Abstract
Patients with childhood leukemia surviving into adulthood have elevated risk of developing thyroid cancer, brain cancer, and non-Hodgkin lymphoma (NHL); these risks cannot automatically be extrapolated to patients surviving adult leukemia. We tested whether survivors of adult leukemia are at increased risk of developing thyroid cancer, brain cancer, and NHL. We included the entire adult Danish population (14 years of age or older), in a 28-year follow-up period from 1980 through 2007, composed of 6 542 639 persons; during this period, 18 834 developed adult leukemia, 4561 developed thyroid cancer, 13 362 developed brain cancer, and 15 967 developed NHL. In nested studies using Cox regression models on individual participant data, we found that, after adult leukemia, the multivariate adjusted hazard ratios were 4.9 (95% confidence interval [CI], 2.8-8.5) for thyroid cancer, 1.9 (95% CI, 1.2-3.1) for brain cancer, and 3.3 (95% CI, 2.5-4.4) for NHL. Corresponding hazard ratios after childhood leukemia were 10.4 (95% CI, 0.4-223) for thyroid cancer, 7.2 (95% CI, 2.0-26) for brain cancer, and 6.5 (95% CI, 0.4-110) for NHL. Patients with adult leukemia have excess risk of thyroid cancer, brain cancer, and NHL, similar to patients with childhood leukemia.
Introduction
Patients with childhood leukemia surviving into adulthood have elevated risk of developing thyroid cancer, brain cancer. and non-Hodgkin lymphoma (NHL).1-6 Aggressive radiotherapy and chemotherapy given to ensure survival of patients with childhood leukemia are among the main risk factors contributing to the observed elevated risks.5,7-9
Adult leukemia differs from childhood leukemia: whereas chronic lymphocytic leukemia (CLL) and acute myeloid leukemias (AMLs) are the most common leukemia subtypes in adults, acute lymphoblastic leukemia (ALL) is the most common leukemia subtype in children.10,11 In addition, adult patients with ALL are generally treated with other radiotherapy and chemotherapy protocols than those commonly used in patients with childhood leukemia.12-15 Because of these differences, the known risks of thyroid cancer, brain cancer, and NHL in survivors of childhood leukemia1-6 cannot automatically be extrapolated to adult patients with leukemia.
We tested the hypothesis that survivors of adult leukemia are at increased risk of developing thyroid cancer, brain cancer, and NHL. For this purpose, we studied the entire adult Danish population, 14 years of age or older, from 1980 through 2007, for a total of 6.5 million adults. We used information from the national Danish Civil Registration System, the national Danish Cancer Registry, and Statistics Denmark; these 3 registries were all complete from 1980 through 2007. As a positive control, we measured risk of thyroid cancer, brain cancer, and NHL in patients treated for childhood leukemia (< 14 years), using information from the same registries.
Methods
These studies were approved by Herlev Hospital, Copenhagen University Hospital, the Danish Data Protection Agency, the Danish Health Ministry, and Statistics Denmark; in Denmark, approval by ethics committees of such anonymous nationwide studies is not necessary.
Unique identification of inhabitants in Denmark
The national Danish Civil Registration System records all births, immigrations, emigrations, and deaths in Denmark through the civil registration number, which uniquely identifies all inhabitants of Denmark and includes information on age and sex. The national Danish Civil Registration System is 100% complete; that is, for practical purposes no persons are lost to follow-up.
National Danish Cancer Registry
The national Danish Cancer Registry identifies 98% of all incident cancers in Denmark.16,17 All diagnoses in the registry are assigned based on histologic examination by a fully trained pathologist. During 1980 to 2003, the national Danish Cancer Registry used the International Classification of Diseases (7th edition) and during 2004 to 2007 the 10th edition. Therefore, discrimination between, for example, the chronic or acute forms of lymphoid leukemia is not possible using the 7th edition alone. However, parallel to the registration in the national Danish Cancer Registry, acute and chronic lymphoid leukemia diagnoses were recorded in the Danish Patient Registry using International Classification of Diseases 8th edition (1980-1994) and 10th edition codes (1995-2007), wherein this distinction is possible. The national Danish Cancer Registry has compared records between these 2 registries and for acute and chronic lymphoid leukemia augmented the records with equivalent 10th edition codes covering the entire period of 1978 to 2003, whereas the 10th edition was used thereafter.18 Furthermore, for each registered cancer, the pathologic morphology code is available, recorded using the International Classification of Diseases for Oncology (SNOMED). NHLs were during the period 1980 to 2003 primarily recorded using this classification system.
Leukemia
Persons diagnosed with incident leukemia from January 1, 1980 through December 31, 2007 were identified using the national Danish Cancer Registry. After common practice in hematologic oncology, we subdivided adult leukemia into 2 main subgroups: CLL and non-CLL. The non-CLL was further subgrouped into: ALL, AML (including both the acute and subacute forms of myeloid and monocytic leukemias), chronic myeloid leukemia (CML, including chronic forms of myeloid and monocytic leukemias), and a group of the remaining rarer forms of leukemias (others). CLL was classified according to the International Classification of Diseases 10th edition codes C91.1 (1980-2007). ALL was 10th edition codes C91.0 and C91.2 (1980-2007). AML was 10th edition codes C92.0, C92.2, C, C92.4, C92.5, C93.0, and C93.2 (1980-2007). CML was 10th edition codes C92.1 and C93.1 (1980-2007). The remaining rarer forms of leukemia (others) were classified as leukemia's C91 to C95.9 (1980-2007) not categorized into the other groups.
Before 2001, AML was diagnosed based on the French-American-British (FAB) classification and thereafter based on the World Health Organization (WHO) classification. In the FAB classification, at least 30% blasts in the bone marrow are required for diagnosis, whereas in the subsequent WHO classification only at least 20% blasts are required. The national Danish Cancer Registry does not take these classification differences into account and records the diagnosis as reported by clinicians regardless of classification system used.
Endpoints: thyroid cancer, brain cancer, and NHL
Persons with incident thyroid cancer, brain cancer, and NHL diagnosed from January 1, 1980 through December 31, 2007 were also identified using the national Danish Cancer Registry. Thyroid cancer was classified using the International Classification of Diseases 7th edition codes 194.0 to 195.9 (1980-2003) and 10th edition codes C37.0 to C37.9, C73.0 to C75.9, and D09. Brain cancer was 7th edition codes 192.0 to 193.9 (1980-2003) and 10th edition codes C69.0 to C72.9 (2004-2007). NHL was 7th edition codes 200.0 to 200.9, 202.0 to 202.9 (1980-2003), 10th edition codes C82 to C85.9, C96.0 to C96.9 (2004-2007), and in addition classified using the International Classification of Diseases for Oncology (SNOMED) morphology codes based on cell type: B cell, T cell, and unspecified cell type. B-cell NHLs were SNOMED codes M9670 to M9680, M9690 to M9699, and M9760 to M9769 (1980-2007). T-cell NHLs were codes M9700 to M9729 (1980-2007). NHLs of unspecified cell type were codes M9590 to M9591 (1980-2007).
Cancers before leukemia
Leukemia patients with a history of cancer before the first diagnosis of leukemia were determined using the national Danish Cancer Registry, by searching for any cancer from 1980 until date of leukemia diagnosis. Similar controls with a history of nonleukemia cancer were determined by searching for any nonleukemia cancer from 1980 until the date of study entry in the nested study (date of leukemia diagnosis for the leukemia patient in the matched set).
Cancer treatment
During 1980 to 2003, the national Danish Cancer Registry recorded dichotomous treatment information (non/any) for both radiotherapy and chemotherapy started within 4 months after a leukemia diagnosis; however, no details are available regarding the specific type of treatment administered to the leukemia patient. From 2004 and onwards, the registration was transferred to the Danish National Health Ministry that unfortunately no longer validated the information on treatment. Therefore, only treatment data for the period 1980 to 2003 have been validated and are used in the present analysis.
Other covariates: demographics
Since 1980, Statistics Denmark has gathered information concerning descent, highest obtained level of education, and geographic residence of all persons living in Denmark. The Danish Central Person Registry defines Danish descent as a person born in Denmark with Danish Citizenship, whose both parents are born in Denmark and are Danish Citizens.
Statistical analyses
All analyses were performed on individual participant data. Statistical analyses were performed with Stata Version 11.0 MP software. All tests were 2-sided. Standardization of incidence rates was calculated using the WHO world standard population.19 We used an Epanechnikov kernel function for smoothing the density curves of incident leukemias. We used log-rank tests, Wald tests, and Cox regression models with delayed entry at age of leukemia diagnosis; Cox regression models calculated hazard ratios with 95% confidence intervals (CIs) as a measure of relative risk. We used multivariate models adjusted for age at diagnosis, date of birth, sex, descent, highest obtained level of education, and geographic residency. For Cox proportional hazards regression analyses, we assessed the assumption of proportional hazards graphically by plotting log (cumulative hazard) as a function of follow-up time. We detected no major violations of the proportional hazards assumption (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). For further validation of regression models, conditional hazard ratios were estimated using time intervals of 0-10, 10-20, and 20-28 years after start of follow-up (supplemental Figure 2). For each interval all events outside were censored, time was started at the beginning of the interval, and follow-up was stopped at the end of the interval. Cumulative incidence curves were estimated by the method of Kaplan-Meier with censoring on death or emigration. We used Poisson regression models for estimation of absolute incidence of second malignancies. Absolute 5-year risks were estimated for both leukemia patients and matched controls as the percentage within 5 years from the start of follow-up who developed a second malignancy.
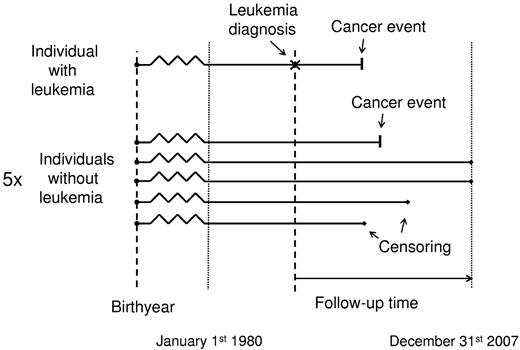
Matched analyses were based on nested studies, where each leukemia patient with the specific subtype was matched on sex, birth year, and age at diagnosis for the leukemia patient with up to 5 general population controls without a diagnosis of the leukemia subtype in question (but could have had other leukemia subtypes; Figure 1). The matching information was only used to form the matched cohorts and was not used in the regressions. All 6 persons in each matched subset were followed from the date of the leukemia diagnosis for the leukemia patient and censored at the date of emigration, death, December 31, 2007, or diagnosis of the cancer endpoint in question, whatever came first. Thus, competing risk of death was accounted for in the analysis by censoring at the date of death (or emigration), information that is 100% complete in the Danish registries.
Design of nested studies for matched analyses, where each leukemia patient with the specific subtype was matched on sex, birth year, and age at diagnosis for the leukemia patient with 5 general population controls. For both leukemia patient and controls, there are 4 types of outcomes: cancer event, censoring because of death, censoring because of emigration, and end of follow-up (here illustrated for the controls).
Design of nested studies for matched analyses, where each leukemia patient with the specific subtype was matched on sex, birth year, and age at diagnosis for the leukemia patient with 5 general population controls. For both leukemia patient and controls, there are 4 types of outcomes: cancer event, censoring because of death, censoring because of emigration, and end of follow-up (here illustrated for the controls).
Age categories for stratified analyses were designated as 14-39, 40-54, 55-64, and > 65 years. For these analyses stratified by age, a leukemia patient and the corresponding 5 controls entered into an age category based on the age at the leukemia diagnosis (ie, the time when follow-up started). For analyses stratified by radiotherapy or chemotherapy, the status of the leukemia patient determined which group the corresponding 5 controls entered. Analyses stratified by radiotherapy and chemotherapy were limited to the time period 1980 to 2003, wherein valid treatment data were available. For analysis of risk of second malignancy without a history of cancer before leukemia, both leukemia patients and controls with history of cancer before date of leukemia diagnosis were excluded. For analysis of risk of second malignancy among leukemia patients and controls with prior history of cancer, the persons without prior cancers were excluded. To further investigate treatment effects, analyses with latent periods of 1, 2, 5, and 10 years were performed (ie, within these periods, an event among those persons with or without leukemia only censored the person). For analyses stratified on calendar periods, a leukemia patient and the corresponding 5 controls entered into the calendar period based on the date of diagnosis of leukemia.
Results
We included the entire Danish population, 14 years of age and older, in a 28-year follow-up period from 1980 through 2007, composed of 6 529 277 adults and 137 million person-years of follow-up; for use as a positive control, we also included 2 825 237 children. Baseline characteristics of the entire population are shown in Table 1. The age distributions of the leukemia cases and the controls are not comparable; the age group 14-39 years represents 65% of controls because that group includes all controls who reached age 14 any time during 1980 through 2007. The age difference between cases and controls is corrected for by matching on age.
Leukemia
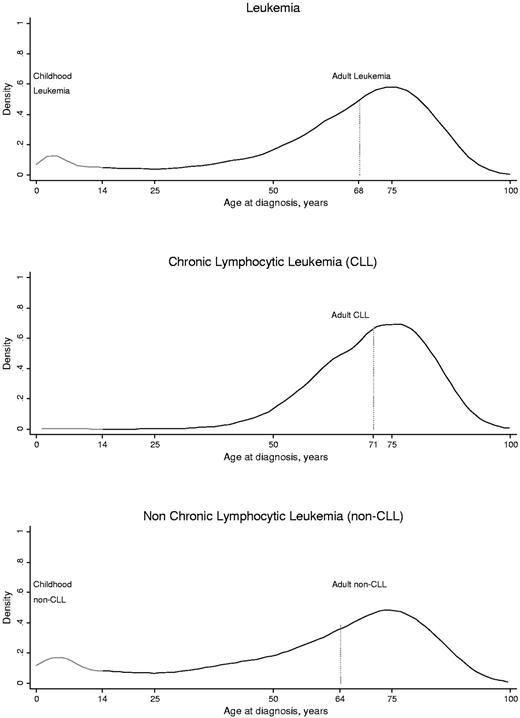
Age distributions of incident adult and childhood leukemia diagnoses in the entire Danish population are shown in Figure 2. We identified 8582 adults diagnosed with CLL and 10 252 adults diagnosed with non-CLL. Median age at diagnosis for adults with CLL was 71 years (interquartile range, 63-79 years), with a standardized incidence rate of 0.26 (95% CI, 0.25-0.27) per 10 000 person-years. Median age at diagnosis for adults with non-CLL was 64 years (range, 55-76 years) with a standardized incidence rate of 0.38 (0.37-0.39) per 10 000 person-years.
Distributions of adult and childhood leukemia, CLL, and non-CLL as relative densities as a function of age at diagnosis in the entire population. We included the entire Danish population in a 28-year follow-up period from 1980 through 2007. Among 7 492 989 total persons, 20 062 people developed leukemia either as a child (n = 1228) or as an adult (n = 18 834). The age distribution shown here for leukemia patients is identical to the age distribution of controls in the nested studies.
Distributions of adult and childhood leukemia, CLL, and non-CLL as relative densities as a function of age at diagnosis in the entire population. We included the entire Danish population in a 28-year follow-up period from 1980 through 2007. Among 7 492 989 total persons, 20 062 people developed leukemia either as a child (n = 1228) or as an adult (n = 18 834). The age distribution shown here for leukemia patients is identical to the age distribution of controls in the nested studies.
Thyroid cancer, brain cancer, and NHL
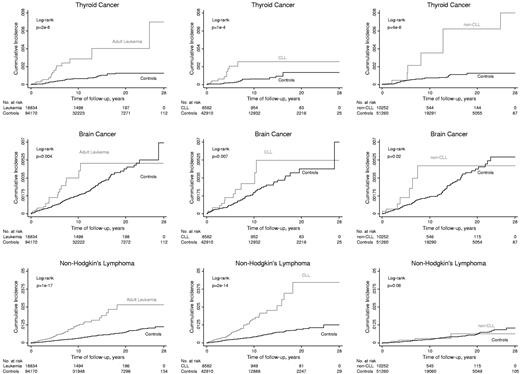
The cumulative incidence of thyroid cancer, brain cancer, and NHL after adult leukemia compared with controls is shown in Figure 3. The cumulative incidences of thyroid cancer, brain cancer, and NHL were higher after a diagnosis of adult leukemia than for matched controls never diagnosed with adult leukemia (Figure 3 left panels); results were similar for the subtypes of CLL and non-CLL (Figure 3 middle and right panels), except that the cumulative incidence of NHL was not higher in those with non-CLL versus controls (Figure 3 bottom right figure).
Kaplan-Meier cumulative incidences as a function of follow-up time in nested studies of thyroid cancer, brain cancer, and NHL after adult leukemia. Subtypes of adult leukemia were CLL and non-CLL.
Kaplan-Meier cumulative incidences as a function of follow-up time in nested studies of thyroid cancer, brain cancer, and NHL after adult leukemia. Subtypes of adult leukemia were CLL and non-CLL.
Thyroid and brain cancers were mainly observed in a relatively short period within 8 years and 12 years after a diagnosis of adult leukemia or any of the 2 subtypes. Because of few events in the control group after this period, there is little statistical power in the following period to exclude that hazard ratios are constant over time. By contrast, NHLs were observed up to 20 years after a diagnosis of adult CLL, comparable to the median follow-up time of 21 years.
Risk of thyroid cancer, brain cancer, and NHL
For risk of thyroid cancer after adult leukemia versus controls, the multivariate adjusted hazard ratio was 4.9 (95% CI, 2.8-8.5; Table 2). Corresponding values were 4.5 (2.0-9.8) after adult CLL and 6.9 (3.0-16) after non-CLL. For comparison, the multivariate adjusted hazard ratio of thyroid cancer after childhood leukemia versus controls was 10 (0.4-223).
For risk of brain cancer after adult leukemia versus controls, the multivariate adjusted hazard ratio was 1.9 (1.2-3.1). Corresponding values were 1.7 (1.0-3.2) after adult CLL and 2.3 (1.1-4.9) after adult non-CLL. For comparison the multivariate adjusted hazard ratio of brain cancer after childhood leukemia versus controls was 7.2 (2.0-26).
For risk of NHL after adult leukemia versus controls, the multivariate adjusted hazard ratio was 3.3 (2.5-4.4). Corresponding values were 3.4 (2.5-4.6) after adult CLL and 1.9 (0.95-3.9) after adult non-CLL. For comparison the multivariate adjusted hazard ratio of NHL after childhood leukemia versus controls was 6.5 (0.4-110).
The increased risk of NHL in those with CLL was primarily the result of B-cell lymphomas (Table 2). Details on the number of specific subtypes of NHL are found in supplemental Table 1.
Absolute 5-year risk of thyroid cancer, brain cancer, and NHL
The absolute 5-year risks of thyroid cancer after adult leukemia were 0.14% and 0.03% for matching controls (Table 3); that is, 1 of 714 adult leukemia patients developed thyroid cancer within 5 years of the leukemia diagnosis, and in comparison 1 of 3333 matched controls developed thyroid cancer within the same 5-year period. Corresponding values were 0.18% after CLL and 0.02% for matching controls, and 0.07% after non-CLL and 0.04% for matching controls.
The absolute 5-year risks of brain cancer after adult leukemia were 0.16% and 0.08% for matching controls. Corresponding values were 0.16% after CLL and 0.09% for matching controls and 0.17% after non-CLL and 0.08% for matching controls.
The absolute 5-year risks of NHL after adult leukemia were 0.46% and 0.16% for the matching controls. Corresponding values were 0.59% after CLL and 0.23% for matching controls, and 0.24% after non-CLL and 0.11% for matching controls.
Treatment and risk of thyroid cancer, brain cancer, and NHL
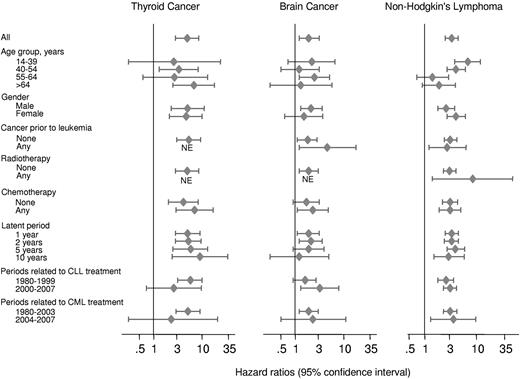
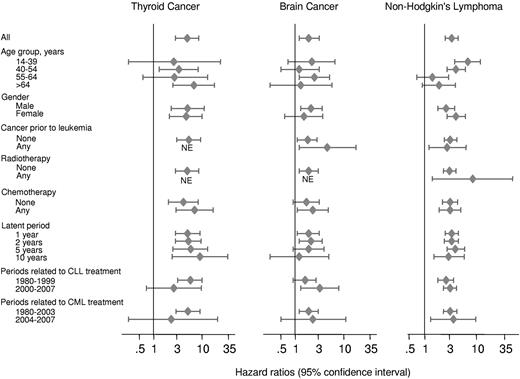
In an attempt to investigate the impact of treatment versus individual person attributable factors, Figure 4 and supplemental Table 2 present risk of thyroid cancer, brain cancer, and NHL after leukemia, CLL, and non-CLL stratified into different categories related to treatment and the person. Treatment protocols for CLL changed in Denmark around year 2000, and for CML around year 2003. Figure 4 and supplemental Table 2, therefore, also show risk estimated before and after years 2000 and 2003.
Risk of thyroid cancer, brain cancer, and NHL in Danish adults, after a diagnosis of leukemia matched with 5 population controls, stratified by age group, sex, history of cancer before leukemia, treatment of leukemia, latent entry, and calendar periods reflecting changes in leukemia treatment protocols in Denmark. NE indicates not available because of no events in the leukemia patient group.
Risk of thyroid cancer, brain cancer, and NHL in Danish adults, after a diagnosis of leukemia matched with 5 population controls, stratified by age group, sex, history of cancer before leukemia, treatment of leukemia, latent entry, and calendar periods reflecting changes in leukemia treatment protocols in Denmark. NE indicates not available because of no events in the leukemia patient group.
Although risk of thyroid cancer, brain cancer, and NHL after adult leukemia differs slightly from stratum to stratum, the 95% CIs of the different hazard ratios overlap (Figure 4). This suggests that the increased risk of thyroid cancer, brain cancer, and NHL after adult leukemia is elevated irrespective of age, sex, cancer before leukemia, radiotherapy, chemotherapy, latent periods of 1, 2, 5, and 10 years, or whether leukemia developed before or after years 2000 and 2004.
Discussion
The principal finding of this Danish nationwide study is that patients with adult leukemia have excess risk of developing thyroid cancer, brain cancer, and NHL, similar to patients with childhood leukemia. These findings are novel.
After adult leukemia versus controls, we observed that patients had the highest risk of thyroid cancer, followed next by risk of NHL, and finally risk of brain cancer. This order is similar to results for childhood leukemia.5
Several cohort studies of patients with childhood leukemia have shown that the primary risk factors for second malignancies are radiotherapy and chemotherapy,5,20,21 with a combined attributable fraction of up to 80% of all second malignancies with a median latency of ∼ 10 years.22 Different tissues respond differently to radiation, but risks of thyroid and brain cancer are strongly associated with the amount of radiation.23 Brain cancer has been reported to develop after radiation treatment of patients with childhood lymphoid leukemia, with a median latency of 7-11 years.5,24,25 In Denmark, this was particularly relevant in the mid-1980s where irradiation of the brain was the preferred prophylaxis against spread of leukemia cells to the brain. In this period, patients with childhood ALL received radiation to the thyroid gland because of poor radiation shielding and subsequent risk of thyroid cancer was radiation dose-dependent,26 and highest among the patients treated before the age of 10 years.27
For the assessment of risk of secondary malignancies in leukemia, the applied treatment modalities are essential; however, this study does not permit an assessment of the specific treatment for each patient. In asymptomatic patients with CLL, a “wait-and-watch” strategy was applied, whereas symptomatic patients in the period before 2003 were treated with chlorambucil usually in combination with glucocorticoids. From 2003 to 2005, the first-line treatment, at least in nonelderly groups, was changed to fludarabine-based regimens. AML treatment was basically the same in the follow-up period, although more patients nowadays receive bone marrow transplantation. In CML, tyrosine kinase inhibitors have been the main treatment since 2001, where imatinib was registered. Before that, interferon, hydroxyurea, busulphan, and allogeneic bone marrow transplantation were used routinely when possible. ALL therapy in adults in Denmark has gradually increased in intensity from 1980 to 2007, but based on the same drug combinations, although high-dose methotrexate has through the years replaced brain irradiation as prophylactic treatment. This is in contrast to regimens at childhood ALL containing much higher doses of nonmyeloablative drugs, such as vincristine, high-dose methotrexate, asparaginase, and glucocorticoids, which in addition are less DNA-damaging. The tendency today is that adolescents and young adults are treated with pediatric-like regimens, but this change in treatment strategy has only taken place in the very last few years and therefore has not influenced the results in the present study. Consequently, the majority of the adult leukemia patients included in this study have received some form of cancerogenic leukemia therapy, including alkylating drugs, DNA-topoisomerase II inhibitors, and irradiation.
It is difficult to compare the risks of secondary malignancies among children and adults with various leukemias because of the substantial differences in leukemia types in these age groups. ALL is the dominant form of leukemia in children, whereas the leukemia types that predominate in adults are either nonexistent or very rare in children. Consequently, ALL is rare in adults. Interestingly, our risk estimates of thyroid and brain cancer after adult leukemia are lower than those observed after childhood leukemia, consistent with a different treatment strategy, including more irradiation of the brain in children.
Our observed median latency between an adult leukemia diagnosis and development of thyroid and brain cancer is similar to what is observed for patients with childhood leukemia.5,23,24 In addition, a stronger association was found for patients with adult non-CLL who presumably received higher treatment dosages, and the lowest association was found for patients with CLL not usually treated with radiation to the head and neck region, or intensive chemotherapy. This observation is in concordance with treatment-driven associations and other studies.28
Ionizing radiation, direct exposure to carcinogenic chemicals, and cancer chemotherapy probably account only for a small attributable fraction of the causation of NHL.29,30 In contrast, the main risk factors for development of NHL are long-term immunosuppression or infection with EBV, HIV, or other viruses.31-34 Independently of the treatment of leukemia, there is a strong association between risk of childhood leukemia and NHL,3,5,35,36 as also the close pathobiologic pathways and stem-cell origin would suggest.11
Major strengths of this nationwide study include the size and long follow-up time of the cohort, the completeness of the national Danish Cancer Registry, the 100% complete follow-up, and our ability to account for competing risk of death as dates of death (and emigration) are complete for all persons in the entire country. Theoretical possible limitations include selection bias; however, as we followed the entire adult Danish population without losses to follow-up, this is not an issue. Potential limitation of register-based studies could be misclassification or incompleteness of cancer diagnoses; however, this is unlikely to have a major effect in the present study as all cancer diagnoses from the national Danish Cancer Registry are assigned based on histologic examination by a fully trained pathologist and the national Danish Cancer registry captures 98% of all cancer diagnoses in Denmark.16,17
Another potential limitation is that CLL with secondary NHL are probably regarded as Richter transformation because ∼ 5% of patients with CLL will develop such a transformation to a fast-growing diffuse large B-cell lymphoma.37 In the national Danish Cancer Registry, any such disease development will not always be registered; therefore, the risk of NHL we observe in CLL patients is probably an underestimate. In addition, in the period from 1980 to 1990, the Kiel classification was used routinely in Denmark, in which lymph node biopsies were mandatory for the diagnosis, sometimes leading to confusion on the distinction between CLL and NHL.
Furthermore, a minor limitation could be that, before 2001, the AML diagnosis was based on the FAB classification and thereafter on the WHO classification. The biggest difference here was that the FAB classification required at least 30% blasts in the bone marrow to make the diagnosis, whereas the WHO classification required at least 20% blasts, unlikely to have a major influence on the present results.
In conclusion, in the entire Danish population, patients with adult leukemia have excess risk of thyroid cancer, brain cancer, and NHL, like patients with childhood leukemia. These findings may be useful for discussion during revision of guidelines for treatment programs and follow-up surveillance for adult leukemia patients.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the national Danish Cancer Registry for providing access to their data records.
This work was supported by the Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital.
Authorship
Contribution: S.F.N., S.E.B., and B.G.N. designed the study; S.F.N. collected data, performed all analyses, and wrote the first draft of the paper; S.E.B., H.S.B., and B.G.N. oversaw all analysis, contributed to the interpretation of data, and edited the paper; and all authors approved this paper in its final form.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Børge G. Nordestgaard, Department of Clinical Biochemistry, 54M1, Herlev Hospital, Copenhagen University Hospital, Herlev Ringvej 75, DK-2730 Herlev, Denmark; e-mail: brno@heh.regionh.dk.