Abstract
The appropriate therapy for limited-stage nodular lymphocyte predominant Hodgkin lymphoma (NLPHL) is unclear. In contrast to classical Hodgkin lymphoma (CHL), chemotherapy is often omitted; however, it is unknown whether this impacts the risk of relapse. Herein, we compared the outcome of patients with limited-stage NLPHL treated in an era in which ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine) chemotherapy was routinely incorporated into the primary therapy to an earlier era in which radiotherapy (RT) was used as a single modality. Using the British Columbia Cancer Agency Lymphoid Cancer Database, 88 patients with limited-stage NLPHL (stage 1A/1B or 2A, nonbulky disease < 10 cm) were identified. Treatment followed era-specific guidelines: before 1993, (n = 32) RT alone; and 1993 to present (n = 56), ABVD-like chemotherapy for 2 cycles followed by RT with the exception of 14 patients who received ABVD chemotherapy alone. Most patients were male (75%) with stage I disease (61%). In an era-to-era comparison, the 10-year time to progression (98% vs 76% P = .0074), progression-free survival (91% vs 65% P = .0024), and OS (93% vs 84%, P = .074) favored the ABVD treatment era compared with the RT alone era. Treating limited-stage NLPHL similarly to CHL may improve outcome compared with the use of radiation alone.
Introduction
Nodular lymphocyte predominant Hodgkin lymphoma (NLPHL) is an uncommon subtype of Hodgkin lymphoma (HL), representing only 5% of all cases.1 It is distinguished from the much more common classical Hodgkin lymphoma (CHL) based on distinct pathologic and clinical features.1 Patients typically present with limited-stage disease in peripheral lymph nodes, often in a single lymph node region. B symptoms, bulky disease, and extranodal involvement are rare. NLPHL is characterized by the presence of lymphocyte predominant (LP) cells, previously referred to as “popcorn cells,” that express B-cell associated antigens, including CD20 and surface immunoglobulin but lack CD15 and CD30, in contrast to the Hodgkin Reed-Sternberg cells of CHL. The LP cells form either a nodular or a nodular and diffuse pattern on a cellular background rich in small B lymphocytes with few admixed T lymphocytes. The importance of the combination of morphology and immunophenotypic criteria for the diagnosis of NLPHL was highlighted in the European Task Force on Lymphomas study, which reported that only approximately half of the submitted cases that were presumed to be NLPHL actually retained this diagnosis after expert review.2
Because of disease rarity and resultant lack of randomized controlled trials, management guidelines are typically based on single or multi-institution series or subgroup analyses often with short follow-up and/or inadequate pathology review. As a result, treatment recommendations in NLPHL are diverse and range from noncurative options, such as watchful waiting or single-agent rituximab, through involved field radiation alone or combined modality therapy.3 This wide spectrum of therapeutic approaches is based on a presumed difference in natural history compared with CHL. Further, given the presence of B-cell surface markers, there is a perception that the behavior of NLPHL might be more similar to follicular lymphoma with frequent relapses and incurability.4 In addition, it has been claimed that ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine) chemotherapy may be inadequate in NLPHL. However, this assertion has been based on small studies, often with the inclusion of advanced-stage patients. Moreover, there has been an underappreciation of the inherent risk of transformation to aggressive lymphoma, typically diffuse large B-cell lymphoma (DLBCL), compared with CHL,5,6 and this can also compound the confusion in the interpretation and comparison of outcome series. In the absence of randomized prospective trials, useful insights into the natural history and the appropriate therapy of NLPHL can be discerned by evaluating NLPHL in a defined, uniformly treated population.
In British Columbia, treatment approaches for lymphoproliferative disorders follow guidelines derived at the British Columbia Cancer Agency (BCCA) enabling population-based outcome analyses in a given treatment era. The purpose of the current study was to evaluate the outcome of patients with limited-stage NLPHL to determine the impact using ABVD chemotherapy.
Methods
Patients with NLPHL diagnosed between February 1966 and May 2009 in British Columbia were identified in the BCCA Lymphoid Cancer Database. British Columbia legislation mandates reporting of all cancers to a central registry. Cross-comparison of the British Columbia Cancer Registry and the BCCA Lymphoid Cancer Database verified that all cases of NLPHL seen in British Columbia during the study period are included in this report. Patients were included if they were older than 15 years and had limited-stage disease: stage 1A (plus 1B starting in 2001) or stage 2A and all were nonbulky (< 10 cm). Diagnoses were based on the Revised European American Lymphoma/World Health Organization classification of NLPHL.7 This study was approved by the University of British Columbia-BCCA Research Ethics Board.
On initial screen, 121 cases of limited-stage NLPHL were identified. However, after pathology rereview, 33 were excluded: CHL (n = 15) (lymphocyte-rich, n = 8; mixed cellularity, n = 6; nodular sclerosis, n = 1); composite lymphoma (n = 1); non-Hodgkin lymphoma (n = 2; DLBCL, n = 1, non-Hodgkin lymphoma not otherwise specified, n = 1); diffuse morphology and blocks not available for immunohistochemistry (n = 2); concurrent malignancy (n = 1); benign (n = 2); diagnosis out of province (n = 9); and treatment records not available (n = 1). Of the remaining 88 patients, 78 (89%) had a confirmed diagnosis of NLPHL by pathology review, and in 10 cases (11%) the slides or paraffin blocks were unavailable for review. All 10 of the nonreviewed cases were from the radiation alone era (1966-1993).
All patients underwent clinical staging, which included lymphangiography, computed tomography, or both, and 5 patients underwent staging laparotomy.
Limited-stage patients were typically treated according to the following era-specific BCCA treatment guidelines. In the radiation alone era (1966-1993; n = 32), patients were treated with extended field radiotherapy. Beginning in 1993, the ABVD era (n = 56), patients were treated the same as those with CHL: ABVD-like chemotherapy for 2 cycles followed by either extended field radiotherapy (1993-1995), involved field RT (1995-2001) or involved nodal RT (2001-2005) as previously defined.8 Since 2005, patients were treated with 2 cycles of ABVD after which 18F-fluoro-2-deoxyglucose positron emission tomography (PET) was performed. If the PET scan was negative, treatment was completed with 2 further cycles of ABVD for a total of 4, and if it was positive, treatment was changed to involved nodal RT.
Statistical analysis
Overall survival (OS) was calculated from the date of diagnosis of NLPHL to the date of last follow-up or death from any cause. The progression-free survival (PFS) was measured from the date of diagnosis to the date of last follow-up, progression, or relapse of any type of lymphoma or death because of any cause. The time to progression (TTP) was measured from the date of diagnosis to the date of last follow-up, progression or relapse of lymphoma (including aggressive lymphoma), or death because of acute toxicity of primary therapy. The Kaplan-Meier method was used for calculation of all survival endpoints. Survival comparisons were made using the log-rank test. Baseline characteristics were compared between patient groups using the χ2 test. All statistical analyses were performed using SPSS Version 11.5.
Results
Clinical characteristics and treatment of limited-stage NLPHL
The presenting clinical features of the 88 patients with limited-stage NLPHL are shown in Table 1. Of these, 56 (64%) were treated in the ABVD era and 32 (36%) in the radiotherapy alone era. As anticipated, patients were typically male (75%), with a median age of 36.5 years and most had stage I disease (61%). There were no differences in the presenting clinical features between patients in the 2 treatment eras (Table 1).
Most patients were treated using the aforementioned guidelines with a few exceptions. One patient with stage 1A disease was treated with MOPP (mechlorethamine, vincristine, procarbazine, and prednisone) and extended field radiotherapy in the radiotherapy era at the discretion of the treating physician. Two patients in the radiotherapy era initially underwent surgical resection alone because the diagnosis of NLPHL was made retrospectively through pathologic review at the time of relapse. In the ABVD treatment era, 6 patients were treated with radiotherapy alone (initial therapy out of province, n = 1; cardiac disease, n = 1; chemotherapy refusal, n = 1; high neck node presentation, n = 3). All other patients in the ABVD era were treated with standard ABVD, except for 4 patients who received a hybrid regimen known to have similar efficacy (MOPP/ABV).9 Of note, one patient received a single cycle of R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) outside of British Columbia for a presumed diagnosis of T cell-rich B-cell lymphoma. However, on pathologic rereview at the BCCA, the diagnosis was corrected to NLPHL and treatment was continued with ABVD.
Outcome of patients with limited-stage NLPHL
With a median follow-up of 6.4 years for living patients (range, 1.2-40.5 years), the 10-year TTP, PFS, and OS for all 88 patients were 87%, 79%, and 90%, respectively. Excluding the 2 patients who underwent surgical resection alone, there was no difference in outcome between patients with stage I versus 2 disease (10-year TTP, 88% vs 88%, P = .991; 10-year PFS, 74% vs 88%, P = .781). In univariate analysis, there was no impact on PFS by male sex (P = .27), age more than 40 years (P = .53), and bulk more than 5 cm (P = .98).
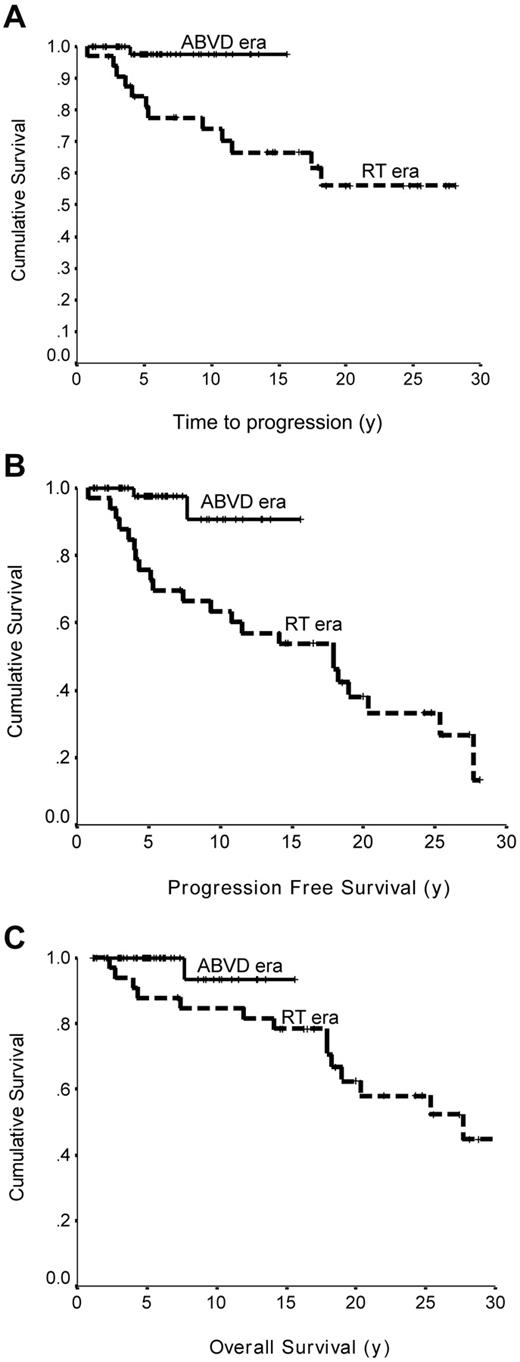
We evaluated the impact of our BCCA decision to treat NLPHL the same as CHL on the natural history of NLPHL in 2 ways. First, using our own institutional results, we compared the outcome of patients treated in the ABVD chemotherapy era (n = 56) with that for those treated in the radiotherapy alone era (n = 32), regardless of actual treatment. We found a striking improvement in the 10-year TTP (98% vs 76%, P = .0074), PFS (91% vs 65%, P = .0024), and OS (93% vs 84%, P = .074; Figure 1) for patients treated in the ABVD era. Of note, similar results were obtained if the 10 cases in which diagnostic material was not available for review were removed (results not shown). The only lymphoma relapse observed in the ABVD era was in a patient with stage 1A disease who received radiotherapy alone. In contrast, with a median TTP of 5.3 years, there have been 12 lymphoma relapses in the radiotherapy alone treatment era, including 5 cases of transformation to aggressive lymphoma. Similarly, in an “as treated” analysis, patients who had chemotherapy incorporated into their primary therapy (n = 51, median follow-up of living patients, 5.7 years; ABVD, n = 46; MOPP/ABV, n = 4; MOPP, n = 1) had a superior 10-year TTP (100% vs 77.5%, P = .0029) with no lymphoma relapses observed in patients who received chemotherapy compared with multiple relapses (n = 10) in patients who received radiotherapy alone (n = 35, median follow-up of living patients, 18.6 years). Of note, the majority of the relapses in the radiotherapy group occurred within the first 5 years of diagnosis, and > 65% of patients in the ABVD-treated patients have already been followed for > 5 years, without detecting any relapses. Further, the 10-year PFS (93% vs 66.5%, P = .0012) and OS (93% vs 85%, P = .1006) were also more favorable in patients who actually received chemotherapy.
Outcome of limited stage NLPHL. (A) Era-to-era comparison of TTP of limited-stage NLPHL treated in the RT alone era (n = 32) or ABVD chemotherapy era (n = 56; P = .0074). (B) Era-to-era comparison of PFS of limited-stage NLPHL treated in the RT alone era (n = 32) or ABVD chemotherapy era (n = 56; P = .0024). (C) Era-to-era comparison of OS limited-stage NLPHL patients treated in the RT alone era (n = 32) or ABVD chemotherapy era (n = 56; P = .074).
Outcome of limited stage NLPHL. (A) Era-to-era comparison of TTP of limited-stage NLPHL treated in the RT alone era (n = 32) or ABVD chemotherapy era (n = 56; P = .0074). (B) Era-to-era comparison of PFS of limited-stage NLPHL treated in the RT alone era (n = 32) or ABVD chemotherapy era (n = 56; P = .0024). (C) Era-to-era comparison of OS limited-stage NLPHL patients treated in the RT alone era (n = 32) or ABVD chemotherapy era (n = 56; P = .074).
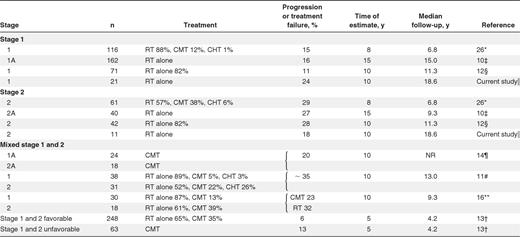
Our second approach to discerning the impact of the decision to treat NLPHL the same as CHL was to compare our results in patients who received radiotherapy alone with those obtained in recently reported large series, with a focus on those that used radiation alone in limited-stage patients and had long follow-up. We chose to compare the outcomes at 10 years, if possible, because of the long natural history of NLPHL and because our data, with a median follow-up for living patients of 6.4 years, are at least as mature as the other series. As shown in Table 2, our institutional results for radiation alone closely match those seen in other series, with an overall recurrence risk at 10 years of ∼ 15%-30%. For example, Wirth et al reported the 15-year progression rates for 202 patients with NLPHL who were treated with radiotherapy alone.10 Eighty percent of the patients in this study had stage I disease and experienced a 16% 15-year progression rate. The 20% of patients with stage II disease had a 15-year progression rate of 27%. In stark contrast, we have not had any lymphoma relapses since introducing planned chemotherapy, regardless of stage.
Fifteen patients who received ABVD chemotherapy and one who received a hybrid regimen were treated based on a PET-adapted protocol. After 2 cycles of ABVD, 12 of 16 (75%) cases were PET negative and 11 completed their treatment with 2 further cycles of chemotherapy for a total of 4 cycles. One patient received radiotherapy despite a negative PET at the treating physician's discretion. The remaining 4 (25%) cases were PET positive and received involved nodal RT. None of these 16 patients has relapsed (median follow-up 41 months). Of note, 3 additional patients received ABVD alone (4 cycles, n = 2; 6 cycles, n = 1) before the PET-adapted protocol (intra-abdominal adenopathy, n = 2; contraindication to RT, n = 1) and have not relapsed.
Of interest, the 20-year actuarial risk of transformation to aggressive lymphoma was 20% with transformations noted at 2, 11, 11, 17, and 18 years, respectively, and occurred in patients treated with radiotherapy alone (n = 4) or surgical resection (n = 1). Interestingly, all of the relapses after 10 years were the result of aggressive lymphoma, and this represented their first manifestation of relapse.
Sixteen patients have died, none because of NLPHL; however, 3 deaths were the result of transformation to DLBCL. The remaining deaths were the result of second malignancies (n = 5) (lung cancer, n = 3; pancreatic cancer, n = 1; prostate cancer, n = 1), cardiac disease (n = 4), or other (n = 4). Of note, all 3 of the patients who developed lung cancer had received radiation, including the sole death in the ABVD treatment era.
Treatment of relapsed NLPHL
In total, 7 patients developed recurrent NLPHL, 6 of whom had been treated with radiotherapy alone and one had undergone surgical resection. The median time to relapse was 4 years (2.1-9.3 years). Patients were treated either with chemotherapy alone (MOPP/ABVD, n = 1; MOPP, n = 1), combined modality therapy (ABVD + RT, n = 1; MOPP + RT, n = 1), or radiotherapy alone (n = 3). Four patients have developed a second relapse and subsequently received a prolonged course of chemotherapy (MOPP [or CVPP (cyclophosphamide, vincristine, procarbazine, and prednisone)]/ABV, n = 2; MOPP, n = 1; CVP-R [cyclophosphamide, vincristine, prednisone and rituximab], n = 1) and remain in remission.
Discussion
The optimal therapy of NLPHL has been the subject of great debate. Historically, limited-stage HL, including NLPHL, has been most commonly treated with radiotherapy. In recent years, cumulative evidence from multiple clinical trials supports treating limited-stage CHL with an approach that incorporates chemotherapy, typically ABVD. As a result, combined modality therapy (CMT) has replaced radiotherapy alone as the standard of care in the treatment of limited-stage CHL, but this approach has not been universally adopted in limited-stage NLPHL. We found that, since using ABVD chemotherapy for the primary treatment of patients with limited-stage NLPHL, similar to our recommendations for limited-stage CHL, we have observed a more favorable outcome compared with our prior experience with radiation alone. Further, our results in the earlier treatment era are in keeping with other reports in the literature that have evaluated the outcome of limited-stage NLPHL using radiotherapy alone where a relapse rate of 15%-30% was commonly seen.10-12
A large part of the favorable outcome observed in NLPHL probably reflects primarily localized, low-risk disease. Indeed, when the German Hodgkin Lymphoma Study Group compared the outcome of CHL and NLPHL within the subgroups early favorable, early unfavorable, and advanced stage, there were no differences observed.13 Similarly, limited-stage (1A or 2A) patients with CHL without mediastinal involvement have a similar prognosis to patients with limited-stage NLPHL.14 However, there has been a widespread reluctance to incorporate chemotherapy into the treatment approach of patients with NLPHL, presumably because of fear of “overtreatment.” This is highlighted in the recent National Comprehensive Cancer Network guidelines, which endorse radiotherapy alone for early-stage NLPHL (stage 1A and 2A), reserving chemotherapy for those who have B symptoms or who have advanced-stage disease.3 Similarly, the European Society of Medical Oncology has also dichotomized the treatment of CHL and NLPHL where involved field RT (30 Gy) alone is the recommended therapy for NLPHL patients with stage IA disease, without risk factors.15 Interestingly, our results of NLPHL patients with stage II disease are better than those reported in other series (Table 2), some of which have reported an inferior survival with radiotherapy alone,10,12 suggesting that chemotherapy may overcome any negative influence of stage.
A minority of studies have evaluated the outcome of CMT or compared the outcome of CMT or chemotherapy with radiotherapy alone. The M. D. Anderson Cancer Center compared the survival of 48 patients with limited-stage NLPHL by treatment received (CMT alone n = 11 vs radiotherapy alone n = 37) and found no difference in outcome (10-year relapse-free survival 77% vs 68%, P = .89); however, patient numbers were small and the chemotherapy used may be inferior to ABVD (MOPP or NOVP (mitoxantrone, vincristine, vinblastine, and prednisone).16 Feugier et al reported a 10-year FFP (failure from progression) of 80% using combined modality therapy in 42 patients with stage IA (n = 24) or 2A (n = 18) disease, but treatment was variable in some cases using only 1 cycle of ABVD or alternate chemotherapy EBVM, which also may not have similar efficacy to ABVD14 (Table 2). A recently published population-based study with pathologic review evaluated 113 patients, with “early”-stage NLPHL, including 93 patients treated with radiotherapy alone and 20 who had received either CMT (n = 13) or chemotherapy alone (n = 7), and found that the small group of patients treated with chemotherapy alone had an inferior outcome.12 However, the relative contribution of systemic treatment in this study is difficult to assess because few patients received chemotherapy, a wide variety of chemotherapy regimens were used, and the indication for selecting patients for chemotherapy was unclear. In our study, patients were managed after prospectively chosen, uniformly applied provincial practice guidelines minimizing selection bias and allowing assessment of the contribution of brief ABVD chemotherapy.
Given the overall excellent prognosis in NLPHL, the goals of therapy should be to maintain high cure rates but also to avoid future secondary complications, similar to the treatment paradigm for limited-stage CHL. Use of brief ABVD chemotherapy minimizes both the reliance on radiotherapy and the cumulative effect of anthracyclines and bleomycin. Radiotherapy doses and fields have been reduced in recent years in an effort to reduce potentially fatal, secondary complications; however, the risk using modern radiotherapy remains unknown. A recent randomized controlled study demonstrated that after brief chemotherapy the dose of radiotherapy can be reduced from 30 Gy to 20 Gy without compromising efficacy, which may decrease long-term complications.17 However, it is unknown whether this dose reduction affects relapse risk if radiation is given as a single modality treatment. With growing concerns of the long-term effects of radiotherapy and an unknown “safe dose,” more recently attention has turned to using ABVD alone in the treatment of limited-stage HL.18 In the present study, using the guidance of a negative PET scan after 2 cycles of chemotherapy, 11 patients received ABVD alone (total = 4 cycles) with no observed relapses; however, further follow-up is still needed. Regardless, our results suggest that incorporation of brief systemic treatment into the primary management of NLPHL substantially improves outcome compared with radiation alone.
Watchful waiting has also been endorsed by some groups, particularly in the treatment of pediatric patients with NLPHL; however, this approach may be associated with an unacceptable rate of relapse.19 Single-agent rituximab has also been explored; but despite a high response rate, it is likewise associated with a high rate of relapse, possibly including transformation to large cell lymphoma.20-22 From these analyses, it appears unlikely that rituximab alone is curative.
Randomized prospective trials remain the “gold standard” to define the best treatment in oncology and would be clearly desirable for identification of an optimal strategy for limited-stage NLPHL. In their absence, it is appropriate to ask whether this uncommon subtype should be treated differently from limited-stage CHL. Using the same strategy used for CHL, namely, brief ABVD chemotherapy, we observed a substantial improvement in outcome for this rare HL subtype such that relapses have been markedly reduced and survival may have been improved. The most desirable approach would be to examine this question in randomized clinical trials. Until such investigations have been conducted, it seems reasonable to consider treating limited-stage NLPHL with the same approach as is recommended for limited-stage CHL.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Jeremey Hamm from the BCCA Surveillance and Outcomes Unit for statistical assistance.
Authorship
Contribution: K.J.S. designed and performed the research and wrote the manuscript; B.S. performed the pathologic review; M.A.-M. collected the clinical data; L.H.S. analyzed the data; R.D.G. performed the pathologic review; and J.M.C. designed the research and analyzed the data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kerry J. Savage, British Columbia Cancer Agency, 600 West 10th Avenue, Vancouver, BC V5Z 4E6; e-mail: ksavage@bccancer.bc.ca.