Abstract
Using population-based data from Sweden, we identified all multiple myeloma (MM) patients (n = 8740) and 5652 monoclonal gammopathy of undetermined significance (MGUS) patients diagnosed between 1986 and 2005. We calculated standardized incidence rates (SIRs) for all subsequent hematologic and nonhematologic malignancies for MM patients diagnosed before/after 1995 (introduction of high-dose melphalan/autologous stem cell transplantation [HDM-ASCT]) and 2000 (introduction of immunomodulatory drugs [IMiDs]), respectively. MM patients had an 11.51-fold (95% confidence interval: 8.19-15.74) increased risk of acute myeloid leukemia (AML)/myelodysplastic syndromes (MDS); risk was very similar before/after 1995 and 2000, respectively. MGUS patients had an 8.01-fold (5.40-11.43) increased risk of AML/MDS. Risk was confined to IgG/IgA, while no IgM MGUS patients developed AML/MDS; patients with monoclonal-protein (M-protein) concentrations > 1.5 g/dL (SIR = 11.12; 3.61-25.96) had higher risk than those < 1.5 g/dL (SIR = 4.67; 1.71-10.16). An excess risk of nonmelanoma skin cancer was observed subsequent to both MM (SIR = 2.22; 1.74-2.80) and MGUS (SIR = 3.30; 2.76-3.90). Our novel observations of an excess risk for AML/MDS following IgG/IgA (but not IgM) MGUS, and the highest risk associated with M-protein concentrations > 1.5 g/dL, support a role for nontreatment-related factors in plasma cell dyscrasias. AML/MDS risk following MM was the same before/after the introduction of HDM-ASCT. Longer follow-up is needed to characterize second tumor risks in the IMiD era.
Introduction
Multiple myeloma (MM) is the second most common hematologic malignancy in Western countries.1 Before the introduction of alkylating agents, median survival time of MM patients was < 1 year.2 In the early 1960s, melphalan was discovered to have an anti-MM effect; until recently, the combination of melphalan and prednisone (MP) has remained the mainstay of therapy in MM.3 High-dose melphalan with autologous stem cell transplantation (HDM-ASCT) in younger patients with MM was first introduced in the late 1980s. HDM-ASCT has been evaluated in both randomized4-10 and population-based studies11 ; improved response rates and progression-free survival have been found in the majority of studies, while improved overall survival was found in only 4 of 8 studies.4,5,7,11 During the last decade, agents with new mechanisms of action, such as thalidomide,12 bortezomib,13 and lenalidomide,14 have increased the therapeutic options in MM and further improved response rate, progression-free survival, and overall survival.
For > 4 decades, an excess of acute myeloid leukemia (AML) and myelodysplastic syndromes (MDS) have been reported following MM.15,16 Some previous population-based studies on patients with hematologic malignancies including MM have reported an increased incidence of second malignancies including myeloid leukemias.17,18 Although the underlying biologic mechanisms of AML/MDS following MM need to be better defined, treatment-related factors including the use of melphalan have been considered to be the main cause of the observed elevated risk.19-21 Based on small numbers, some studies have reported the risk of AML to increase with increasing cumulative melphalan dose, duration of melphalan therapy, or a combination; however, other investigators have found that the risk of AML in MM patients may be independent of cumulative melphalan dose.22,23 In the era of novel MM therapies, an increasing number of patients are living longer, and in turn will receive more types of MM therapies thus extending the natural course of their disease. Consequently, clinicians who treat and follow patients with MM have started to encounter long-term complications as the new challenge in clinical MM management. Indeed, preliminary analyses from recent randomized clinical trials have reported an increased incidence of second malignancies including AML/MDS in MM patients treated with maintenance lenalidomide.24-26
Using high-quality population-based data from Sweden, we have conducted the largest systematic evaluation to date focusing on second hematologic and nonhematologic malignancies following MM. By identifying all (n = 8740) MM patients diagnosed between 1986 and 2005 (follow-up until 2006), we were able to define patterns of second malignancies before and after the introduction of novel therapies, and to compare risks in relation to the Swedish general population. So far, most of the attention has been drawn to various MM treatments and their potential risk of causing second malignancies. However, as pointed out by Bergsagel et al in 1979,20 it is inherently complex to quantify the risks of second tumors in relation to a given therapy because we do not know the baseline incidence of second malignancies in (untreated) MM patients. To overcome this problem, and to test the hypothesis that nontreatment-related factors may play a role in subsequent malignancies following plasma cell dyscrasias, we estimated the risk of subsequent hematologic and solid malignancies among 5652 monoclonal gammopathy of undetermined significance (MGUS) patients diagnosed during the same calendar period and compared risk patterns in relation to the Swedish general population.
Methods
Central registries, patients, and controls
Since the 1950s, Sweden has provided universal medical care for the entire population (independent of socioeconomic status and geographic region), currently approximately 9 million people. In contrast to many other countries, patients with lymphoproliferative malignancies in Sweden are usually diagnosed, treated, and followed clinically by physicians at a few hospital-based hematology or oncology centers. The Nordic Myeloma Study Group (NMSG) was founded in 1987. In 1995, NMSG developed the first guidelines for the diagnosis and treatment of MM (updated in 2001 and 2006), and since 2009 there have been national guidelines in Sweden (latest update in 2011). These guidelines have been universally accepted and followed in Swedish hospitals.27 Given these facts, therapy for MM patients is very similar across the entire nation, independent of socioeconomic status, and geographic variations.
In Sweden, melphalan was introduced in the treatment of MM in the early 1960s. It was initially given as a continuous daily dose and, from the 1970s, was gradually exchanged for the combination with prednisone (MP). In a recent investigation, we obtained detailed information on the primary treatment on all MM patients diagnosed in Malmö (the third largest city in Sweden) between 1950 and 2005.28 In that study, we reported that among patients diagnosed after age 65 years, MP-like therapy was the most dominant primary treatment in all calendar periods. Among patients diagnosed at age 65 years or younger, vincristine-doxorubicin-dexamethasone (VAD)–like therapy (including other high-dose corticosteroid-based regimens) was introduced in the primary treatment in the mid-1990s, and was given to 96% of patients in the last calendar period.28
Since 1958, all physicians and pathologists/cytologists in Sweden have been obliged by law to report each case of cancer diagnosed or treated to the centralized nationwide Swedish Cancer Registry, yielding a comprehensive and accurate (> 90%-95%) database.29,30 Using the Swedish Cancer Registry, we identified all MM patients diagnosed between 1986 and 2005 (Table 1).31
Patients' characteristics
| Variable . | Multiple myeloma . | MGUS . |
|---|---|---|
| Total, n (%) | 8740 (100) | 5652 (100) |
| 65 years or younger, n (%) | 2495 (29) | 1585 (28) |
| Older than 65 years, n (%) | 6245 (71) | 4067 (72) |
| Male, n (%) | 4811 (55) | 2845 (50) |
| Median age at diagnosis, y (interquartile range) | 72 (64-79) | 73 (63-80) |
| Median follow-up time, mo (interquartile range) | 27.9 (10.3-54.0) | 52.4 (22.4-92.6) |
| Year of diagnosis 1986-1994, n (%) | 4228 (48) | 1362 (24) |
| Year of diagnosis 1995-2005, n (%) | 4512 (52) | 4290 (76) |
| Variable . | Multiple myeloma . | MGUS . |
|---|---|---|
| Total, n (%) | 8740 (100) | 5652 (100) |
| 65 years or younger, n (%) | 2495 (29) | 1585 (28) |
| Older than 65 years, n (%) | 6245 (71) | 4067 (72) |
| Male, n (%) | 4811 (55) | 2845 (50) |
| Median age at diagnosis, y (interquartile range) | 72 (64-79) | 73 (63-80) |
| Median follow-up time, mo (interquartile range) | 27.9 (10.3-54.0) | 52.4 (22.4-92.6) |
| Year of diagnosis 1986-1994, n (%) | 4228 (48) | 1362 (24) |
| Year of diagnosis 1995-2005, n (%) | 4512 (52) | 4290 (76) |
MGUS indicates monoclonal gammopathy of undetermined significance.
We also used a nationwide MGUS cohort established from a national hospital network, including MGUS patients diagnosed between 1967 and 2005 in Sweden.32 In the present study, to cover the same period as the MM patients, we restricted the analysis to MGUS patients diagnosed between 1986 and 2005 (Table 1). As described previously,32 because MGUS is generally asymptomatic, it is usually an unexpected finding during a medical work-up for another cause. In Sweden, when a clinician detects an MGUS patient, he/she will typically consult with a hematology specialist at a regional hospital-based center, and, if needed, refer the patient for further work-up, especially to rule out an underlying malignancy. These centers are affiliated with a hospital-based hematology/oncology centers. The first population-based MGUS screening studies were initiated by Dr Waldenström's group in Sweden in the early 1960s.33 Indisputably, these early efforts have played an important role and facilitated to an increasing awareness regarding MGUS among Swedish clinicians. In the nationwide MGUS cohort (including patients diagnosed between 1967 and 2005),32 MGUS patients diagnosed between the late 1960s and the late 1970s were primarily diagnosed by Dr Waldenström's group at Malmö University Hospital. During these years, diagnostic criteria were defined by the presence of a monoclonal protein (M-protein) in serum in the absence of an underlying lymphoproliferative malignancy.33 From the early 1980s, efforts have been made, mainly influenced by Dr Kyle's group at the Mayo Clinic, to establish stringent criteria to distinguish MGUS from asymptomatic forms of myeloma and related disorders.34 MGUS is now defined by the presence of a monoclonal Ig of < 3 g/dL in serum; if BM examination was performed, a plasma cell content of < 10%, no evidence of other lymphoproliferative disorders, and the absence of clinical manifestations related to the monoclonal gammopathy.35 These criteria are essentially the same as have been used at Swedish hospitals during the study period. The following approaches were applied to establish the nationwide MGUS cohort: first, we retrieved information on all incident patients through our national network, which included all outpatient units including all major regional hospital-based hematology/oncology centers in Sweden. For all MGUS patients, we obtained information on sex, date of birth, date of diagnosis, and region/unit where the diagnosis was made. When available, we also collected information on MGUS isotype and concentration of the monoclonal spike at diagnosis. Second, we identified all MGUS patients who were reported in the Swedish Inpatient Registry, which captures information on individual patient-based discharge diagnoses and discharge listings from all inpatient care, with a very high coverage.36 Data on all MGUS patients from these 2 sources was merged into one master database. Using the nationwide Swedish Cancer Registry, which includes information on all incident cancers diagnosed since 1958 (including date of diagnosis and region/hospital where the diagnosis was made),30,31 we obtained data on all cancer diagnoses for all MGUS patients. Lastly, in accord with current diagnostic guidelines,35 we removed any MGUS patient with a recorded preceding lymphoproliferative malignancy.
For all MM and MGUS patients, we conducted record linkage with the Swedish Cancer Registry and the Swedish Cause of Death Registry to obtain data (until December 31, 2006) on second malignancies and of vital status, respectively. In addition, we obtained age- and sex-specific population incidence rates for all hematologic and nonhematologic malignancies from the Swedish Cancer Registry.
Approval was obtained from the Karolinska Institutional Review Board for this study. Informed consent was waived because we had no contact with study subjects. An exemption from institutional review board review was obtained from the National Institutes of Health Office of Human Subjects Research because we used existing data without personal identifiers.
Statistical analysis
To evaluate the risk of malignancies following MM and MGUS, respectively, we calculated standardized incidence ratios (SIRs; observed cancers in the patient cohorts divided by the expected numbers from the general population) with 95% confidence intervals (CIs) for all subsequent hematologic and nonhematologic tumors overall and separately. Expected numbers of events were calculated by applying age- and sex-specific population incidence rates to the person-years observed among our cases. We evaluated the risk of every individual malignancy as well as defined organ-specific subgroups. For all malignancies where we found statistical associations, we conducted sensitivity analyses excluding malignancies diagnosed within a year of MGUS and MM diagnosis, respectively. In all our analyses, SIRs and 95% CIs were based on the first malignancy after MM and MGUS, respectively.
To assess the role of novel MM therapies in relation to the development of second malignancies, the analysis for younger (65 years or younger) patients were stratified before and after the year 1995 (when HDM-ASCT was introduced as standard treatment for younger MM patients in Sweden).28,31 We also conducted exploratory analyses stratified before and after year 2000 (when immunomodulatory drugs [IMiDs] were introduced in Sweden).28,31
Results
Malignancies subsequent to MM
Sixty-nine and 508 of the MM patients were diagnosed with a second hematologic and nonhematologic malignancy, respectively (Figure 1; supplemental Appendix, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Twenty-eight (4.6%) second malignancies were diagnosed within a month of MM diagnosis; the most common tumor types were prostate (n = 11) and renal cancer (n = 5). Risk estimates for second malignancies following MM were virtually unchanged when cases diagnosed within a month were excluded from the analysis (not shown).
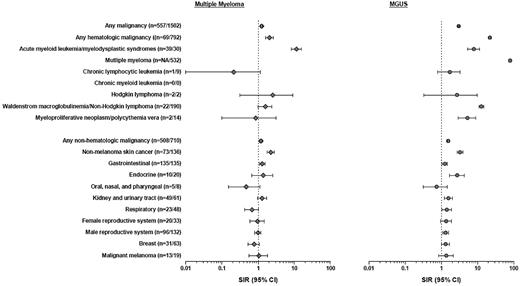
Risk of hematologic and nonhematologic malignancies in MM and MGUS. Unspecified malignancies, unspecified acute leukemia, unspecified chronic leukemia, unspecified leukemia and monocytic leukemia, malignancies of the central and peripheral nervous system, conjunctiva, retina and ocular tumors, retroperitoneal, soft tissue and mediastinal tumors, and tumors with unknown primary are not included in the table due to small numbers. None of these tumor types was significantly associated with MM or MGUS, respectively. SIR indicates standardized incidence ratio; CI, confidence interval; gastrointestinal: esophagus, stomach, small intestine, colon, rectum, anal canal, liver and biliary system, and the pancreas; endocrine: parathyroid, thyroid, adrenals, hypophyses, carotid body, and islet cells; oral, nasal, and pharyngeal: lip, tongue, salivary glands, oral cavity, oropharynx, tonsils, nasopharynx, pyriform fossa, nasal cavity, paranasal sinuses, and the middle ear cavity; kidney and urinary tract: kidney, renal pelvis, ureter, urethra, and urinary bladder; respiratory: trachea, bronchi, lung parenchyma, and the pleura; female reproductive system: uterus, adnexa, cervix, placenta, vagina, vulva, and labia; and male reproductive system: prostate, testis, epididymis, and penis.
Risk of hematologic and nonhematologic malignancies in MM and MGUS. Unspecified malignancies, unspecified acute leukemia, unspecified chronic leukemia, unspecified leukemia and monocytic leukemia, malignancies of the central and peripheral nervous system, conjunctiva, retina and ocular tumors, retroperitoneal, soft tissue and mediastinal tumors, and tumors with unknown primary are not included in the table due to small numbers. None of these tumor types was significantly associated with MM or MGUS, respectively. SIR indicates standardized incidence ratio; CI, confidence interval; gastrointestinal: esophagus, stomach, small intestine, colon, rectum, anal canal, liver and biliary system, and the pancreas; endocrine: parathyroid, thyroid, adrenals, hypophyses, carotid body, and islet cells; oral, nasal, and pharyngeal: lip, tongue, salivary glands, oral cavity, oropharynx, tonsils, nasopharynx, pyriform fossa, nasal cavity, paranasal sinuses, and the middle ear cavity; kidney and urinary tract: kidney, renal pelvis, ureter, urethra, and urinary bladder; respiratory: trachea, bronchi, lung parenchyma, and the pleura; female reproductive system: uterus, adnexa, cervix, placenta, vagina, vulva, and labia; and male reproductive system: prostate, testis, epididymis, and penis.
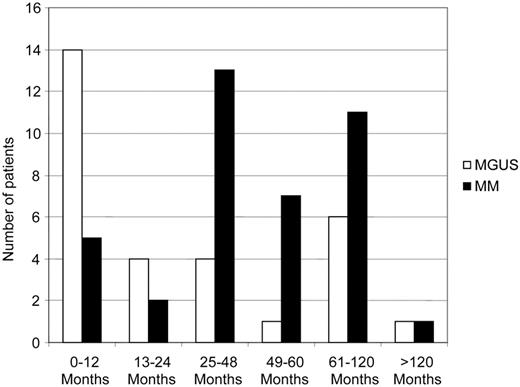
Compared with the Swedish population, MM patients had a 1.26-fold (95% CI 1.16-1.36) increased risk of developing any second malignancy (Figure 1). Hematologic second malignancies were 2.04-fold (95% CI 1.59-2.58) more common, and most of the excess risk was contributed by AML/MDS (SIR = 11.51, 95% CI 8.19-15.74, incidence = 139.0 per 100 000 person-years; Figure 1). In sensitivity analysis excluding malignancies diagnosed within a year of MM diagnosis, the risk of AML/MDS following MM was very similar (SIR = 13.47; 95% CI 9.33-18.82; n = 34). The median time to AML/MDS diagnosis following MM was 45.3 months (interquartile range: 29.8-73.3 months; Figure 2). Furthermore, among MM patients who developed a subsequent nonhematologic malignancy, there were 2 individuals who developed AML/MDS as a third malignancy (following lung and prostate cancer, respectively).
Nonhematologic second malignancies were 1.19-fold (95% CI 1.09-1.30) more common following MM (Figure 1). There was an increased risk for gastrointestinal malignancies (SIR = 1.30; 95% CI 1.09-1.53) and nonmelanoma skin cancer (SIR = 2.22, 95% CI 1.74-2.80).
We analyzed risk for AML/MDS among younger (< 65 years) MM patients diagnosed before/after 1995 (introduction of HDM-ASCT for patients < 65 years), and risks were not significantly different (SIR = 33.34; 95% CI 12.23-72.57 vs SIR = 23.19; 95% CI 11.98-40.50). Similarly, risks for AML/MDS among MM patients (including all age groups) diagnosed before/after 2000 (introduction of IMiDs) were not significantly different (SIR = 13.51; 95% CI 8.83-19.80 vs SIR = 8.35; 95% CI 4.17-14.94).
Malignancies subsequent to MGUS
Seven hundred ninety-two and 710 of the MGUS patients were diagnosed with a subsequent hematologic and nonhematologic malignancy, respectively (Figure 1; supplemental Appendix). As expected,28 the most common malignancy diagnosed after MGUS was MM (n = 532, 9.4%; SIR = 79.86, 95% CI 73.21-86.94). In further accord with the literature,36 190 (3.4%) MGUS patients (dominantly IgM isotype) were diagnosed with a subsequent diagnosis of Waldenström macroglobulinemia (WM)/non-Hodgkin lymphomas (NHL; SIR = 12.85, 95% CI: 11.08-14.81). Eighty-six (7.1%) second malignancies were diagnosed within a month of MGUS diagnosis (46 of these were MM). With the exception of lymphoproliferative hematologic malignancies, risk estimates for malignancies diagnosed subsequent to MGUS were virtually unchanged when patients diagnosed with a malignancy within a month after MGUS diagnosis were excluded (not shown).
We observed an elevated risk for AML/MDS following MGUS (SIR = 8.01, 95% CI 5.40-11.43, incidence 102.0 per 100 000 person-years; Figure 1); none of these MGUS patients developed MM following AML/MDS. When excluding malignancies diagnosed within a year of MGUS diagnosis, the risk of AML/MDS following MGUS was very similar (SIR = 5.13; 95% CI 2.93-8.34; n = 16). The median time to AML/MDS diagnosis following MGUS was 14.4 months (interquartile range: 6.0-87.6 months; Figure 2). The excess AML/MDS risk was confined to patients with IgG/IgA MGUS (n = 13/2293) and none of the IgM MGUS (n = 0/559) patients developed AML/MDS (Table 2). Based on small numbers, we found more pronounced AML/MDS risk among MGUS patients with M-protein concentrations > 1.5 g/dL (SIR = 11.12; 95% CI 3.61-25.96) versus < 1.5 g/dL (SIR = 4.67, 95% CI 1.71-10.16; Table 2). Lastly, we observed an increased risk for myeloproliferative neoplasms/polycythemia vera (SIR = 5.32; 95% 2.91-8.92) subsequent to MGUS (Figure 1); the analysis excluding malignancies diagnosed within a year of MGUS diagnosis revealed virtually the same results (SIR = 4.54, 95% CI 2.18-8.35; n = 10).
Risk of AML/MDS among MGUS patients, in relation to isotype and M-protein concentration
| Variable . | MGUS patients, n (%) . | AML/MDS cases, n . | SIR (95% CI) . |
|---|---|---|---|
| MGUS isotype | |||
| IgG and IgA | 2293 (40.6) | 13 | 8.14 (4.34-13.9) |
| IgM | 559 (9.9) | 0 | |
| Missing | 2798 (49.5) | 17 | 10.06 (5.86-16.1) |
| M-protein concentration | |||
| 1.5 g/dL or greater | 714 (12.7) | 5 | 11.12 (3.61-26.0) |
| < 1.5 g/dL | 1732 (30.6) | 6 | 4.67 (1.71-10.2) |
| Missing | 3206 (56.7) | 19 | 9.44 (5.68-14.8) |
| Variable . | MGUS patients, n (%) . | AML/MDS cases, n . | SIR (95% CI) . |
|---|---|---|---|
| MGUS isotype | |||
| IgG and IgA | 2293 (40.6) | 13 | 8.14 (4.34-13.9) |
| IgM | 559 (9.9) | 0 | |
| Missing | 2798 (49.5) | 17 | 10.06 (5.86-16.1) |
| M-protein concentration | |||
| 1.5 g/dL or greater | 714 (12.7) | 5 | 11.12 (3.61-26.0) |
| < 1.5 g/dL | 1732 (30.6) | 6 | 4.67 (1.71-10.2) |
| Missing | 3206 (56.7) | 19 | 9.44 (5.68-14.8) |
AML indicates acute myeloid leukemia; MDS, myelodysplastic syndrome; MGUS, monoclonal gammopathy of undetermined significance; SIR, standardized incidence ratio; and CI, confidence interval.
Compared with the Swedish population, MGUS patients had a 1.56-fold (95% CI 1.44-1.68) increased risk of developing a nonhematologic malignancy (Figure 1). We found increased risk for nonmelanoma skin (SIR = 2.04; 95% CI 1.63-2.51), endocrine (SIR = 3.34; 95% CI 1.78-5.71), and breast cancer (SIR = 1.32; 95% CI 1.02-1.69). In analysis excluding malignancies diagnosed within a year of MGUS diagnosis, the risks were very similar (data not shown). In addition, MGUS patients had an increased risk of the following organ-specific subgroups: kidney and urinary tract (SIR = 1.58; 95% CI 1.21-2.04), respiratory (SIR = 1.42; 95% CI 1.05-1.89), male reproductive system (SIR = 1.32; 95% CI 1.11-1.57), and gastrointestinal cancer (SIR = 1.25; 95% CI 1.05-1.48). However, no specific malignancy was significantly increased within these organ-specific subgroups. When excluding malignancies diagnosed within a year of MGUS diagnosis, none of the organ-specific subgroups were found to be at significantly increased risk (data not shown).
Discussion
In this largest systematic evaluation of second malignancies following MM to date, we were able to expand our current knowledge regarding the risk of developing AML/MDS. In accord with prior studies,15,16,20,21,30 compared with the general population, we found MM patients to have an 11-fold increased risk of developing AML/MDS reflected in an incidence of 139.0 per 100 000 person-years. On average, AML/MDS occurred approximately 4 years after MM diagnosis.
In our study, we conducted analyses designed to study patterns of second primary malignancies in relation to the introduction of HDM-ASCT (year of 1995 in Sweden).28 More specifically, we estimated the risk for AML/MDS before and after 1995, and found the patterns to be very similar. We have speculated that the observed lack of difference over time could potentially reflect the fact that before the introduction of HDM-ASCT, MM patients typically received oral melphalan in combination with steroids.31 Thus, it may be that lower doses of extended oral melphalan therapy and melphalan concentrated to 1 or 2 high-dose courses could have similar impact on the risk of developing AML/MDS. Unfortunately, because of lack of detailed treatment information for individual patients, we were unable to further examine the role of melphalan dosing in the context of second malignancies. It is possible that the observed patterns are influenced by temporal variations in exposures to other types of therapies. In our exploratory analyses focusing on the introduction of IMiDs (year 2000 in Sweden), we found the risk patterns for AML/MDS to be very similar over time. However, since the actual usage of IMiD therapy in Sweden during the study period was relatively low28,31 and the follow-up time was restricted; the analysis focusing on the introduction of IMiDs should be interpreted with caution. Clearly, there is a need for future analysis when more mature data become available.
For the first time, to our knowledge, we found IgG/IgA (but not IgM) MGUS patients to have an increased risk of developing AML/MDS. None of these MGUS patients developed MM following AML/MDS. This observation is important in that it supports a role for nontreatment-related factors in the causation of AML/MDS in plasma cell dyscrasias. Given that the risk of AML/MDS was higher subsequent to MM compared with MGUS, we have speculated that there may be an interaction between underlying plasma cell disease and treatment-related factors. Alternatively, it is possible that more proliferative plasma cell dyscrasias (ie, MM) carry a higher risk than precursor states (ie, MGUS). In further support for this hypothesis, based on small numbers, AML/MDS risk was higher in MGUS patients with M-protein concentrations > 1.5 g/dL (vs < 1.5 g/dL). To assess the potential influence of detection bias (ie, underlying disease processes which led to the medical work-up for MGUS), we conducted sensitivity analysis excluding malignancies diagnosed within a year of MGUS diagnosis. As expected, we found MGUS patients being diagnosed with AML/MDS during that first year (Figure 2). Importantly, in the sensitivity analysis (excluding malignancies diagnosed within a year of MGUS diagnosis), we estimated the risk of AML/MDS following to be very similar to the risk calculated in the main analysis. In addition, based on small numbers, the absolute number of AML/MDS following MGUS remained elevated during the > 10 years of follow-up (Figure 2).
As expected, we found MM to be the most common malignancy subsequent to MGUS.37 Recently, it has been shown that virtually all MM patients are preceded by MGUS.38,39 Also in accord with the literature, we found the risk of WM/NHL to be highly elevated among MGUS patients. Based on 11 cases, we found MGUS patients to have a 5-fold increased risk of myeloproliferative neoplasms/polycythemia vera. Although prior smaller studies report an excess of myeloproliferative neoplasms/polycythemia vera in plasma cell dyscrasias,40 given the lack of association between myeloproliferative neoplasms/polycythemia vera and MM in our study, we have speculated that this observation potentially could be a reflection of detection bias because complete blood count (CBC) tests are part of the standard monitoring for MGUS patients. Future studies are needed to answer this question.
When we evaluated the risk of nonhematologic malignancies subsequent to MM and MGUS, we found nonmelanoma skin cancer to be increased compared with the general population (SIR = 2.22 and SIR = 3.30). Given the consistent findings between MM and MGUS, this may represent a true biologic association. Furthermore, compared with the general population, MGUS patients had an increased risk for endocrine malignancies (n = 20); 17 of these patients had parathyroid cancer. We have speculated that this observation most likely is because of the fact that serum calcium levels are closely monitored for MGUS patients (ie, detection bias). We also found MGUS patients, but not MM patients, to have an increased risk for malignancies involving certain organ-specific subgroups, including: kidney and urinary, respiratory, and male reproductive system. However, no specific malignancy was significantly increased. Furthermore, when we assessed organ-specific subgroups and we excluded malignancies detected within 1 year of MGUS diagnosis, the risk estimates were no longer statistically significant. Based on these facts, although true biologic associations cannot be excluded, we feel that these patterns, most likely, reflect increased surveillance of patients with MGUS, or they could be spurious findings because of multiple statistical testing.
Our study has several strengths, including the large sample size and use of high-quality data from Sweden. The study included a stable population with access to standardized health care during the entire study period. By using the nationwide register-based design, we were able to rule out recall bias and ensure a generalizability of our findings. As described in detail in “Central registries, patients, and controls,” the MGUS patients in our study were diagnosed at hematology/oncology outpatient units.32 In accordance with clinical practice in Sweden, most MGUS patients typically underwent a BM examination as part of the clinical work-up. In a recent validation study, we have reported that ascertainment and diagnostic accuracy for lymphoproliferative disorders (including MM) is very high (> 90%-95%) in Sweden.29
Also our study has some limitations. For the MM cohort, we lack detailed clinical and treatment data, as well as information on the molecular subtype of MM. Because the MGUS patients come from a clinical cohort established from a national hospital network in Sweden,32 and not from a screening study, one has to be cautious and consider various types of potential bias. For example, it cannot be ruled out that the observed excess of AML/MDS following MGUS is, at least in part, because of underlying disease processes which led to the medical work-up for MGUS (ie, detection bias). Furthermore, given the fact that MGUS patients are followed clinically, and CBC tests are part of the standard annual monitoring,35 it may have contributed to the detection of, at least some, earlier MDS cases (ie, surveillance bias). As discussed above, in the main analysis (but not the sensitivity analysis), we found the risk of certain organ-specific subgroups of nonhematologic malignancies to be increased, and this may represent some bias. However, there was no association between MGUS and subsequent risk of chronic lymphocytic leukemia (CLL)/chronic myeloid leukemia (CML). One future strategy to assess the potential influence of detection and surveillance bias may be the launching of a large record-linkage study based on a screened MGUS population, and to compare patterns of malignancies with population rates from the National Cancer Institute (NCI) SEER database.18 However, such an effort would have other important limitations. For example, the NCI SEER database does not include information on MDS cases until 2001 when the ICD-O-3 coding went into effect, and, as for any large database, there were a few years of delay until the reporting became entirely consistent. Thus, at this time, the possibilities to accurately quantify the risk of MDS following MGUS—compared with the general US population—are very limited. In addition, the M-protein concentrations are relatively lower in a screened MGUS population (the median M-protein concentration is 0.5 g/dL and 0.8 g/dL in the Olmsted county screening study and the present study, respectively).32,41 Because our study found higher AML/MDS risk among MGUS patients with higher M-concentrations, the clinical value of a screened MGUS population becomes harder to interpret in the context of subsequent risk of developing AML/MDS.
The present study was designed and launched during the spring of 2010. At the annual American Society of Hematology meeting in December 2010, preliminary analyses from 3 randomized clinical trials were presented, suggesting that maintenance therapy with lenalidomide may increase the risk of second malignancies in MM.24-26 Based on available interim data from these trials, AML/MDS has been observed as the most prominent second malignancies.24-26 Currently, there are ongoing efforts designed to review and confirm the cases, and to characterize underlying mechanisms. The results from the present study are important in the context of these clinical trials. For example, it is possible that MM patients who subsequently develop second malignancies carry certain disease- and host-related characteristics that remain to be defined. Our novel findings that IgG/IgA (and not IgM) MGUS have an increased risk of AML/MDS, and the greatest risk is among MGUS patients with M-protein concentrations > 1.5 g/dL (vs < 1.5 g/dL), suggest that nontreatment-related factors play an etiologic role in the development of AML/MDS following MM, likely in combination with treatment factors. Our observation that AML/MDS risk following MM is the same before and after the introduction of HDM-ASCT suggests that administration of melphalan over a longer time period, or concentrated to 1 or 2 high-dose courses, carries similar risks. Although we did exploratory analysis on the patterns of AML/MDS after the introduction of IMiDs, our database had limitations in terms of follow-up, and the use of IMiDs given as maintenance therapy has been very limited in Sweden. Future studies are needed to uncover underlying biologic mechanisms of our findings.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the Intramural Research Program (National Cancer Institute, National Institutes of Health); the Adolf H. Lundin Charitable Foundation; Emily Steplowski and Joe Barker (Information Management Services Inc, Silver Spring, MD) for computer programming support; and David Check (Biostatistics Branch, National Cancer Institute) for the illustrations.
National Institutes of Health
Authorship
Contribution: S.M. and O.L. initiated and designed the study; S.Y.K., M.B., I.T., and O.L. provided data; all authors were involved in the interpretation of the results; S.M. and O.L. drafted the manuscript; and all authors reviewed and approved the submitted version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Ola Landgren, Center for Cancer Research, Medical Oncology Branch, National Cancer Institute, National Institutes of Health, 9000 Rockville Pike, Bldg 10/Rm 13N240, Bethesda, MD 20892; e-mail: landgreo@mail.nih.gov.