Abstract
Multiple myeloma is a plasma cell malignancy that is heterogeneous with respect to its causative molecular abnormalities and the treatment response of patients. The Bcl-2 protein family is critical for myeloma cell survival. ABT-737 is a cell-permeant compound that binds to Bcl-2 and Bcl-xL but not to Mcl-1. Using a myeloma cell line collection (n = 25) representative of different molecular translocations, we showed that ABT-737 effectively kills a subset of cell lines (n = 6), with a median lethal dose ranging from 7 ± 0.4nM to 150 ± 7.5nM. Of interest, all sensitive cell lines harbored a t(11;14). We demonstrated that ABT-737–sensitive and ABT-737–resistant cell lines could be differentiated by the BCL2/MCL1 expression ratio. A screen of a public expression database of myeloma patients indicates that the BCL2/MCL1 ratio of t(11;14) and hyperdiploid patients was significantly higher than in all other groups (P < .001). ABT-737 first induced the disruption of Bcl-2/Bax, Bcl-2/Bik, or Bcl-2/Puma complexes, followed by the disruption of Bcl-2 heterodimers with Bak and Bim. Altogether, the identification of a subset of cell lines and primary cells effectively killed by ABT-737 alone supported the evaluation of ABT-263, an orally active counterpart to ABT-737, for the treatment of t(11;14) and hyperdiploid groups of myeloma harboring a Bcl-2high/Mcl-1low profile.
Introduction
Members of the Bcl-2 family are critical regulators of apoptosis, and interactions between prosurvival and proapoptotic members may determine cell fate. Structural and functional characteristics divide these members into 3 subgroups: multidomain antiapoptotic members (Mcl-1, Bcl-2, Bcl-xL, Bcl-W, and A1), multidomain proapoptotic members (Bax and Bak), and BH3-only members (Bim, Bid, Bad, Bik, Puma, and Noxa).1 Antiapoptotic molecules antagonize cell death by sequestering either the BH3-only proteins or multidomain proapoptotic members. The presence of Bax or Bak is required to mediate mitochondrial damage.2 Bax and Bak must be activated to oligomerize and form pores in the mitochondrial outer membrane, allowing the release of cytochrome c to the cytosol.
Multiple myeloma (MM) is a plasma cell malignancy that is heterogeneous with respect to its causative molecular abnormalities and the treatment response of patients. Numerous studies have shown that chromosomal abnormalities including full or partial deletion of chromosomes 13 or 17, 1q21 amplification, recurrent 14q32 translocations, or hyperdiploidy are associated with patient outcome.3,4 Analyses of the global gene expression profile of patients have led to the molecular classification of MM patients into different disease subtypes.5 MM remains an incurable disease that requires new therapeutic approaches. The elevated expression of antiapoptotic proteins was observed in MM cells and provides a block in apoptosis associated with resistance to therapy. In particular, Mcl-1 is overexpressed in MM, thus explaining its major role in myeloma survival and resistance to chemotherapy.6-8 In addition to Mcl-1 overexpression and gene and protein expression profiles indicate that Bcl-2 is overexpressed in a subset of MM in both cell lines and patients.9
Recently, we demonstrated that a large collection of human myeloma cell lines (HMCLs) harbors the heterogeneity found in patients that is mainly reflected by molecular translocations.10
ABT-737 is a cell-permeant compound that selectively binds with high affinity to Bcl-2 and Bcl-xL but not to Mcl-1 and antagonizes their antiapoptotic functions.11 It also exhibits single-agent activity against a variety of cell lines and primary clinical samples, particularly in chronic lymphocytic leukemia (CLL).11,12 Thus, the aim of this study was to investigate the efficiency of ABT-737 on HMCLs (n = 25) with different genetic backgrounds (eg, MMSET/FGFR3 translocation, c-MAF, or MAFB translocation and CCND1 translocation)10 and its relationship with the expression of the antiapoptotic proteins. We found that ABT-737 was highly effective in CCND1 subset of HMCLs and identified potential predictors of ABT-737 sensitivity in these HMCLs.
Methods
Cells and culture conditions
The XG-1, XG-2, XG-5, XG-6, XG-7, NAN-1, NAN-3, NAN-7, and BCN HMCLs were derived in our laboratories from primary myeloma cells cultured in the presence of 5% FCS and 3 ng/mL recombinant IL-6 as described previously.13 The KMS-11, KMS-12-PE, KMS-12-BM, and KMM-1 HMCLs were kindly provided by Dr T. Otsuki (Kawasaki Medical School, Kurashiki, Japan); JJN-3 was provided by Dr I. Van Riet(Vrije Universiteit Brussel, Brussels, Belgium); JIM-3 was provided by Dr I. MacLennan (Birmingham Medical School, Birmingham, United Kingdom); Karpas-620 was provided by Dr A. Karpas (Cambridge Clinical School, Cambridge, United Kingdom); and MM.1S was provided by Dr S. Rosen (Northwestern University, Chicago, Illinois). The AMO1, LP-1, L-363, NCI-H929, U-266, OPM-2, and SKMM-2 HMCLs were from DSMZ, and the RPMI 8226 HMCL from the American Type Culture Collection. All HMCLs derived in our laboratory were cultured in the presence of recombinant IL-6.
Immunoblotting
Cells (5 × 106) were resuspended in 150 μL of lysis buffer (10mM Tris, pH 7.6, 150mM NaCl, 5mM EDTA, and 1% Triton X-100) containing 2mM PMSF and 2 μg/mL aprotinin. After 40 minutes on ice, the lysates were cleared by centrifugation at 12 000g for 30 minutes at 4°C. Equal amounts of total protein were separated by SDS-PAGE, electrotransferred to polyvinylidene difluoride membranes, and analyzed following standard procedures. Signals were detected using an ECL kit (Pierce Chemical). The following antibodies were used: Bcl-2 (DakoCytomation Denmark A/S), caspase-3 (Santa Cruz Biotechnology), Bik (N-19; Santa Cruz Biotechnology), caspase-9 (clone F-7; Santa Cruz Biotechnology), Mcl-1 (S19; Santa Cruz Biotechnology), PUMA (Calbiochem, Merck), Bim (Millipore Bioscience Research Reagents), Bcl-xL (BD Biosciences Transduction Laboratories), Bax (Immunotech, Beckman Coulter), Bak (Calbiochem, Merck), and actin (Millipore Bioscience Research Reagents).
Immunoprecipitation
Cells (25 × 106) were lysed in 1% 3-[(3-cholamidopropyl)dimethylammonio]propanesulfonate containing lysis buffer. Whole cell lysates were obtained, precleared with protein A-Sepharose, and incubated overnight with 5 μg of the specific antibody. Immunocomplexes were captured with either protein A-Sepharose or protein G-Agarose. The beads were pelleted, washed 3 times, and boiled in SDS sample buffer. The presence of immunocomplexes was determined by Western blot analysis.
Assays for detection of apoptotic cells and Bax/Bak activation
Cell death was assessed either by annexin-V–FITC or Apo 2.7 PE-conjugated antibody staining (Beckman Coulter). The flow cytometry analysis was performed on an FACSCalibur flow cytometry system using CellQuest software (BD Biosciences).
For Bax and Bak immunofluorescence staining, 5 × 105 cells were fixed using the Intraprep permeabilization reagent kit (Immunotech) following the manufacturer's recommendations. The cells were incubated with anti-Bax (clone 6A7) mAb, anti-Bak (BD Biosciences Pharmigen), or IgG1 isotype control for 20 minutes. The cells were then incubated with anti–mouse- or anti–rabbit-FITC antibodies (Immunotech) for 20 minutes, washed once in PBS, and resuspended in PBS-1% formaldehyde. The flow cytometry analysis was performed as described in the preceding paragraph.
Primary myeloma cells
Blood or bone marrow samples from MMs were obtained either from the Department of Hematology at University Hospital of Nantes or from untreated patients during standard procedures in Intergroupe Francophone du Myélome after informed consent. Plasma cells were obtained after gradient density centrifugation using Ficoll Hypaque and purification with CD138-immunomagnetic beads. In all cases, purity of the plasma cells was higher than 90%, as assessed by morphology.
Gene expression profiling
The gene expression data were normalized with the MAS5 algorithm. The expression levels of the Bcl-2 and Mcl-1 genes in the HMCLs were obtained from the RAGE database (http://rage.montp.inserm.fr/).14 Bcl-2, Mcl-1, and Bcl-xL probes (203685_at, 200797_s_at, and 212312_at, respectively) were selected based on the major variation in expression level. Affymetrix data from a cohort of 414 untreated patients from the Arkansas Cancer Research Center also was analyzed for BCL2 and MCL1 expression using the Amazonia database (http://amazonia.transcriptome.eu/).15 All microarray data are available in the ArrayExpress database under accessions E-TABM-937 and E-TABM-1088.
RNA interference assays and transient transfections
Control nontargeted small interfering (si)RNA, siMcl-1, siBcl-2, siBax, and siBak were purchased from Thermo Fisher Scientific. siRNAs were transfected into cells using the Lipofectamine RNAiMAX (Invitrogen) according to the manufacturer's instructions. In brief, cells were plated at 1 × 106 cells per well in a 6-well plate. After 24 hours, siRNA (100 pmol) was transfected into the cells using Lipofectamine RNAiMAX reagent. After transfection, the cells were incubated for 72 hours and subjected to various analyses. The gene-silencing effect was evaluated by Western blot analysis. KMM-1 cells were transfected with pRc-CMV Bcl-2 as described previously.9
Statistical analysis
Statistical comparisons were done with the Fisher exact test, the Wilcoxon rank sum test, or the Spearman test.
Results
The CCND1 subset of HMCLs with a Bcl-2high/Mcl-1low gene and protein expression profile is highly sensitive to ABT-737
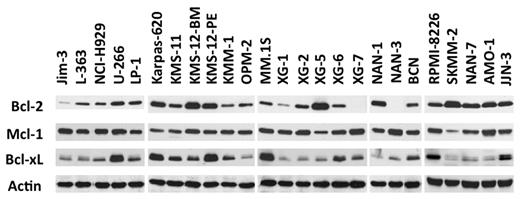
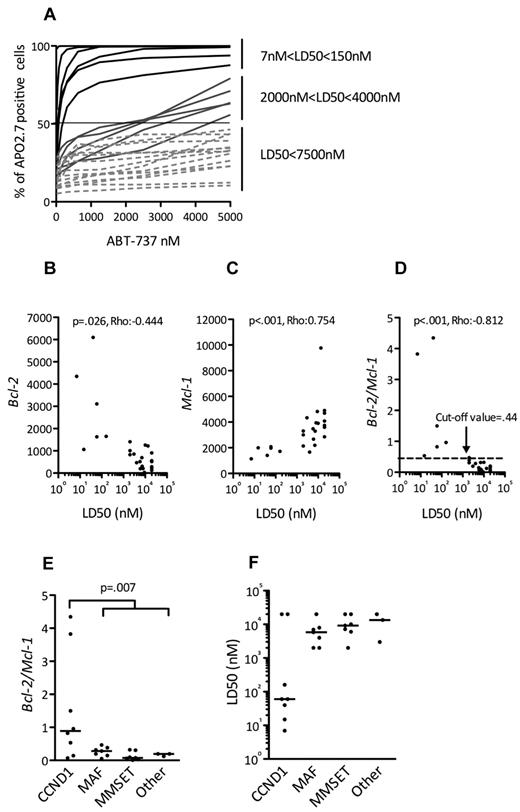
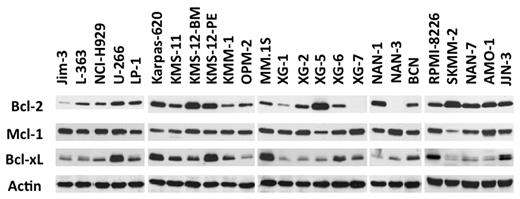
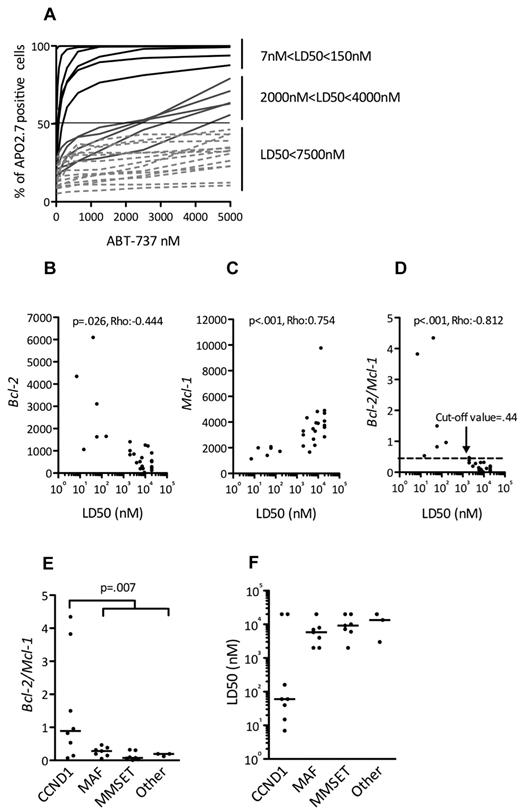
Expression of Bcl-2 antiapoptotic family members was assessed by immunoblotting in a collection of 25 HMCLs. Although Mcl-1 was always expressed, strong levels of Bcl-2 and Bcl-xL were expressed in a subset of cell lines (Figure 1), prompting an evaluation of the in vitro cytotoxic effects of the Bcl-2 antagonist ABT-737, which targets these 2 proteins. The HMCLs were incubated with different concentrations of ABT-737, ranging from 5nM to 20μM, for 48 hours. Cell viability was assessed by Apo 2.7 staining. The cytotoxic effects of ABT-737 were extremely heterogeneous within this collection of HMCLs, with a median lethal dose (LD50) ranging > 3 logs (0.007-20μM; Figure 2A). ABT-737 was highly effective against 6 cell lines, with LD50 values ranging from 7 ± 0.4 to 150 ± 15nM. In contrast, the other HMCLs had either an intermediate (n = 5) or resistant (n = 14) response to ABT-737, with LD50 values ranging from 2-4μM or > 7.5μM, respectively (Table 1). Notably, the LD50 value was not reached for 5 cell lines at a 20μM dose.
An HMCL subgroup highly expresses Bcl-2 and Bcl-xL. Immunoblot analysis of 25 HMCL lysates (70 μg of protein) with the indicated antibodies. Actin was used as a loading control.
An HMCL subgroup highly expresses Bcl-2 and Bcl-xL. Immunoblot analysis of 25 HMCL lysates (70 μg of protein) with the indicated antibodies. Actin was used as a loading control.
All ABT-737–sensitive HMCLs harbor a t(11;14) and could be identified by their BCL2/MCL1 ratio. (A) HMCLs (n = 25) were cultured with increasing doses of ABT-737 for 48 hours then stained with Apo 2.7 for flow cytometry analysis. The means of 3 independent experiments are shown. The LD50 value is determined for each cell line (ABT-737 concentrations where 50% of cells are stained with Apo 2.7). Analysis of BCL2 (B) and MCL1 expression (C) in function of ABT-737 sensitivity (LD50) of HMCLs. Bcl-2 and Mcl-1 expression levels were defined by Affymetrix microarray and plotted against the LD50. The Spearman rank correlation coefficient is indicated. (D) Analysis of BCL2/MCL1 expression ratio in function of ABT-737 sensitivity (LD50) of HMCLs. The Spearman rank correlation coefficient is indicated. The cut-off value (0.44) determined as the mean of Bcl-2/Mcl-1 ratio of resistant cell lines +2 SD (P < .001; sensitivity = 100%; specificity = 94.7%; 85% predictive positive value; and 100% negative predictive value) is indicated. Comparisons of BCL2/MCL1 gene expression ratio (E) and ABT-737 LD50 (F) in the different molecular groups of HMCLs.
All ABT-737–sensitive HMCLs harbor a t(11;14) and could be identified by their BCL2/MCL1 ratio. (A) HMCLs (n = 25) were cultured with increasing doses of ABT-737 for 48 hours then stained with Apo 2.7 for flow cytometry analysis. The means of 3 independent experiments are shown. The LD50 value is determined for each cell line (ABT-737 concentrations where 50% of cells are stained with Apo 2.7). Analysis of BCL2 (B) and MCL1 expression (C) in function of ABT-737 sensitivity (LD50) of HMCLs. Bcl-2 and Mcl-1 expression levels were defined by Affymetrix microarray and plotted against the LD50. The Spearman rank correlation coefficient is indicated. (D) Analysis of BCL2/MCL1 expression ratio in function of ABT-737 sensitivity (LD50) of HMCLs. The Spearman rank correlation coefficient is indicated. The cut-off value (0.44) determined as the mean of Bcl-2/Mcl-1 ratio of resistant cell lines +2 SD (P < .001; sensitivity = 100%; specificity = 94.7%; 85% predictive positive value; and 100% negative predictive value) is indicated. Comparisons of BCL2/MCL1 gene expression ratio (E) and ABT-737 LD50 (F) in the different molecular groups of HMCLs.
Given the variation in ABT-737 sensitivity and the previously shown importance of the relative expression of Bcl-2 family antiapoptotic proteins in predicting ABT-737 sensitivity,16-18 we investigated whether BCL2 and/or MCL1 gene expression, as analyzed by Affymetrix gene expression profiling, could predict ABT-737 sensitivity. High expression of BCL2 was inversely correlated with the LD50 (P = .026; ρ = −0.444, Spearman test), which is in agreement with the strong affinity of ABT-737 for Bcl-2 (Figure 2B). MCL1 expression levels, however, were directly correlated with the LD50 (P < .001; ρ = 0.754, Spearman test), which is consistent with the thought that Mcl-1 is involved in ABT-737 resistance (Figure 2C).17 Of note, no correlation was found between BCLXL gene expression and LD50 (P = .125; ρ = 0.316, Spearman test; see supplemental Methods, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Finally, a direct correlation also was found between LD50 and the BCL2/MCL1 ratio (P < .001; ρ = −0.812, Spearman test; Figure 2D). Indeed, the mean of BCL2/MCL1 ratio of sensitive HMCLs was significantly higher than that of resistant HMCLs: 2 ± 1.65 versus 0.18 ± 0.13 (P < .001). When a cut-off value of 0.44 was used (2 SD above the mean ratio of resistant cell lines), all sensitive HMCLs (6/6) had a ratio > 0.44, whereas only 1 resistant HMCL of 19 had a ratio > 0.44 (P < .001; sensitivity = 100% and specificity = 94.7%; Figure 2D). Thus, the BCL2/MCL1 ratio could be used to discriminate highly ABT-737–sensitive from ABT-737–resistant HMCLs.
An analysis of the BCL2/MCL1 ratios with respect to the different genetic backgrounds of these HMCLs (MMSET translocation, c-MAF or MAFB translocation, CCND1 translocation, or others) revealed that the BCL2/MCL1 ratio of the CCND1 HMCLs was significantly higher than that of all other groups (Wilcoxon test, P = .007; Figure 2E). A further analysis of the relationship between ABT-737 sensitivity and the different HMCL subsets revealed that 6 of the 8 t(11;14) HMCLs were sensitive, whereas none of the other subgroups contained sensitive cell lines (Fisher test, P < .001; Figure 2F). Altogether, these results demonstrated that 6 CCND1 HMCLs (SKMM-2, NAN-7, XG-5, Karpas-620, KMS-12-PE, and KMS-12-BM) displayed a very high sensitivity to ABT-737 that was associated with a BCL2/MCL1 value > 0.44 (Table 1; Figure 2D). Similarly, protein expression analysis showed that these lines have a specific Bcl-2high/Mcl-1low profile (Figure 1).
ABT-737 is also very efficient in a subset of primary myeloma cells
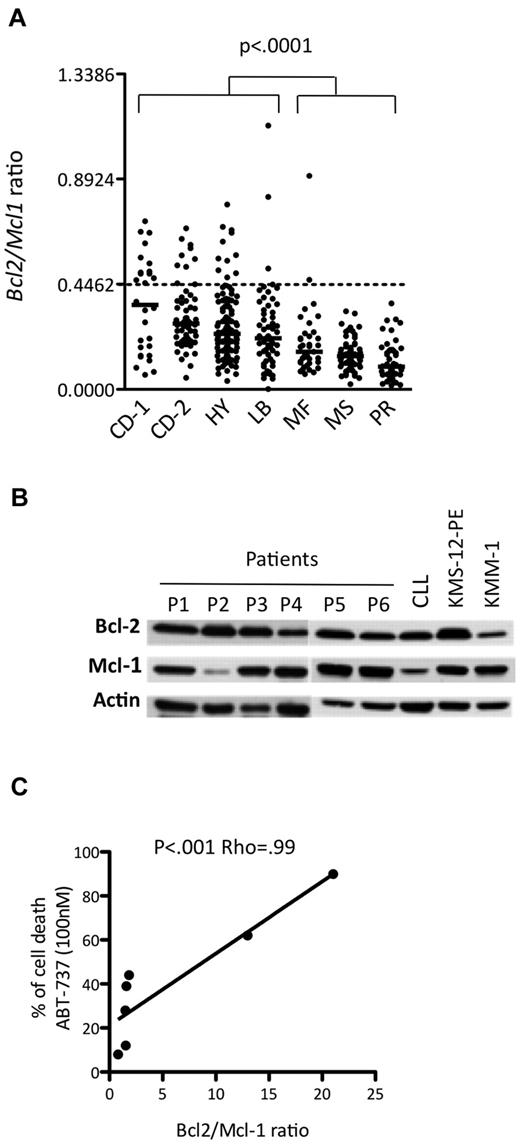
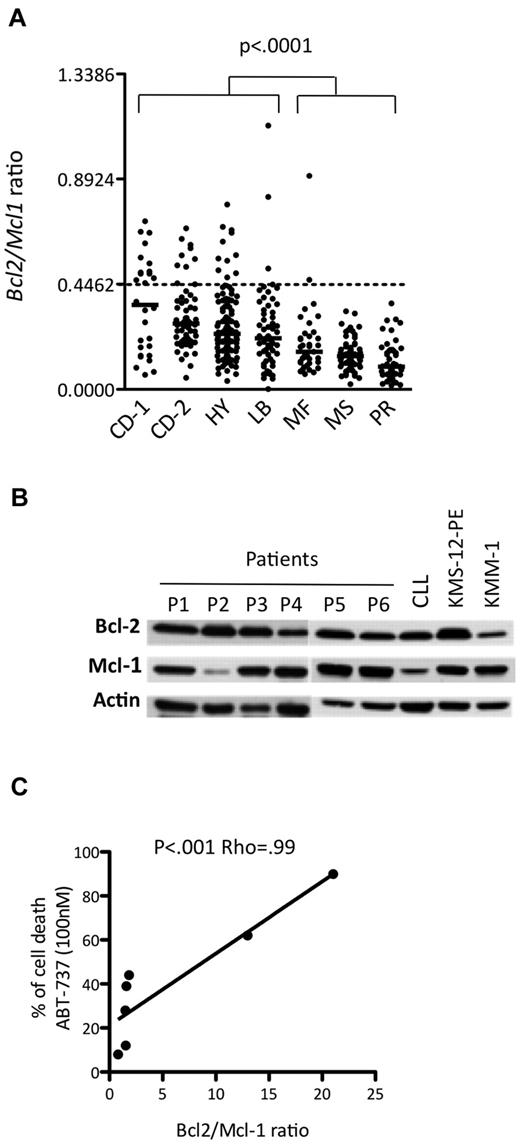
Currently, MM patients are usually classified into 7 groups based on the molecular classification defined by Zhan et al.5 The publicly available Affymetrix gene expression levels of 414 newly diagnosed MM patients were used to determine the BCL2/MCL1 ratio for each patient in each of the different molecular groups, which were CD-1, CD-2, hyperdiploid, low bone disease, proliferation, MAF, and MMSET (Figure 3A). Notably, the CD-1 and CD-2 molecular subtypes include all MM patients with t(11;14) translocation. Thus, the BCL2/MCL1 ratios of the CD-1 (median, 0.36; range, 0.06-0.71), CD-2 (median, 0.28; range, 0.05-0.68), low bone disease (median, 0.22; range, 0.05-1.12), and hyperdiploid (median, 0.24; range, 0.04-0.79) patients were significantly higher than those of all other patients (Wilcoxon test, P < .001). Those patients presenting values higher than the cut-off defined above belonged mainly to the CD-1 (46%), CD-2 (15%), and hyperdiploid (9%) groups, whereas none belonged to either the proliferation or MMSET groups (Figure 3A). Altogether, these results suggested that a subset of primary myeloma cells might be effectively responsive to ABT-737 treatment at a reasonable therapeutic dose. To test this hypothesis, 7 primary purified CD138+ myeloma samples were analyzed both for ABT-737 sensitivity and for the Bcl-2 and Mcl-1 profile. The LD50 values indicated that 2 of the 7 primary myeloma cells were highly sensitive to nanomolar concentrations of ABT-737 (Table 2). Notably, highly sensitive patients with an LD50 ≤ 100nM (patients 2 and 3) presented the highest protein expression ratio of Bcl-2/Mcl-1 (Figure 3B). We found a strong correlation between ABT-737 sensitivity (percentage of cell death at 100nM) and Bcl-2/Mcl-1 ratio (P < .001; ρ = 0.99, Spearman test; Figure 3C). Thus, the Bcl-2/Mcl-1 protein expression profile was also a strong indicator of ABT-737 sensitivity in primary myeloma cells.
ABT-737 is efficient against a subset of primary myeloma cells. (A) Affymetrix gene expression profiles of purified myeloma cells from 414 newly diagnosed patients, publicly available from the Arkansas Cancer Research Center. The ratio of BCL2 to MCL1 is indicated for each patient in the different molecular groups classified according to Zhan et al5 as follows: CCND1-1 (CD-1), CCND1-2 (CD-2), hyperdiploid (HY), low bone disease (LB), MAF (MF), MMSET (MS), and proliferation (PR). (B) Western blot analysis of Bcl-2 and Mcl-1 expression in CD138+ purified primary MM cells. A sensitive HMCL (KMS-12-PE) and a resistant HMCL (KMM-1) as well as a CLL sample were included in the panel as controls. Actin was used as a loading control. (C) Analysis of primary myeloma cell sensitivity to ABT-737 (100nM) in function of Bcl-2/Mcl-1 ratio. The Spearman rank correlation coefficient is indicated.
ABT-737 is efficient against a subset of primary myeloma cells. (A) Affymetrix gene expression profiles of purified myeloma cells from 414 newly diagnosed patients, publicly available from the Arkansas Cancer Research Center. The ratio of BCL2 to MCL1 is indicated for each patient in the different molecular groups classified according to Zhan et al5 as follows: CCND1-1 (CD-1), CCND1-2 (CD-2), hyperdiploid (HY), low bone disease (LB), MAF (MF), MMSET (MS), and proliferation (PR). (B) Western blot analysis of Bcl-2 and Mcl-1 expression in CD138+ purified primary MM cells. A sensitive HMCL (KMS-12-PE) and a resistant HMCL (KMM-1) as well as a CLL sample were included in the panel as controls. Actin was used as a loading control. (C) Analysis of primary myeloma cell sensitivity to ABT-737 (100nM) in function of Bcl-2/Mcl-1 ratio. The Spearman rank correlation coefficient is indicated.
Modulation of Bcl-2 or Mcl-1 level of expression modulated ABT-737 sensitivity
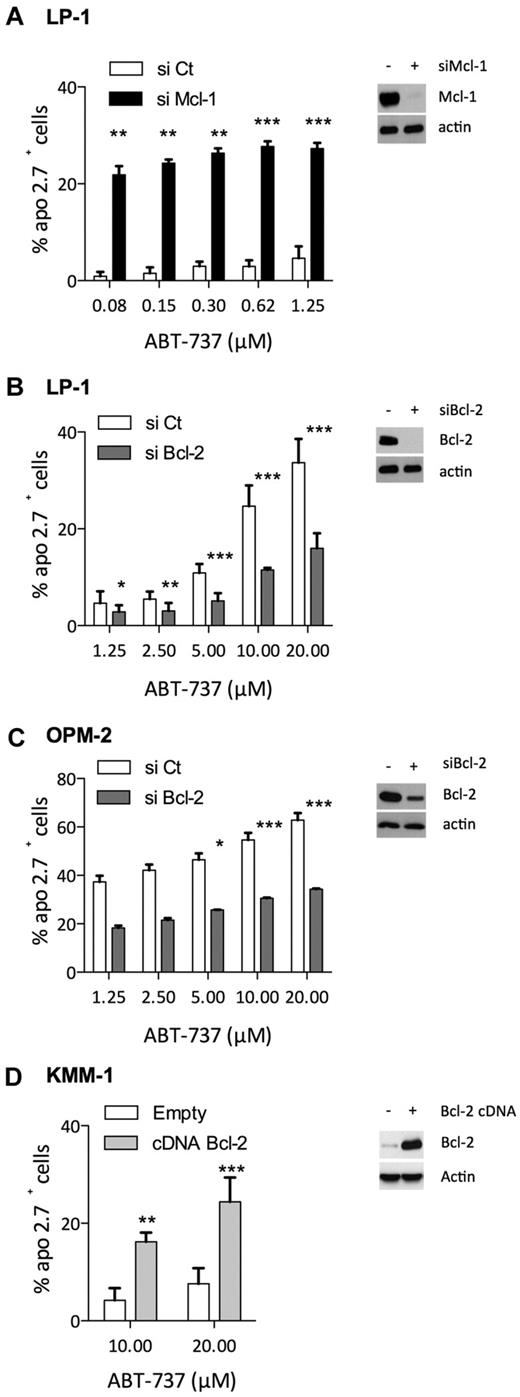
To further demonstrate that Bcl-2/Mcl-1 ratio predicted ABT-737 sensitivity, we either silenced or overexpressed Bcl-2 or Mcl-1 in HMCLs, although myeloma cells are known to be difficult to transfect. Thus, LP1 and OPM-2 myeloma cells were selected to modulate the expression of Bcl-2 and Mcl-1 because siRNA strategies were attainable in these cells. In addition, myeloma cells were shown to be highly dependent on Mcl-1 expression for survival6,7 ; and indeed, down-regulation of Mcl-1 by siRNA induced complete cell death in OPM-2 cells (data not shown). In contrast, LP1 cells underwent moderate apoptosis under knockdown of Mcl-1, allowing us to analyze the role of Mcl-1 expression in ABT-737 resistance. Mcl-1 knockdown resulted in a significant sensitization of LP-1 cells to ABT-737 at doses as low as 80nM compared with the negative control siRNA, indicating that Mcl-1 confers ABT-737 resistance in myeloma cells (Figure 4A). siRNAs against Bcl-2 were transfected in both LP-1 and OPM-2 cells, resulting in a strong decrease of Bcl-2 in LP-1 and a moderate decrease of Bcl-2 in OPM-2 cells (Figure 4B-C). The strong decrease of Bcl-2 in LP-1 significantly prevented ABT-737–induced cell death at doses ranging from 1.25 to 20μM (Figure 4B). Although incomplete, the silencing of Bcl-2 in OPM-2 cells also led to a significant increase of ABT-737 resistance at doses ranging from 5 to 20μM (Figure 4C). Conversely, Bcl-2 was transiently overexpressed in KMM-1 cells, a highly ABT-737–resistant cell line, with an LD50 > 20μM. We observed a significant increase of apoptosis induction in KMM-1 cells overexpressing Bcl-2 at both 10 and 20μM of ABT-737 (Figure 4D). Altogether, these results indicated that the level of both Bcl-2 and Mcl-1 played a significant role in the sensitivity to ABT-737; decreasing Mcl-1 led to an important sensitization to ABT-737 and conversely, increasing Bcl-2 levels sensitized myeloma cells to ABT-737.
Involvement of Bcl-2 and Mcl-1 levels in ABT-737 induced apoptosis. (A-C) Seventy-two hours after, Bcl-2 or Mcl-1 siRNA transfection in myeloma cells as indicated, protein levels were examined by immunoblotting, and cells were treated with ABT-737 for 24 hours. Apoptosis was quantified by Apo 2.7 staining. Results represent the mean of 4 independent experiments. (D) KMM1 were transfected with pRc-CMV Bcl-2 or empty vector for 48 hours before to be cultured with ABT-737 for 24 hours. Results represent the mean of 5 independent experiments. Statistical analysis were performed by paired Student t test: *P < .05; **P < .005; and ***P < .0005.
Involvement of Bcl-2 and Mcl-1 levels in ABT-737 induced apoptosis. (A-C) Seventy-two hours after, Bcl-2 or Mcl-1 siRNA transfection in myeloma cells as indicated, protein levels were examined by immunoblotting, and cells were treated with ABT-737 for 24 hours. Apoptosis was quantified by Apo 2.7 staining. Results represent the mean of 4 independent experiments. (D) KMM1 were transfected with pRc-CMV Bcl-2 or empty vector for 48 hours before to be cultured with ABT-737 for 24 hours. Results represent the mean of 5 independent experiments. Statistical analysis were performed by paired Student t test: *P < .05; **P < .005; and ***P < .0005.
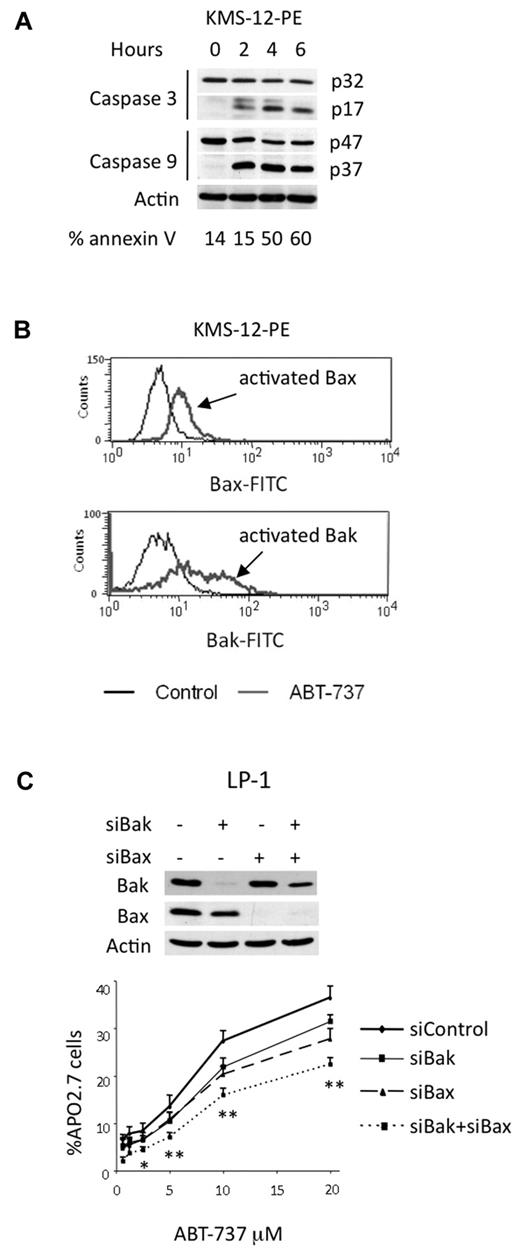
ABT-737 triggered a rapid induction of cell death because of the release of either multidomain or BH3-only proapoptotic proteins from Bcl-2
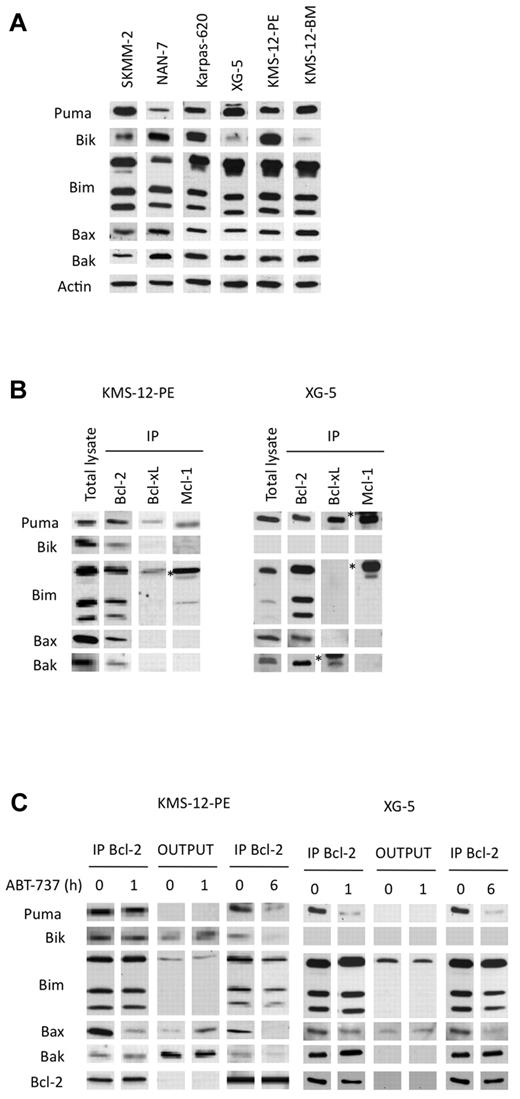
To characterize cell death induced by ABT-737 in myeloma cells, a kinetic study of caspase activation was performed in a sensitive t(11;14) HMCL. The cleavage of caspases-9 and -3 was detected after a 2 hour treatment in KMS12-PE, indicating that apoptosis induction involved the intrinsic pathway (Figure 5A). In addition, using antibodies against conformational active forms of Bax and Bak, we demonstrated an activation of both effector proteins (Figure 5B). We then investigated the role of these effector proteins in ABT-737–induced apoptosis by silencing Bax or Bak alone or both in LP-1 cells (Figure 5C). The silencing of Bax or Bak led to a complete decrease of each protein, whereas simultaneous silencing resulted in a complete decrease of Bax and a less efficient decrease of Bak. The silencing of either Bax or Bak reduced apoptosis induced by ABT-737, but only the silencing of both effector proteins led to a significant and stronger reduction of apoptosis in LP-1 cells (Figure 5C). This result confirmed that ABT-737–induced apoptosis occurred through the mitochondrial pathway, in which both effectors were involved. The cell death induced by ABT-737 was detected after 4 hours, indicating an early triggering of apoptosis (Figure 5A). Letai and colleagues proposed that early apoptosis induced by ABT-737 is the consequence of cells primed for death that involves the sequestration of prodeath members by Bcl-2.16,19,20 Therefore, the expression of proapoptotic proteins in sensitive t(11;14) HMCLs was examined. In all sensitive cell lines, the expression of Bim and Puma, which are activator BH3-only proteins, was high, whereas the expression of Bik, a sensitizer BH3-only protein, was heterogenous (Figure 6A). In addition, uniform expression of Bax and Bak was found in all sensitive HMCLs (Figure 6A). To evaluate the relative abundances of endogenous complexes in sensitive cell lines, immunoprecipitates of antiapoptotic proteins were analyzed in both KMS12-PE and XG-5 cells that mainly differ by the expression of Bik. XG-5 cells did not express Bik in contrast to KMS12-PE. Bcl-2 was complexed with Bim, Bax, Bak, and Puma in both cell lines (Figure 6B). In addition, Bcl-2 was found complexed with Bik in KMS12-PE. Only a small fraction of the Bim cellular pool also was found complexed with either Mcl-1 or Bcl-xL in both cell lines; however, an important complex Puma/Mcl-1 was found in XG-5 cells (Figure 6B). Altogether, these data suggested that multidomain or BH3-only proapoptotic proteins bound to Bcl-2 prime myeloma cells for ABT-737 killing. To determine which proapoptotic protein was first displaced from Bcl-2 by ABT-737, KMS-12-PE and XG-5 cells were treated with ABT-737 for a short time (1 hour), and the dynamic of the Bcl-2 complexes was analyzed by immunoprecipitation. An examination of Bcl-2 heterodimers in KMS12-PE after a 1-hour ABT-737 treatment showed a decrease in both Bcl-2/Bax and Bcl-2/Bik complexes (Figure 6C). Consistent with the decrease of these complexes, there was an increase of Bax and Bik in the supernatant. After a longer ABT-737 exposure (6 hours), all of the Bcl-2 heterodimers were disrupted, and only a small amount of Bim remained bound to Bcl-2 (Figure 6C). In XG-5 cells, a 1-hour treatment of ABT-737 triggered a dissociation of both Bcl-2/Bax and Bcl-2/Puma complexes (Figure 6C). The early displacement of Bax, Bik, or Puma from Bcl-2 can be explained by their lower affinity for Bcl-2 in comparison with that of Bim, as determined previously in different studies.19,21 The displacement of Bax can lead to its oligomerization and activation, whereas Bik can probably act through further displacement of Bim and Puma from Bcl-2, according to its role as a sensitizer BH3-only molecule. Finally, Puma could either act as a sensitizer or directly activate Bax as reported previously.22
ABT-737–induced apoptosis through the mitochondrial pathway. (A) KMS-12-PE cells were treated with 40nM ABT-737 for the indicated times. Cell death was analyzed by annexin-V staining. Cleavage of caspases-3 and -9 correlated with cell death. (B) Bax and Bak activation was determined by staining with antibodies against active forms in KMS-12-PE cells either treated or not with ABT-737. One representative experiment of 3 is shown. (C) LP-1 cells were transfected with the indicated siRNA for 72 hours before being treated with ABT-737 for 24 hours. Equivalent amounts of cell lysates were separated by SDS-PAGE and then immunoblotted with the indicated antibodies. Cell death was assessed by Apo 2.7 staining. Results represent the mean of 3 independent experiments. Statistical analysis were performed by paired Student t test: *P < .05; **P < .005.
ABT-737–induced apoptosis through the mitochondrial pathway. (A) KMS-12-PE cells were treated with 40nM ABT-737 for the indicated times. Cell death was analyzed by annexin-V staining. Cleavage of caspases-3 and -9 correlated with cell death. (B) Bax and Bak activation was determined by staining with antibodies against active forms in KMS-12-PE cells either treated or not with ABT-737. One representative experiment of 3 is shown. (C) LP-1 cells were transfected with the indicated siRNA for 72 hours before being treated with ABT-737 for 24 hours. Equivalent amounts of cell lysates were separated by SDS-PAGE and then immunoblotted with the indicated antibodies. Cell death was assessed by Apo 2.7 staining. Results represent the mean of 3 independent experiments. Statistical analysis were performed by paired Student t test: *P < .05; **P < .005.
BH3-only and multidomain proapoptotic proteins sequestered by Bcl-2 were displaced by ABT-737. (A) Immunoblot analysis of the indicated proteins (70 μg). Actin was used as a loading control. (B) Immunoprecipitation of KMS-12-PE or XG-5 cell lysates (300 μg) using the indicated antibodies. The immunoprecipitates were analyzed for the presence of antiapoptotic, BH3-only proteins or multidomain proapoptotic proteins. The asterisk (*) corresponds to the immunoglobulin light chain. (C) Bcl-2 immunoprecipitation of KMS-12-PE or XG-5 cell lysates treated or not with 40nM ABT-737 for both 1 and 6 hours. The immunoprecipitates and the outputs (IP supernatants) were analyzed for the presence for the indicated proteins by immunoblotting.
BH3-only and multidomain proapoptotic proteins sequestered by Bcl-2 were displaced by ABT-737. (A) Immunoblot analysis of the indicated proteins (70 μg). Actin was used as a loading control. (B) Immunoprecipitation of KMS-12-PE or XG-5 cell lysates (300 μg) using the indicated antibodies. The immunoprecipitates were analyzed for the presence of antiapoptotic, BH3-only proteins or multidomain proapoptotic proteins. The asterisk (*) corresponds to the immunoglobulin light chain. (C) Bcl-2 immunoprecipitation of KMS-12-PE or XG-5 cell lysates treated or not with 40nM ABT-737 for both 1 and 6 hours. The immunoprecipitates and the outputs (IP supernatants) were analyzed for the presence for the indicated proteins by immunoblotting.
BH3-only propapoptotic proteins also were displaced from Bcl-2 by ABT-737 in a resistant cell line but recaptured by Mcl-1
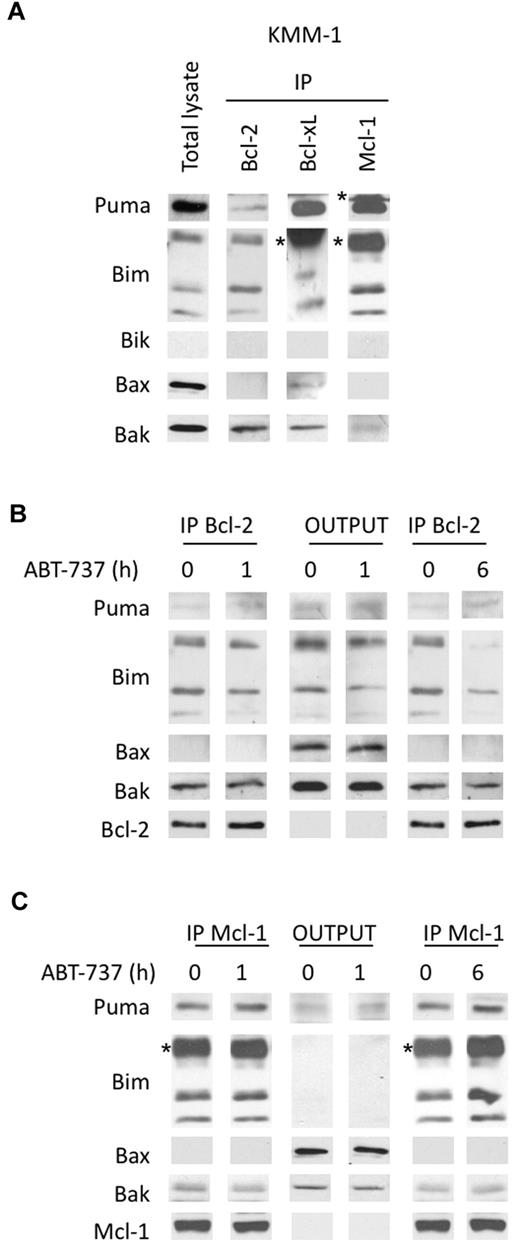
Our findings demonstrated that the silencing of Mcl-1 overcame ABT-737 resistance, highlighting the role of Mcl-1 in the resistance to ABT-737. To further investigate the role of Mcl-1 in ABT-737 resistance, we determined the endogenous complexes in KMM-1–resistant cell line characterized by an LD50 > 20μM. KMM-1, a Bik negative cell line, displayed a weak amount of Bcl-2 in contrast to high Mcl-1 levels. Importantly, Bcl-2 mainly interacted with Bim and in a lesser extent with Puma but not with Bax (Figure 7A). The major complexes observed in this cell line were Mcl-1/Bim, Mcl-1/Puma, and Bcl-xL/Puma (Figure 7A). We further analyzed the modifications in Bcl-2 heterodimers induced by a short ABT-737 treatment. After a 1-hour treatment, a small decrease of Bcl-2/Bim complexes was observed, but after a longer exposure the majority of Bcl-2/Bim complexes was disrupted (Figure 7B). This result indicated that ABT-737 also reached its target, Bcl-2, in this resistant cell line. Because Mcl-1 is involved in ABT-737 resistance, we postulated that Mcl-1 was potentially able to sequester Bim released from Bcl-2. To test this hypothesis, we analyzed Mcl-1/Bim complexes in the supernatant of Bcl-2 immunoprecipitation (Figure 7C). Although Mcl-1 level remained constant under ABT-737 treatment, we observed an increase of the Mcl-1/Puma and Mcl-1/Bim complexes, indicating that Mcl-1 was able to sequester BH3-only proteins released from Bcl-2 (Figure 7C). Sequestration of both Bim and Puma by Mcl-1 was readily detectable after 6 hours of ABT-737 treatment as shown in the Mcl-1 immunoprecipitate. Altogether, these results might explain the role of Mcl-1 in the resistance to ABT-737.
Analysis of the dynamics of anti and proapoptotic Bcl-2 protein complexes in KMM-1 resistant cells treated by ABT-737. (A) Immunoprecipitation of KMM-1 cell lysates using the indicated antibodies. (B) Bcl-2 immunoprecipitation of KMM-1 cell lysates (300 μg) treated or not with 40nM ABT-737 for both 1 and 6 hours. The immunoprecipitates and the outputs (IP supernatants) were analyzed for the presence for the indicated proteins by immunoblotting. (C) Supernatants of Bcl-2 immunoprecipitation (300 μg) were used for Mcl-1 immunoprecipitation. Immunoblotting was preformed as described. The asterisk (*) corresponds to the immunoglobulin light chain.
Analysis of the dynamics of anti and proapoptotic Bcl-2 protein complexes in KMM-1 resistant cells treated by ABT-737. (A) Immunoprecipitation of KMM-1 cell lysates using the indicated antibodies. (B) Bcl-2 immunoprecipitation of KMM-1 cell lysates (300 μg) treated or not with 40nM ABT-737 for both 1 and 6 hours. The immunoprecipitates and the outputs (IP supernatants) were analyzed for the presence for the indicated proteins by immunoblotting. (C) Supernatants of Bcl-2 immunoprecipitation (300 μg) were used for Mcl-1 immunoprecipitation. Immunoblotting was preformed as described. The asterisk (*) corresponds to the immunoglobulin light chain.
Discussion
MM initially responds to chemotherapy, but relapse and chemoresistance always occurs. Therefore, improvement in long-term survival will depend on novel therapeutic approaches. BH3 mimetics represent a novel class of drugs able to promote apoptosis, and ABT-737 is the prototypic drug in this class. ABT-737 has monotherapeutic toxicity against leukemia and lymphoma malignancies. Indeed, primary cells from patients with chronic lymphocytic leukemia,12 acute myeloid leukemia,23 acute lymphocytic leukemia,24 and B lymphoma25 have been shown to be extremely sensitive to ABT-737. In addition to these pathologies, ABT-737 efficiency was investigated in myeloma cells and demonstrated substantial antimyeloma activity,26-28 although its efficacy was proven only using higher concentrations. In the present study, we provided the first evidence that a subset of HMCLs is extremely sensitive to ABT-737 in a nanomolar concentration range. MM seems very heterogeneous with respect to ABT-737 sensitivity, with LD50 values ranging > 3 logs (0.007-20μM). ABT-737 sensitivity was strongly correlated with either high BCL2 expression or low MCL1 expression, but no correlation was found with BCLXL. Furthermore, the BCL2/MCL1 ratio as determined by gene expression profiling allowed the discrimination between sensitive and resistant HMCLs, with a cut-off value of 0.44. In a similar manner, an analysis of the relative expression of Bcl-2 and Mcl-1 by immunoblotting seems to be a very good indicator of ABT-737 sensitivity. These findings are in agreement with previous studies in different cell types, showing that indicators of sensitivity correlated with high levels of Bcl-2 and low levels of Mcl-1.16-18,23,29 Furthermore, our study demonstrated that changing the relative abundance of Bcl-2 compared with Mcl-1 by silencing either Bcl-2 or Mcl-1 modified the sensitivity to ABT-737. Indeed, silencing Bcl-2 decreased the sensitivity to ABT-737, whereas silencing Mcl-1 strongly increased the sensitivity to ABT-737. Our results once again confirm the essential role of Mcl-1 in the resistance to ABT-737. Finally, HMCLs expressing a high Bcl-2/Mcl-1 ratio were all found in the t(11;14) group, and all sensitive HMCLs in our study belonged to this molecular group. The identification of this molecular subset of HMCLs that are highly sensitive to ABT-737 was only possible through the analysis of our large collection of HMCLs, which covers most of the known myeloma heterogeneity. Although high Bcl-2 expression was found in the t(11;14) group, there has been no documented explanation supporting this finding. Of note, myeloma cells do not usually have a translocation that directly leads to increased Bcl-2 expression. Our analysis in a limited number of primary myeloma cells also highlighted the existence of a subset of MM that robustly responded to very low concentrations of ABT-737 (nanomolar range). These results confirmed and extended the study of Trudel et al28 showing that 4 of 15 MM patients were sensitive to treatment with 0.25μM ABT-737. Finally, our immunoblotting analysis confirmed that the relative expression of Bcl-2 to Mcl-1 in the lysates of purified primary myeloma is a strong indicator of ABT-737 sensitivity in the 7 samples analyzed (P < .001).
Using BCL2/MCL1 ratio as an indicator of ABT-737 sensitivity, we evaluated a proportion of patients susceptible to ABT-737 in the different molecular groups of patient defined by Zhan et al5 and showed that patients harboring MMSET or MAF translocations will not be eligible for ABT-737 therapy, which is consistent with our study of HMCLs. Analysis of the hyperdiploid group of patients, which was not represented in the group of 25 HMCLs, indicated that a subset of these patients could be efficiently targeted by ABT-737. Notably, hyperdiploid patients represent half of myeloma patients. Finally, the highest proportion of patients able to respond to ABT-737 were found in the CD-1 (CD20−) and CD-2 (CD20+) molecular subtypes, both characterized by a t(11;14). Although t(11;14) MM has been considered so far to be a favorable MM prognostic based on clinical response, a recent study indicated that CD-1 and CD-2 have very different clinical responses.30 Although the CD-1 group is characterized by the highest level of complete response, the duration of complete response is as short as that of the proliferation group. In contrast, CD-2 has the longest duration of complete response among all molecular entities. Because the CD-1 group of patients relapse very quickly, the development of new therapeutic approaches is important. Altogether, our data suggest that a proportion of patients in the CD-1, CD-2 and hyperdiploid groups expressed a Bcl-2high/ Mcl-1low profile, which is compatible with the use of ABT-737 as a single agent.
To better understand the apoptotic events induced by ABT-737 in myeloma cells, we investigated both the expression of prodeath proteins and the abundance of endogenous heterodimers between pro- and antiapoptotic proteins. Although the Bim, Puma, Bax and Bak proteins seemed to be uniformly expressed among t(11;14) HMCLs, Bik was either highly expressed or absent. No correlation between ABT-737 sensitivity and Bik expression, however, can be made. We also investigated whether prodeath proteins were indeed bound by Bcl-2. Coimmunoprecipitation experiments in KMS-12-PE and XG-5 cells demonstrated that the different prodeath proteins, either BH3-only or multidomain proteins, were all sequestered by Bcl-2. To determine the molecular events initiated by ABT-737, any changes in the abundance of the Bcl-2 heterodimers after a short exposure to ABT-737 were analyzed in sensitive cell lines expressing or not Bik. ABT-737 first displaced Bax, Bik, and Puma from Bcl-2. After a longer exposure, the majority of Bcl-2 heterodimers also were disrupted, whereas Bcl-2/Bim complexes were still detected, probably because of both the abundance and the high affinity of these complexes. The lower affinity of Bik, Bax, and Puma for Bcl-2 in comparison with the affinity of Bim can probably explain why the Bcl-2/Bax, Bcl-2/Bik, and Bcl-2/Puma heterodimers were first disrupted by ABT-737. Because Bim displays the highest affinity for Bcl-2, Bcl-2/Bim should be the most difficult complex to be disrupted by a BH3-mimetic, a hypothesis that was confirmed by our observation that Bcl-2/Bim complexes were still detectable after a 6-hour ABT-737 exposure. The disruption of Bcl-2/Bax heterodimerization could directly promote the permeabilization of the outer mitochondrial membrane. In contrast, Bik, a sensitizer BH3-only protein, should be involved in the release of direct activators like Bim and possibly Puma from Bcl-2. Finally, our results did not allow us to discriminate whether Puma act as sensitizer BH3-only or a direct activator of Bax. The involvement of ABT-737 in the displacement of Bax was also reported in lymphoma cells in which the level of Bcl-2/Bax complexes also was correlated with ABT-737 response, suggesting that Bcl-2 can be primed with Bax in these cells.16 Other strong evidence of Bax involvement in ABT-737–induced apoptosis was provided by Konopleva et al23 in acute myeloid leukemia cells, who demonstrated that ABT-737 induced the disruption of the Bcl-2/Bax complex and activated the intrinsic apoptotic pathway via a Bim-independent process. In agreement with that study, we showed that Bax displacement was the initiator event, but our data also suggested that a later occurring Bim and Puma displacement was involved, probably amplifying the apoptotic process. Altogether, our mechanistic studies indicated that the expression ratio of Bcl-2/Mcl-1 governs, in large part, the sensitivity to ABT-737, a sensitivity that can be additionally modulated by the nature and the abundance of the prodeath proteins complexed with Bcl-2. In contrast, resistant cell lines expressed high levels of Mcl-1 and low levels of Bcl-2 that consequently influenced the nature of Bcl-2 heterodimeres. Indeed, in KMM-1–resistant cell line only the Bcl-2/Bim and Bcl-2/Bak complexes can be evidenced, indicating that low levels of Bcl-2 favored the formation of heterodimeres with proteins that displayed a high affinity for Bcl-2. Of major interest, no Bcl-2/Bax complex can be observed, which can explain in part the ABT-737 resistance of this cell line. In addition, despite the weak levels of Bcl-2/Bim complexes, they were still dissociated by ABT-737, indicating that ABT-737 also reached its target in a resistant cell line. Finally, the capture of released Bim and Puma by Mcl-1 can also contribute to explain the resistance of this cell line to ABT-737. Mcl-1 is a very short half life protein that always need de novo translation to maintain its level of expression, its high turnover allowed the recapture of free Bim or Puma.
Finally, the better understanding of the resistance mechanism explained why the Bcl-2/Mcl-1 ratio of expression is the best predicting factor for ABT-737 sensitivity.
Although the combination of several drugs usually seems to be the most efficient chemotherapeutic regimen, the identification of a subset of MM (Bcl-2high/Mcl-1low) that can be effectively targeted by ABT-737 as a single agent supported the evaluation of ABT-263,31,32 an orally active counterpart to ABT-737, for the treatment of CD-1, CD-2, and hyperdiploid MM harboring a Bcl-2high/Mcl-1lowprofile. With respect to the ABT-737 response in a xenograft MM model, 2 previous independent studies showed very different results: ABT-737 either efficiently suppressed My5 tumor growth28 or inefficiently altered OPM-2 tumor growth.26 These results highlighted the variability in the MM response to ABT-737 but were in agreement with the sensitivity of both cell lines to ABT-737; ie, My5 is a sensitive cell line (LD50 = 200 nM)28 in contrast to OPM-2, a resistant HMCL in our study (LD50 = 6 μM). Clinical trials with ABT-263 as a single agent have shown substantial single agent therapeutic activity, particularly in CLL, and encourage clinical trials for other pathologies.32-34 Altogether, the major progress made in the stratification of MM patients should be now used in concert with new therapeutic approaches to propose targeted and individualized therapies.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs E. Rouffiac and R. Humerickhouse from Abbott Laboratories for providing ABT-737.
This work was supported by Ligue Contre le Cancer (Equipe labellisée 2008). L.B. was supported by CYMATH and the French Society of Hematology.
Authorship
Contribution: L.B. and P.G.-B. contributed to the design of experiments, performed the experiments, and helped write the paper; G.D. and S.M. performed siRNA experiments; C.T. and C.D. helped analyze the data; P.M., H.A.-L., and S.L.G. provided myeloma samples; R.B. helped write the paper; and C.P.-D. and M.A. contributed to the design of experiments and helped analyze the data and write the paper.
Conflict of interest: The authors declare no competing financial interests.
Correspondence: Martine Amiot, Inserm, UMR_S892, Centre de Recherche en Cancérologie Nantes/Angers, 8, quai Moncousu Nantes, BP70721 F-44007, France; e-mail: mamiot@nantes.inserm.fr.
References
Author notes
L.B. and P.G.-B. contributed equally to this work.