Abstract
We conducted this study to determine the feasibility and safety of cladribine followed by rituximab in patients with hairy cell leukemia including the vari-ant form (HCLv). Cladribine 5.6 mg/m2 given IV over 2 hours daily for 5 days was followed ∼ 1 month later with rituximab 375 mg/m2 IV weekly for 8 weeks. Responses were recorded and BM minimal residual disease (MRD) was evaluated after the completion of rituximab. Thirty-six patients have been treated including 5 with HCLv. Median age was 57 years (range, 37-89). All patients (100%) have achieved complete response (CR), defined as presence of no hairy cells in BM and blood with normalization of counts (absolute neutrophil count [ANC]> 1.5 × 109/L, hemoglobin [Hgb] > 12.0 g/dL, platelets [PLT] > 100 × 109/L), as well as resolution of splenomegaly. There were no grade 3 or 4 nonhematologic adverse events directly related to the treatment. Only 1 patient (with HCLv) has relapsed; median CR duration has not been reached (range,1+-63+ months). Three patients with HCLv died including 1 with relapsed disease and 2 from unrelated malignancies. Median survival duration has not been reached (range, 2+-64+ months). Treatment with clad-ribine followed by rituximab is effective tk;4and may increase CR rate. This study was registered at www.clinicaltrials.gov as NCT00412594.
Introduction
Over the past quarter century, we have witnessed remarkable progress in the treatment of patients with hairy cell leukemia (HCL).1,2 The nucleoside analogs cladribine and pentostatin have made a significant impact in the treatment of HCL producing complete response (CR) rates of 80% to 90%.3,4 Long-term follow-up of patients treated with these agents have shown that about one-fourth to one-third of patients relapse after a follow-up of 3 to 4 years with little difference between the 2 drugs, in terms of durability of response.5-7 Previous reports have suggested that the quality of the initial response can be predictive of the outcome, with a longer disease-free survival in those achieving a CR after initial therapy as opposed to those with lesser responses.7-9 This has led to the suggestion by some authors to persist with therapy, in the absence of toxicity, until a CR is attained,7 and to efforts to eradicate minimal residual disease (MRD) after the initial therapy. This is particularly important as the median age of patients with HCL is in the 50s, and subsequent CRs may be shorter in duration with successive therapy.5,10,11
Determination of the risk of relapse based on the persistence of MRD determined by immunohistochemistry (IHC) using anti-CD45RO, anti-CD20, and DBA.44 in paraffin-embedded BM sections was first reported by the group from Northwestern University.12 More recently, more precise methods using immunophenotyping by flow cytometry, and consensus primer or clone-specific PCR analysis of Ig receptors, have been used to detect MRD in patients with HCL.13-15 Whether eradication of MRD should be a goal of therapy in the initial management of patients with HCL remains a subject of discussion.16 Sigal et al have reported that among 19 patients with HCL in continuous CR after 1 course of cladribine (median time from therapy, 16 years, [range, 11-21 years]) in whom BM samples were available, 7 (37%) had MRD, and 3 (16%) had morphologic evidence of HCL suggesting that patients with MRD or even morphologic disease can live many years without a hematologic relapse.17
We have previously reported on the efficacy of rituximab in eradicating MRD in patients with HCL treated with cladribine.14 Among the 13 patients, reported MRD assessed by consensus primer PCR or flow cytometry was eradicated in 92% at 3 months. We have reported a brief summary of this phase 2 trial including patients with relapsed disease recently for a collective report of a meeting of hairy cell leukemia experts at the National Institutes of Health.18 Here we report on the cohort of previously untreated patients and in greater detail. This includes 11 untreated patients reportedly previously and 5 patients with previously untreated variant HCL (HCLv). All previously treated patients reported in prior reports were excluded from this study.
Methods
Eligibility
Patients were eligible if they had a new diagnosis of HCL with active disease. Diagnosis of HCL was based on morphologic evaluation of peripheral blood, BM aspirates, and core biopsies, in combination with a characteristic flow cytometric immunophenotype (bright positivity for CD22, CD11c, and CD103). Most cases were also stained for tartrate-resistant acid phosphatase (TRAP) on aspirate smears, and all tested cases were positive. Patients were also eligible to participate if they had the HCLv.19 Active disease was defined as 1 or more of the following: (1) hemoglobin (Hgb) < 10 g/dL or transfusions of at least 2 units of packed RBCs per month, absolute neutrophil count (ANC) < 1.5 × 109/L, platelet (PLT) count < 100 × 109/L, or > 25% decline from baseline over 3 months in 1 or more cell lines; (2) circulating hairy cells at least 1 × 109/L or extramedullary HCL; and (3) recurrent infections, progressive decline in performance status, or symptomatic splenomegaly. Patients were required to have an Eastern Cooperative Oncology Group (ECOG) performance status of 2 or less and adequate organ function with a serum bilirubin of ≤ 3.5 mg/dL, aspartate aminotransferase/alanine aminotransferase (AST/ALT) < 3 times the upper limit of normal and creatinine ≤ 2.0 mg/dL. Higher values were acceptable if they were directly related to the disease. Patients with known infection with HIV, or hepatitis B and C were excluded from the study.
Treatment plan
Cladribine 5.6 mg/m2 was given intravenously over 2 hours daily for 5 days. Approximately 28 days after initiation of cladribine, BM examination was repeated and then 8 weekly doses of rituximab 375 mg/m2 were administered. After the completion of therapy with rituximab a repeat BM examination for assessment of response and evaluation of MRD was performed. Further follow-up has been conducted every 3 months for the first year, then every 6 months with peripheral blood evaluations including flow cytometry assays for MRD.
Supportive care
Prophylactic antibiotics including levofloxacin, valcyclovir, and fluconazole (or equivalent agents) were administered at the discretion of the treating physician. Tumor lysis prophylaxis with allopurinol (or alternative similar agents) and growth factors such G-CSF or its long-acting version was administered at the discretion of treating physician. Transfusion support with irradiated and filtered packed RBCs and/or platelets as well as appropriate intravenous antibiotics was provided when indicated.
Criteria for response and statistical considerations
CR was defined as the absence of hairy cells on BM aspirate smears and biopsy specimens or the presence of < 1% atypical cells (not definitively called hairy cells) in BM and blood, and the disappearance of all evidence of HCL on physical examination. Achievement of CR required an ANC of ≥ 1.5 × 109/L; Hgb at least 12.0 g/dL (at least 11.0 g/dL for females); and platelet count at least 100 × 109/L without growth factor or transfusion support. Responses were evaluated after completion of therapy with rituximab generally at ∼ 3 months after the initiation of treatment.
The study was conducted with early stopping rules to monitor futility and excess toxicity. These stopping boundaries were not reached. Differences in subgroups by different covariates were evaluated using the χ2 test or Fisher exact test for categorical variables, and the Mann-Whitney U for continuous variables.
BM histology and IHC
Formalin-fixed, paraffin-embedded BM core biopsies were stained with H&E for morphologic evaluation, at all posttherapy time points. For the majority of time points, IHC staining was performed on biopsy sections by standard techniques, for a pan-B-cell marker other than CD20. Such Abs included PAX-5 (1:35; BD Biosciences Transduction Laboratories) or CD79a (1:50, DAKO). Cases showing B-cell clusters or aggregates (∼ 20 cells or more) by IHC were scored as positive for residual disease.
MRD and immune status assessment by multiparameter flow cytometry
Peripheral blood and BM specimens for MRD assays were stained with a 4-color panel of Abs with the following combinations (FITC/PE/PerCP-Cy5.5/allophycocyanin): CD20/CD103/CD45/CD19, CD22/CD11c/CD45/CD19, CD20/CD25/CD45/CD19, Igκ/CD22/CD45/CD19, Igλ/CD22/CD45/CD19, and Igκ/Igλ/CD19/CD22. All Abs were from BD Biosciences. Abs were added to 106 mononuclear cells per tube in 100 μL of whole blood or marrow (diluted with PBS and 1% FBS as needed), and incubated for 10 minutes at room temperature. Erythrocyte lysis was performed using BD PharmLyse (BD Biosciences), followed by a wash with PBS containing 0.1% sodium azide, using a Sorvall Cell Washer 2. Cells were resuspended for acquisition in PBS containing 1% formaldehyde. For tubes containing anti-κ or anti-λ Abs, lysis, and washing were performed before cell-surface staining. Data were acquired on FACSCalibur flow cytometers (BD Biosciences) using BD CellQuest Pro software. CD19-positive B-cells were selectively gated, with from 40 000 to 600 000 total cells acquired per tube, depending on sample quality. Data were analyzed using CellQuest Pro (BD Biosciences).
Hairy cells were identified on the basis of bright coexpression of CD11c and CD22, positivity for CD103 and CD25 (moderate to bright), and clonal expression of surface Ig light chains on CD22 bright B cells. Hairy cell variants were negative for CD25. The limit of sensitivity was established using control specimens consisting of 10 BM aspirates, acquired for staging of patients with other B-lineage lymphomas, all negative for malignancy, stained with the same Ab panel. There were small subsets of CD19-positive cells in most control marrows which were also positive for CD11c bright/CD22 bright. Thus, this marker combination was noncontributory for MRD studies. However, the background of CD103+ cells in control marrows was low (< 0.02% of total cells), and CD25 bright+ cells showed similarly low levels. Therefore, in cases with background populations of normal B cells, the limit of detection of residual HCL was 0.02%. In specimens with no normal background B cells, HCL populations with multiple phenotypic aberrancies could be detected at a level of 0.05% to 0.003% (20 aberrant cells of 40 000-600 000 collected).
Establishment of sensitivity limits.
For many MRD assays by multiparameter flow cytometry (MFC), the critical factor in determining sensitivity is actually the level of rare background normal cells (or nonspecific events) with a similar phenotype to the neoplastic cells. Before undertaking the assay, we therefore studied the levels of normal marrow cells with various Ag combinations. We found the lowest levels with CD19+CD103+ coexpression, with up to 0.02% of normal BMA cells showing this pattern. We can collect enough cells to identify 20 aberrant cells in 200 000 or more (0.01%), so our sensitivity is not limited by technical factors, but by normal background.
CD20 expression and its potential loss after rituximab.
We based our quantitation of residual hairy cells on coexpression of CD19 and CD103, and CD19 and CD25 coexpression, as well as kappa/λ staining on CD19 bright and CD22 bright cells. We considered it of interest to examine CD20 expression on the residual hairy cells, so CD20 was included in the tube with CD19 and CD103. We did not fail to detect MRD because of loss of CD20.
Immune status assessment.
For immune status assessment, PBLs were stained with the following Ab combinations (FITC/PE/PerCP-Cy5.5/APC): CD8/CD4/CD45/CD3 and CD3/CD56/CD45/CD19. All Abs were from BD Biosciences. Erythrocytes were lysed using FACSLyse (BD Biosciences). Data were acquired and analyzed as described above.
MRD assessment by PCR
Total DNA was extracted from PB or BM aspirate samples using an automated method (Autopure; Genta). B-cell clonality was determined using a PCR method using V primers derived from the framework 1 (FR1), framework 2 (FR2) and framework 3 (FR3) regions, in combination with either a consensus JH or CH primer with detection by capillary electrophoresis.20
IGHV gene analysis
Total RNA was extracted from BM aspirate or peripheral blood using TRIzol, converted to cDNA using Superscript II (Invitrogen) and used to amplify the clonal IgH gene rearrangements by a PCR method using V primers derived from the leader region with either a consensus JH or CH primer.21 The dominant IgH variable region (IGHV) clone product(s) was then sequenced by standard Sanger methods. Divergence from germline IGHV segments (IMGT database) was calculated using VBASE (VBASE Sequence Directory, I. M. Tomlinson, MRC Center for Protein Engineering, Cambridge, United Kingdom; www2.mrc-lmb.cam.ac.uk/vbase); 2% or less changes over codons 1-94 of IGHV were regarded as unmutated. The VH segment used was also recorded.
Soluble IL-2R analysis
Soluble IL-2R (soluble CD25 [sCD25]) as well as other soluble receptors such as sCD22 have been previously reported to be useful in monitoring disease burden in patients with lymphoid neoplasms including HCL.22 The assay was performed by Focus Diagnostics Inc using the Quantikine (R&D Systems Inc) ELISA kit. This kit uses a sandwich enzyme immunoassay methodology consisting of an immobilized mAb against sCD25 and a polyclonal Ab against sCD25 that is linked to HRP. Addition of a substrate results in the production of color, the intensity of which is proportional to the concentration of sCD25 in the sample. Results are calculated by comparison to a standard curve.
Results
Patients
Between June 2004 and October 2009, 36 consecutive patients with untreated HCL or HCLv were treated. All patients signed an informed consent form in accordance with the Declaration of Helsinki, approved by the University of Texas M. D. Anderson Cancer Center Institutional Review Board. They included 31 pati-ents with newly diagnosed HCL and 5 with untreated HCLv. The median age of the patients was 57 years (range, 37-89 years). Twenty-one of 25 evaluable patients had mutated IGHV, whereas IGHV was unmutated in 4 patients (5 patients had an inadequate specimen and 6 were not done). FISH was positive for the presence of a clone with p53 deletion/monosomy 17 in 4 of 5 patients with HCLv. IGVH was mutated in all patients with HCLv. Patient characteristics are summarized in Table 1.
Response and follow-up
All 36 patients treated achieved a complete response after the completion of treatment with rituximab. The morphologic and/or immunohistochemical examination of the first BM examination which was performed ∼ 1 month after the initiation of cladribine showed persistent disease in 12 (44%) of 27 evaluable patients. This became negative in all patients in the subsequent BM evaluations.
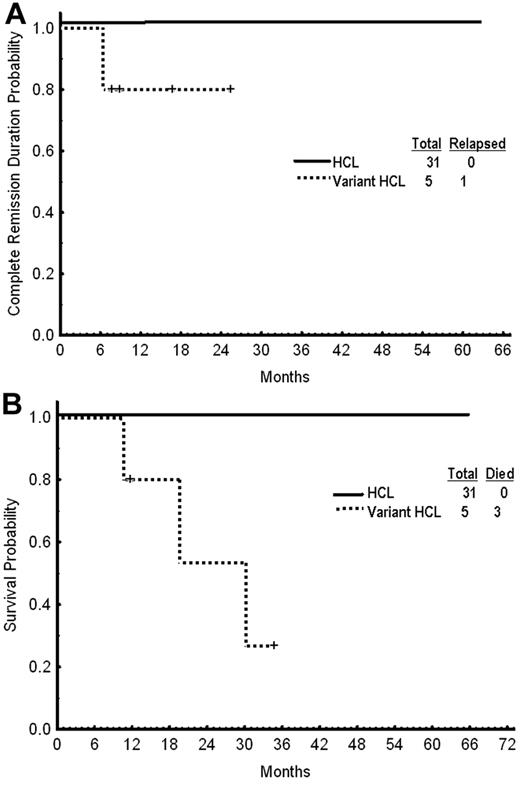
With a median follow-up of 25 months (range, 2-68), only 1 patient (with HCLv) has relapsed and the median CR duration has not been reached (range, 1-63 months; Figure 1). Three patients with HCLv have died including the 1 with relapsed disease and 2 from unrelated malignancies (Figure 1B). One patient with HCLv developed pancreatic cancer and another developed metastatic lung cancer. No other secondary cancer has been reported among the rest of the 36 patients.
Outcome after treatment. (A) Complete remission duration. (B) Overall survival by subgroup.
Outcome after treatment. (A) Complete remission duration. (B) Overall survival by subgroup.
Monitoring residual disease
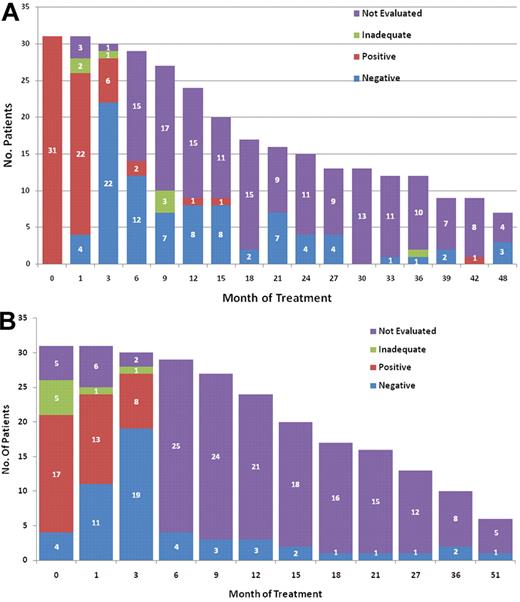
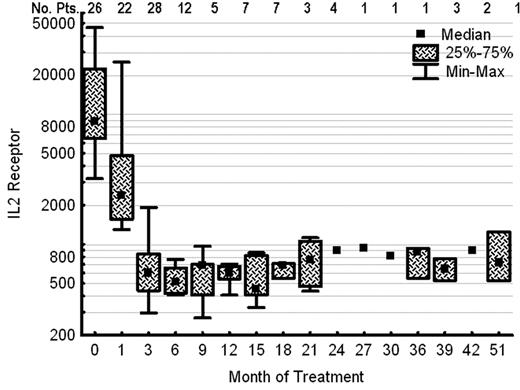
Figure 2 shows the follow-up MRD assessments in patients with newly diagnosed classic HCL. MRD by MFC was positive in 22 (85%) of 26 evaluable patients in a BM specimen obtained ∼ 1 month after therapy with cladribine and became negative in 22 (79%) of 28 evaluable patients in a subsequent BM performed after the completion of treatment with rituximab (Figure 2A). MRD assessed by consensus primer PCR was positive in 13 (54%) of 24 evaluable patients after treatment with cladribine and be-came negative in 19 (70%) of 27 evaluable patients after ritux-imab (Figure 2B). Median serum IL-2R level (reference range, 200-1100 U/mL) before initiation of therapy was 8753 U/mL (range, 3183-46 234). This fell to 2376 U/mL (range, 1298-25 071), after cladribine and before the initiation of treatment with rituximab, and to 602 U/mL (range, 295-1910) after rituximab (Figure 3). Because of small patient numbers and as the majority of the patients became MRD negative and their sCD25 level decreased to within the normal range, it is impossible to correlate these values to time to CR or MRD negativity. Follow-up BM or peripheral blood specimens for the evaluation of MRD by MFC was available in selected patients and remained negative in the majority, as shown in the Figure 2. So far, conversion from a negative to a positive has not been predictive for relapse.
Monitoring MRD. (A) MRD by multiparameter flow cytometry (MFC) for patients with classic hairy cell leukemia (n = 31). Testing on the first 3 time points was done on BM specimens and the rest on peripheral blood. (B) MRD by IgH PCR for patients with classic hairy cell leukemia (n = 31; testing not done for all patients at diagnosis). The results for MFC and PCR at various time points do not always belong to the same patients because of sample inadequacy or lack of testing. MRD evaluations for patients with HCLv were not included in these figures.
Monitoring MRD. (A) MRD by multiparameter flow cytometry (MFC) for patients with classic hairy cell leukemia (n = 31). Testing on the first 3 time points was done on BM specimens and the rest on peripheral blood. (B) MRD by IgH PCR for patients with classic hairy cell leukemia (n = 31; testing not done for all patients at diagnosis). The results for MFC and PCR at various time points do not always belong to the same patients because of sample inadequacy or lack of testing. MRD evaluations for patients with HCLv were not included in these figures.
Serum IL-2R levels (units per milliliter) before and after therapy in patients with hairy cell leukemia (n = 26).
Serum IL-2R levels (units per milliliter) before and after therapy in patients with hairy cell leukemia (n = 26).
Comparing MFC to IHC for MRD detection
Given the availability of a high-sensitivity MFC assay, many pathologists did not routinely perform IHC on specimens from HCL patients posttherapy. However, IHC studies were performed at the discretion of the evaluating pathologist, and thus several posttherapy specimens in this study were tested by both MFC on aspirates and IHC on trephine biopsies. Twenty-four cases were studied by IHC and MFC after therapy with cladribine, and an additional 20 were studied by both techniques after rituximab. Of these 44 cases, 32 (73%) showed concordant results with both IHC and MFC (13 positive for MRD, and 19 negative). Eleven (25%) of 44 were positive by flow cytometry but nondiagnostic by IHC, which highlighted only scattered B-lineage cells, in most specimens accounting for < 5% of cellularity. Only one specimen of 44 (2.2%) was positive by IHC and negative by MFC.
Comparing MRD detection in peripheral blood and BM
To determine the sensitivity of MRD detection in peripheral blood versus BM aspirate specimens: we compared information on concurrent specimens from the patients, including 28 paired peripheral blood and BM aspirate specimens (11 after initial treatment with cladribine and 17 after a course of rituximab). Twenty-three (82%) showed concordant results for residual HCL (10 positive and 13 negative), while the remaining 5 were positive in the BM and negative in peripheral blood. Thus, peripheral blood in this study was less sensitive for MRD assessment than BM, but was of course more convenient to the patients undergoing repeated sampling.
Toxicity and immunosuppression
The regimen was well tolerated with no severe or unexpected toxicity. There were no grade 3 and 4 nonhematologic toxicity related to therapy. Twelve patients (33%) had 15 episodes of reversible grade 3 and 4 infections including neutropenic fever, cellulitis, and herpes zoster dermatitis. All were considered possibly related to the treatment with the median time from start of therapy to infection being 9 days (range, 4-71 days). Only 7 (19%) patients did not receive prophylactic antibiotics. However, there was no difference between the incidence of grade 3 and 4 infections among those who did or did not receive prophylaxis (P = .2). Furthermore, there was no difference in the median pretreatment CD4 counts (786 vs 726, P = .8) and the median lowest CD4 count at months 0 to 4 (223 vs 220, P = .9) between patients who did or did not have grade 3 or 4 infections. Other adverse events were grade 1 and 2 and included nausea, rash, fatigue, weight gain, weight loss, and fever.
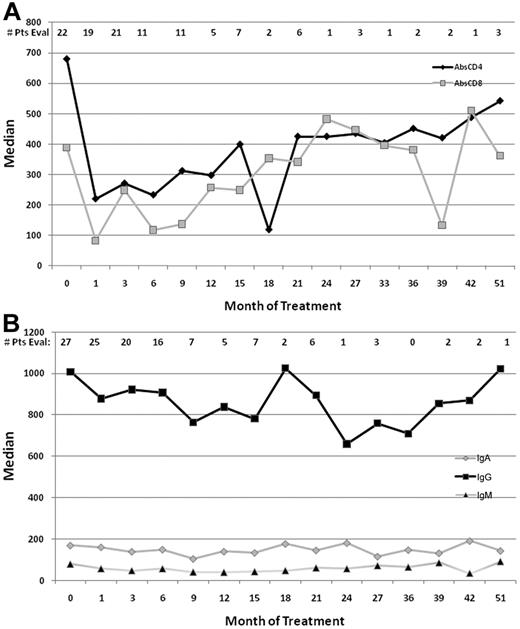
The effect of the addition of an extended course of rituximab on immune function, was evaluated by measuring peripheral blood CD4 and CD8 lymphocyte subsets as well as Ig levels at various time points during the therapy (Figure 4). Treatment with cladribine led to a statistically significant decline in the CD4 count, which remained stable after therapy with rituximab but recovered slowly over a period of ∼ 1 year. Both CD4 and CD8 counts dropped markedly at day 30 (after cladribine; P < .0001 and P = .0003, respectively) and day 90 (after rituximab), compared with day 0 (before starting therapy). However, neither CD4 nor CD8 counts dropped significantly from day 30 to day 90 (by paired Wilcoxon test; Figure 4A).
Immune function parameters. (A) Median absolute CD4 and CD8 counts (cells per microliter) before and after therapy. (B) Ig levels (milligrams per deciliter) pre- and posttherapy.
Immune function parameters. (A) Median absolute CD4 and CD8 counts (cells per microliter) before and after therapy. (B) Ig levels (milligrams per deciliter) pre- and posttherapy.
The median IgG levels declined after therapy with cladribine and rituximab but rapidly reversed with further follow-up. There was no significant drop for IgM and IgA levels (Figure 4B).
Discussion
The nucleoside analogs cladribine and pentostatin are highly effective in the treatment of patients with HCL. However, a minority of patients are resistant to the initial therapy and another significant proportion relapse after an initial response with the relapse-free survival curve not showing a plateau.7,16 In this report we demonstrate the high efficacy of sequential therapy with cladribine and rituximab in patients with HCL including those with HCLv, a group historically resistant to therapy with nucleoside analogs. All (100%) of patients achieved a CR and all (with the exception of 1 patient with relapsed HCLv) remain in CR at a median follow-up of 25 months. It would be impossible to determine whether the responses assessed at 3 months were attributable to the combination and not cladribine alone and whether persistent morphologic disease or MRD after 1 month would predict a lack of response to cladribine eventually.
A recent report suggested that patients with HCL may harbor MRD and even morphologically evident disease many years after initial therapy without experiencing overt relapse.17 This raises the question of whether eradication of MRD should be the goal of therapy in HCL. However, several studies have suggested that patients who achieve a morphologic CR have a better outcome than those with lesser responses.10,11 It remains debatable whether the outcome can be further improved by eliminating all evidence of the disease by seeking and eradicating MRD.
The role of rituximab in the management of indolent lymphoid neoplasms is now well established. Several trials have explored its use after the achievement of a response to chemotherapy and in a maintenance strategy.23-26 In general, these reports have suggested a benefit for prolonging the CR duration and progression-free survival (PFS) in patients with low grade lymphoproliferative disorders. This has not been associated with any significant in-crease toxicity or immune dysfunction. Our report also suggests that a protracted course of rituximab after induction with cladribine may be beneficial in converting residual disease to CR; whether this translates to a universal benefit for all patients with HCL in prolonging CR, PFS, and most importantly survival, requires larger randomized trials with longer follow-up. Considering the relatively high number of infections, further evaluation of infection prophylaxis should also be studied in future trials.
The important question is the cost of this therapy in all patients with HCL and whether it is justified to treat the whole population when the benefits are likely to be limited to a minority of patients with the disease. Recent studies have examined potential predictors of outcome in HCL and have identified subsets at higher risk of failure following treatment with nucleoside analogs.27,28 In a study from the National Institutes of Health, patients with VH4-34 IGHV gene rearrangement were more frequently unmutated, had a greater white blood cell count (WBC) at diagnosis, and significantly lower response rate (P < .001) and PFS (P = .007) after initial cladribine treatment, as well as shorter overall survival (P < .001).28 In another study, among 58 patients with newly diagnosed disease, 6 expressed unmutated IGHV and this was associated with a higher likelihood of failure to respond to cladribine (P < .001).27 Whether these predictors can be used to assign patients more at risk of relapse to receive rituximab should be further investigated.
Historically, patients with HCLv have had a worse prognosis with limited response to the nucleoside analogs. In this study, all 5 patients with HCLv treated with cladribine followed by rituximab achieved CR. Interestingly, the only relapse in the study occurred in a patient with HCLv and another 2 patients with this disease died of a secondary cancer. Furthermore, the majority of patients with this disease had evidence of p53 mutation. Of interest, patients with CLL and chromosome 17 deletions/p53 dysfunction have a poor response to fludarabine-based regimens.29
The treatment of patients with HCL leukemia has been a major success in oncology practice. However, whether we can improve on this success will depend on better understanding the biology of this intriguing disease. Several recent studies have better characterized the cell of origin and molecular events occurring in HCL and modern techniques have allowed a better characterization of disease biology.30,31 Here, we have demonstrated the feasibility and potential benefit from chemo-immunotherapy in these patients. Longer follow-up of our patients is needed to clearly determine the superiority of the combination over cladribine alone. Whether rituximab should be administered concurrently with cladribine or sequentially to obtain the maximum benefit also remains unclear. Future studies further exploring these strategies are encouraged. Clearly, the excellent outcome of the majority of patients with HCL who receive cladribine as first-line therapy mandates further, well-designed trials before such a strategy is considered as “standard of care” for all patients.
Presented in part at the annual meeting of the American Society of Hematology, Orlando, FL, December 4-7, 2010.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by a grant from the Hairy Cell Leukemia Research Foundation and by a research grant from Genentech Pharmaceuticals.
Authorship
Contribution: F.R. designed the study, treated patients, collected and analyzed data, and wrote the manuscript; S.O. and H.K. provided advice in designing the study, treated patients on the study, and reviewed and approved the manuscript; J.J., P.C., and R.L. designed and performed MRD studies, collected and analyzed data, wrote the manuscript, and approved the final manuscript; S.P. and M.B. collected and analyzed data; S.Y. collected data; and S.F., A.F., C.K., J.B., D.T., and M.K. treated patients on the study.
Conflict-of-interest disclosure: F.R., S.O., and A.F. have received research support from Genentech Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Farhad Ravandi, Department of Leukemia, The University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Blvd, Unit 428, Houston, TX 77030; e-mail: fravandi@mdanderson.org.