). Survival probability at 3 years was 36% (SE ± 8%) in 36 patients transplanted in CR after nelarabine compared with CR patients without transplantation in CR after nelarabine (N = 9) with 0% and failure or PR patients with 4% (SE ± 2%; N = 81) survival probability (P < .0001; Figure 2B).
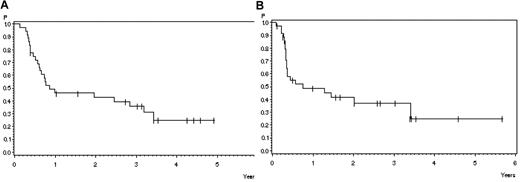
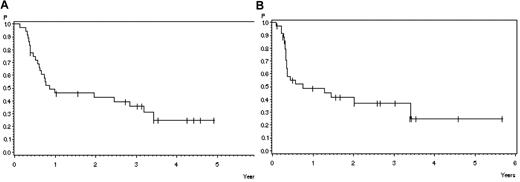
Twelve of 36 patients transplanted in CR are in continuous remission after SCT (33%). Four patients died in CR (11%) related to transplantation (GVHD N = 1, infection N = 3) and 20 patients relapsed (56%). The probability of survival 3 years after transplantation in 36 patients transplanted in CR after nelarabine is 31% (SE ± 8%; Figure 3A), and the relapse free survival at 3 years is 37% (SE ± 9%; Figure 3B). The median time to relapse after SCT was 4 months (range, 1-24) months. In patients alive after SCT, the median survival was 41 months (range, 13-85 months).
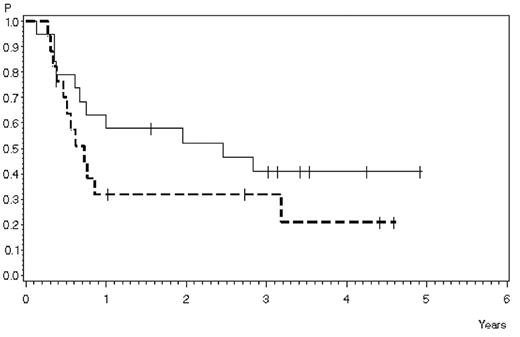
Outcome after stem cell transplantation in patients transplanted in CR after helarabin. (A) Survival: 46% ± 8% after 1 year, 36% ± 8% after 3 years (N = 36). (B) Remission duration: 49% ± 9% after 1 year, 37% ± 9% after 3 years (N = 36).
Outcome after stem cell transplantation in patients transplanted in CR after helarabin. (A) Survival: 46% ± 8% after 1 year, 36% ± 8% after 3 years (N = 36). (B) Remission duration: 49% ± 9% after 1 year, 37% ± 9% after 3 years (N = 36).
Prognostic factors
Pretreatment characteristics were analyzed as potential prognostic factors for achievement of CR and survival (Table 3). Female sex (P = .03), initial diagnosis of T-ALL compared with T-LBL (P = .0004), thymic compared with mature and early T-ALL (P = .02), and BM involvement versus combined or isolated extramedullary involvement (P = .01) had a significant impact on achievement of CR. Age, disease status, and pretreatment had no significant effect on CR rate.
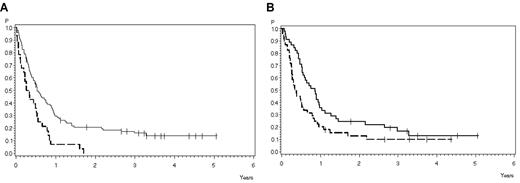
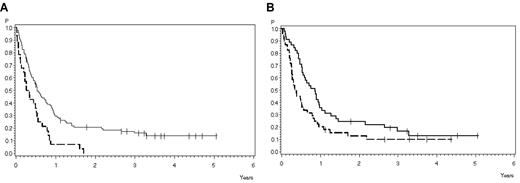
Survival at 3 years was significantly influenced by achievement of CR (see “Overall outcome”). A significant difference in terms of survival was observed between patients younger (16% ± 4%) and older (0%) than 45 years (P = .0007; Figure 4A). Patients with thymic T-ALL had a better survival of 17% (SE ± 6%) compared with 10% (SE ± 5%) in early T-ALL or mature T-ALL (P = .03; Figure 4B). All other factors had no significant influence on survival.
Overall survival according to prognostic factors. (A) According to age: 18-45 years (solid line), 30% ± 5% after 1 year, 16% ± 4% after 3 years (N = 98); older than 45 years (dashed line), 7% ± 5% after 1 year, 0% after 3 years (N = 28). P = .0007. (B) According to subtype. Thymic T-ALL (solid line), 36% ± 7% after 1 year, 17% ± 6% after 3 years (N = 45); and early/mature T-ALL (dashed line), 18% ± 6% after 1 year, 10% ± 5% after 3 years (N = 46). P = .03
Overall survival according to prognostic factors. (A) According to age: 18-45 years (solid line), 30% ± 5% after 1 year, 16% ± 4% after 3 years (N = 98); older than 45 years (dashed line), 7% ± 5% after 1 year, 0% after 3 years (N = 28). P = .0007. (B) According to subtype. Thymic T-ALL (solid line), 36% ± 7% after 1 year, 17% ± 6% after 3 years (N = 45); and early/mature T-ALL (dashed line), 18% ± 6% after 1 year, 10% ± 5% after 3 years (N = 46). P = .03
In the group of CR patients, we analyzed the impact of the number of treatment cycles with nelarabine on outcome of SCT. Survival after SCT at 3 years was 41% (SE ± 11%) for 19 patients treated with > 1 cycle nelarabine compared with 32% (SE ± 11%) for 17 patients treated with only 1 cycle (P > .05; Figure 5).
Survival after SCT. Two or more cycles of nelarabine (solid line), 58% ± 11% after 1 year, 41% ± 11% after 3 years (N = 19); 1 cycle of nelarabine (dashed line), 32% ± 11% after 1 and 3 years (N = 17). P > .05
Survival after SCT. Two or more cycles of nelarabine (solid line), 58% ± 11% after 1 year, 41% ± 11% after 3 years (N = 19); 1 cycle of nelarabine (dashed line), 32% ± 11% after 1 and 3 years (N = 17). P > .05
Toxicity
Toxicity was evaluated in 201 cycles administered in 126 patients. Hematologic toxicity was evaluated in patients without cytopenias at the start of nelarabine. Grade III-IV leukopenia, granulocytopenia, or thrombocytopenia was observed after 41%, 37%, and 17% of the cycles, respectively. A relevant number of patients had elevated glutamic oxaloacetic transaminase (GOT) or glutamic pyruvic transaminase (GPT) before treatment cycles. Hepatic toxicity was analyzed in patients with normal values at the start of treatment. Increases of GOT, GPT, or bilirubin were observed after 6%, 8%, and 3% of the cycles, respectively. After 6% of the cycles, infections grade III-IV were observed. Surprisingly, 1 patient developed pronounced increases of creatine kinase (grade IV), which later decreased without sequelae.
Neurologic toxicities of any degree were observed after 26 cycles (13%) in 20 patients (16%). The majority of events were grade I or II. In 4% of the cycles (N = 4) and 7% of the patients (N = 9), grade III-IV neurotoxicities were observed. In 4 patients, treatment was repeated despite neurotoxicity. In 1 patient, treatment had to be stopped because of a Guillain-Barré–like syndrome with tetraparesis, hallucinations, and reduced vigilance, which developed at day 3 of the first cycle. The symptoms improved slowly after withdrawal. The type of other neurotoxicities is described in Table 4. The most frequent ones were in 6% of the cycles dizziness (N = 13) and mood alterations (N = 12). Syndromes with confusion, cognitive disturbances, impaired consciousness, or memory were observed in up to 4% of the cycles. Most of the symptoms were of transient nature and reversible.
Discussion
We report the so far largest cohort of adults with T-ALL or T-LBL treated with nelarabine. As in previous studies with nelarabine, both T-ALL and T-LBL had been included because of the similar biologic background of these entities.12-15 Overall, the patient group was negatively selected because the majority of patients (74%) had refractory disease, in most cases even refractory to high-dose chemotherapy regimens. A total of 21% had a relapse after SCT. In this setting, a CR rate of 36% and an overall response rate of 46% appear to be a favorable result for a single-drug treatment. Results from a previous study in adult ALL with 39 patients were comparable with CR rates of 31% and an overall response rate of 41%.12 In a pediatric study with 106 patients, the CR rate was 26% and the response rate 33%.11 The lower response rates in pediatric patients may be the result of a different disease biology, more intensive pretreatment, but also to the different schedule, particularly dose reductions in patients with extramedullary involvement.
Of note is the observation that 42% of the patients with a PR after the first cycle achieved a CR after the second cycle compared with 14% in those with failure. These data support the strategy to administer a second cycle in all patients with partial remission and acceptable tolerability to achieve additional complete remissions and to improve remission quality. The CR rate in patients with relapse after SCT was still 33%. In this situation, treatment options are limited and the limited BM toxicity of nelarabine is a favorable feature. Additional treatment approaches (eg, the use of donor lymphocyte infusions or more rapid realization of a second transplantation) may improve the outcome.
Several clinical parameters influenced response to treatment. The chance to achieve a CR was best in patients with thymic T-ALL (56%), which underlines the better chemosensitivity of this subgroup, which is apparently maintained at relapse in some of the patients. Age had no prognostic impact on CR rate but on overall survival. This underlines the good tolerability of nelarabine independent of age on one hand. On the other hand, survival is mainly influenced by the realization rate of SCT, which was lower in older patients. There was no difference in terms of transplantation-related mortality. In addition, in older patients with relapsed T-ALL/T-LBL, nelarabine is a promising option and a higher SCT rate should be sought.
None of the patients with initial diagnosis of T-LBL achieved a CR. The differentiation of T-ALL and T-LBL was a matter of definition in the previous studies with nelarabine. In 1 previous study in adults, patients were classified as T-ALL if they had > 25% BM blasts at diagnosis or at relapse.12 In a pediatric study, relapsed patients with only extramedullary involvement were classified as T-LBL independent of their presentation at diagnosis.15 Classification of T-ALL or T-LBL according to the status at first diagnosis, as in our trial, takes into account that disease biology of T-LBL may be different from T-ALL as underlined by studies showing different gene expression profiles for T-ALL and T-LBL.16,17 On the other hand, patients with any type of extramedullary involvement at relapse achieved a lower CR rate compared with those with BM involvement only (21% vs 43%). In addition, in a pediatric trial, the response rate appeared to be low (14% PR, no CR) in case of extramedullary involvement compared with BM involvement, which may be however also because of the lower dose (400 mg/m2 daily for 5 days) used in the former group.11 The poorer response of extramedullary manifestations may be the result of different reasons (eg, poorer penetration of the drug into lymphomatous masses), specific locations (eg, pleural effusions), or a different biology of diseases prone to infiltrate extramedullary locations. Additional local treatments, such as intrapleural chemotherapy or local irradiation, drugs with prolonged activity, such as liposomal preparations or alternative schedules (eg, with prolonged infusion times or treatment duration) may be of relevance in these patients.
Intensity of prior treatment could be another important factor for response to nelarabine, as it indirectly indicates the degree of chemotherapy resistance. In adult patients treated with nelarabine, a higher response rate was observed for patients with 1 prior treatment approach (55%) compared with patients with at least 2 prior regimens (36%).12 In pediatric patients, a phase 2 study revealed a better CR rate in first compared with second relapse (48% vs 23%).11 We did not observe a difference in terms of response rate comparing patients with first relapse, second relapse, or relapse after SCT. Patients with primary refractory disease, representing a selection of patients with resistance to all ALL-specific standard drugs, however, rarely responded (7% CR).
Patients with CNS involvement were not included in our trial as well as in another trial for adult ALL, and parallel intrathecal therapy was prohibited to avoid potential cumulative neurotoxicity. In a pediatric phase 2 study, 8 of 22 patients with cerebrospinal fluid involvement showed blast cell clearance after nelarabine at a dose reduced schedule (400 mg/m2 daily for 5 days). In these patients, intrathecal therapy was scheduled at day 7 of the nelarabine cycle and then repeated weekly. No additional neurologic toxicities were reported.11 In addition, data from nonhuman primates showed an excellent penetration of nelarabine and ara-G into the cerebrospinal fluid.15 Therefore, studies of nelarabine in patients with CNS involvement would be of interest, and future trials may address the question whether and how different doses of nelarabine can be combined with intrathecal prophylaxis in the interval between nelarabine cycles.
The overall survival after 1 year was 24% with a median survival of 6 months, which is in line with other reports on nelarabine with 28% survival after 1 year and 5 months median survival,12 overall results of relapsed adult ALL4-6 or relapsed T-ALL,3 although this patient group was negatively selected. Results compare favorably with another cohort of adult ALL patients with second attempt of salvage therapy (N = 53) with 18% CR and 3 months median survival.18 Survival was mainly affected by achievement of CR and realization of SCT. The better outcome of younger patients and those with thymic T-ALL is certainly confounded by these factors. The prognostic value of CR achievement and realization of SCT underlines the relevance of these endpoints for future clinical trials with new drugs.
For the first time, data on a large cohort of patients with SCT after nelarabine could be presented. The realization of transplantation in CR patients was very high (80%) compared with other studies11,12 because the donor search for related or unrelated donor had already taken place beforehand. This also applies to the high rate of second transplantations in patients with relapse after SCT (55%). This result indicates that optimal logistics for transplant preparation are essential for treatment optimization in relapsed ALL. The overall survival after transplantation was 36% at 3 years with a probability of long-term remission of 37%. This is a reasonable cure rate for relapsed adult ALL, which requires however further improvement.
Of note is the fact that no unusual problems with engraftment occurred and transplantation-related mortality was very low (11%). The major problem was the high relapse rate after transplantation (56%), which was also described in other studies on relapsed ALL.4-6 The attempt was made to perform SCT as soon as possible after nelarabine single-drug treatment because the remission duration was expected to be short. Therefore, median time to transplantation was only 21 days. This approach can be questioned now. For further treatment optimization, it may be essential to give more cycles of nelarabine before SCT. It has been demonstrated in pediatric patients with relapsed ALL that the relapse rate after transplantation is correlated to the MRD level before transplantation.19,20 In our study, CR patients treated with 2 cycles before SCT compared with those treated with 1 cycle showed a trend toward a better survival (41% vs 32%). The difference was however not statistically different, which may be the result of the small patient number. In addition, patients with negative MRD status after nelarabine tended to have a lower relapse rate after transplantation.
It is remarkable that, in these heavily pretreated patients, the toxicity of nelarabine treatment was limited. No toxicity-related death on treatment was observed. The incidence and duration of grade III-IV neutropenia (37%) and thrombocytopenia (17%) was low in this and other trials with nelarabine, with incidences of 37% and 26%, respectively.12 This feature makes nelarabine a suitable compound for combination treatments, for relapse after SCT, and for older patients.
In previous trials, neurotoxicity had been the dose-limiting toxicity. In this study, most patients had been pretreated with several CNS directed approaches, including CNS irradiation as part of front-line therapy, intrathecal therapies, high-dose cytarabine or methotrexate, and all patients had been pretreated with vincristine. A total of 16% of all patients experienced neurotoxicities, but only in 7% of the patients did toxicity reach grade III or IV. Individual patients tended to develop several neurologic symptoms, but they were generally transient and reversible. The incidence of severe neurotoxicities was also limited in other adult series with only 1 grade IV event in 39 patients,12 whereas 18% of pediatric patients experienced grade III-IV toxicities despite the dose reduced schedule.11 This may indicate a higher vulnerability of pediatric patients. Because severe neurologic toxicities may occur also in adults21 and cannot be predicted so far, all patients should be closely observed.
In conclusion, nelarabine has an impressive single-drug activity in highly resistant relapsed T-ALL and is well tolerated, even in heavily pretreated patients. Because a durable remission cannot be expected with chemotherapy alone, a high proportion of CR patients were transferred to SCT, and long-term relapse-free survival was achieved in one-third of the patients. Because SCT is the only chance of cure in relapsed ALL, an immediate donor search should be initiated in all eligible patients. For future optimized use of nelarabine, several issues should be considered. The mechanisms of resistance to nelarabine are so far not clear. It might be possible, however, to predict response by evaluation of intracellular ara-GTP accumulation.22 Such predictive tests would be helpful to reasonably focus salvage therapies on patients with expected benefit. Optimal combination regimens have so far not been defined, but interim results for nelarabine with multidrug combination regimens for pediatric ALL did not show excess toxicity.23 In relapse, nelarabine should be given earlier, already at first relapse or at MRD relapse. The application of 2 cycles may improve the CR rate and reduce the level of MRD in responding patients before transplantation. In older relapse patients without indication for SCT, maintenance therapy with nelarabine could be an option because of the good tolerability. Finally, the integration of nelarabine in front-line therapy is of interest and will be evaluated in the forthcoming GMALL study 08/2011 for adult ALL.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Ms Roswitha Kotthoff for study coordination and data management; the representatives of the National Cancer Institute Cancer Treatment Evaluation Program, particularly M. Boron, for their organizational support and provision of the study drug; and the GMALL study group for referring patients to the participating study centers and for follow-up documentation.
Authorship
Contribution: N.G. and D.H. designed the research; N.G. coordinated the study, performed the statistical analysis, and wrote the manuscript; and all coauthors recruited study patients, performed study procedures, collected and verified data, and reviewed and approved the manuscript.
Conflict-of-interest disclosure: N.G. and D.H. have received research support from GlaxoSmithKline. N.G. and R.N. have received speakers honorarium from GlaxoSmithKline. The remaining authors declare no competing financial interests.
For a complete list of referring centers, see the supplemental Appendix (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
The current affiliation for R.N. is Stiftungsklinikum Mittelrhein, Koblenz, Germany. The current affiliation for N.B. is Malteser Krankenhaus St Franziskus-Hospital, Flensburg, Germany.
Correspondence: Nicola Gökbuget, Goethe University Hospital, Department of Medicine II, Hematology/Oncology, Theodor Stern Kai 7, 60590 Frankfurt, Germany; e-mail: goekbuget@em.uni-frankfurt.de.