Abstract
We report the findings from the first 10 patients with chemotherapy-refractory chronic lymphocytic leukemia (CLL) or relapsed B-cell acute lymphoblastic leukemia (ALL) we have enrolled for treatment with autologous T cells modified to express 19-28z, a second-generation chimeric antigen (Ag) receptor specific to the B-cell lineage Ag CD19. Eight of the 9 treated patients tolerated 19-28z+ T-cell infusions well. Three of 4 evaluable patients with bulky CLL who received prior conditioning with cyclophosphamide exhibited either a significant reduction or a mixed response in lymphadenopathy without concomitant development of B-cell aplasia. In contrast, one patient with relapsed ALL who was treated in remission with a similar T-cell dose developed a predicted B-cell aplasia. The short-term persistence of infused T cells was enhanced by prior cyclophosphamide administration and inversely proportional to the peripheral blood tumor burden. Further analyses showed rapid trafficking of modified T cells to tumor and retained ex vivo cytotoxic potential of CD19-targeted T cells retrieved 8 days after infusion. We conclude that this adoptive T-cell approach is promising and more likely to show clinical benefit in the setting of prior conditioning chemotherapy and low tumor burden or minimal residual disease. These studies are registered at www.clinicaltrials.org as #NCT00466531 (CLL protocol) and #NCT01044069 (B-ALL protocol).
Introduction
Despite currently available therapies, most patients with B-cell leukemias, including chronic lymphocytic leukemia (CLL) and B-cell acute lymphoblastic leukemia (B-ALL), are incurable.1,2 For this reason, novel therapeutic strategies are needed. The adoptive transfer of genetically engineered immune effector cells aims to rapidly establish T cell–mediated tumor immunity.3,4 In this approach, the patient's own T cells are targeted to tumor cells through a transgene-encoded Ag receptor consisting of either TCR chains or a chimeric Ag receptor (CAR). CARs are composed of an extracellular Ag recognition domain, most commonly a single chain fragment variable derived from a mAb, fused to a transmembrane domain, and a cytoplasmic signaling domain, most commonly including that of the CD3ζ chain.3-10 When expressed in T cells, CARs efficiently redirect T-cell specificity and cytotoxicity to cells expressing the targeted Ag in HLA-independent manner.11-18
We have previously generated a series of CARs specific for the CD19 Ag,11,12 a member of the Ig superfamily and component of a B-cell surface signal transduction complex.19 Expression of CD19 is restricted to B-lineage cells and possibly follicular dendritic cells and is found in most B-cell malignancies, including CLL and B-ALL.19-23 Significantly, CD19 is not expressed in hematopoietic stem cells. The immunologic targeting of CD19 therefore carries a minimal risk of autoimmunity or BM toxicity other than the potential induction of B-cell aplasias.
In preclinical studies, human T cells expressing CD19-specific CARs efficiently lysed a wide panel of human CD19+ tumor cell lines as well as freshly isolated patient B-cell tumors.11 Significantly, intravenously administered CD19-targeted human peripheral blood T cells eradicated systemic CD19+ tumors established in SCID-Beige mice.11,12 Our in vivo studies further showed enhanced antitumor efficacy by providing costimulatory signals to adoptively transferred T cells. Because most B-cell leukemias fail to express ligands for activating costimulatory receptors,24,25 we overcame this limitation by replacing the inert CD8 transmembrane domain with the transmembrane and cytoplasmic signaling domains of the T-cell costimulatory CD28 receptor,26 resulting in the 19-28z CAR, which enhances antitumor efficacy in SCID-Beige mice bearing CD19+ leukemias.12 On the basis of these preclinical data we chose to translate this approach to the clinical setting with the use of the 19-28z CAR.
After the validation of a robust process for large-scale human T-cell transduction and expansion,27 we enrolled 10 patients with either chemotherapy refractory CLL or relapsed B-ALL on 2 phase 1 dose escalation clinical trials. The primary objective of these trials is to assess the safety of infusing 19-28z+ T cells with or without prior cyclophosphamide-conditioning chemotherapy. Secondary objectives include assessment of clinical responses to therapy and the effect of cyclophosphamide conditioning on disease response, T-cell persistence, and T-cell function. Herein, we report our findings on the first 10 patients, 9 of which were infused with the manufactured T cells. We were able to generate sufficient CAR-transduced T cells from leukapheresis products derived from all enrolled patients. Either a marked reduction in tumor burden stable disease or B-cell aplasia was observed in 4 of the 5 evaluable patients given cyclophosphamide before T-cell infusion. We demonstrate rapid trafficking of 19-28z+ T cells to sites of tumor involvement and report on the short-term persistence and function of the adoptively transferred T cells. Collectively, these data show the promise of CD19-targeted T cells for the treatment of B-cell malignancies and provide insights into how to optimally apply this strategy in the clinical setting.
Methods
Clinical protocols
CLL protocol (NCT00466531).
Patients with relapsed purine analog-refractory CLL are eligible for enrollment. This is a phase 1 dose escalation trial. Patients initially undergo leukapheresis for T-cell collection. In the first step, one cohort of 3-6 patients is treated with the lowest initial dose of T cells, level 1 (1.2-3.0 × 107 19-28z+ T cells/kg), without prior cyclophosphamide administration. In the second step with 2 cohorts, patients are treated with dose-escalating cyclophosphamide chemotherapy (1.5 and 3.0 g/m2) followed 2 days later by infusion of modified T cells at dose level 1. After the death on study of the first patient treated in the second cohort,28 the subsequent 3 patients were treated at the −1 T-cell dose level (0.4-1.0 × 107 19-28z+ T cells/kg) with T-cell infusions split over 2 days to enhance safety. Enrollment on this second cohort has been completed.
B-ALL protocol (NCT01044069).
Adult patients with CD19+ B-ALL are eligible for enrollment. Patients can be either enrolled in first complete remission (CR1) after ≥ 1 round of consolidation therapy or on presentation with relapsed or refractory disease, defined as no CR after ≥ 2 induction regimens. Patients enrolled in CR1 underwent leukapheresis but are treated with T cells only after relapse. Patients enrolled with relapsed or refractory B-ALL leukapheresed, treated with a salvage reinduction regimen, and regardless of remission status, receive cyclophosphamide (3.0 g/m2) followed 2 days later by split dose infusion of autologous 19-28z+ T cells. This is a standard phase 1 dose-escalation trial with 3 planned T-cell doses, 3 × 106, 1 × 107, and 3 × 107 19-28z+ T cells/kg. Patients enrolled on this protocol are not precluded, if eligible, to undergo additional therapy with allogeneic stem cell transplantation after the modified T-cell infusion.
Both trials were approved by the Memorial Sloan-Kettering Cancer Center Institutional Review Board, the Recombinant DNA Advisory Committee of the National Institutes of Health, and are supported by BB-IND 13266 approved by the US Food and Drug Administration. In both trials, informed consent is obtained from eligible patients in accordance with the Declaration of Helsinki. Patients enrolled on these trials do not receive IL-2. Adverse events during and after therapy were assessed according to the National Institutes of Health Common Terminology Criteria for Adverse Events Version 3.0 (http://ctep.cancer.gov/). The Memorial Sloan-Kettering Cancer Center Data and Safety Monitoring Board review all safety data every 6 months.
Generation and expansion of genetically modified T cells
19-28z–transduced T cells were generated as described.27 Briefly, T cells were isolated from leukapheresis product and activated with Dynabeads ClinExVivo CD3/CD28. Release criteria include 19-28z CAR expression by flow cytometry; average vector copy number by quantitative PCR; vector identity by Southern blot; absence of replication competent retrovirus by PCR and marker rescue cell culture assay; residual Dynabeads ClinExVivo CD3/CD28; negative bacterial, fungal, and Mycoplasma cultures; endotoxin level no > 5 EU/kg; Gram stain–negative on day of infusion; > 80% cell viability; and CD3+, CD8+, CD5+ (patients with CLL), CD10+ (patients with ALL), and CD19+ phenotype by flow cytometry and CD19-specific cytotoxicity. The assays were performed as described in Hollyman et al27 and Taylor et al.29
Restimulation of T cells from postinfusion PBMCs with Dynabeads ClinExVivo CD3/CD28
PBMCs collected in cell preparation tubes (BD Biosciences) were purified from whole blood according to the manufacturer's recommendations. Patient CD3+ T cells were subsequently selected and activated with Dynabeads ClinExVivo CD3/CD28 (Invitrogen) at ratio of 3 beads to 1 CD3+ T cell. CD3+ T cells were cultured in X-VIVO 15 medium (Lonza) supplemented with 100 U/mL IL-2. Cell samples were taken at different time points to determine the average vector copy number by quantitative RT-PCR and the expression of 19-28z CAR by flow cytometry.
Restimulation of T cells from postinfusion PBMCs with CD19+CD80+ artificial APCs
Human CD19 and CD80 expressing fibroblasts (3T3-CD19-CD80)11 were irradiated at 30 Gy. Postinfusion PBMCs were thawed and plated on the 3T3-CD19-CD80 cells in X-VIVO 15 media (Lonza) supplemented with 5% human AB serum (GEMINI) and 100 U/mL IL-2. Lysis of 3T3 cells was monitored, and PBMCs were fed on day 3. Cells were counted 7 days after restimulation and stained with anti–human CD3-FITC, CD8-eFluor 450, CD4-PE-Cy7, biotinylated goat anti–mouse IgG F(ab)2 followed by APC-labeled streptavidin, and 7-amino-actinomycin D (7-AAD) with the use of standard staining procedures. Data acquisition was performed on a LSRII flow cytometer, and data analysis was performed with FlowJo software (TreeStar Inc).
Characterization of cytokine profiles secreted by EOP 19-28z–transduced T cells
Samples of patient T cells transduced with 19-28z CAR were taken at the end of production (EOP). T cells (1.0 × 106) were plated on 3T3 artificial APCs (AAPCs) expressing CD19 or untransduced 3T3 fibroblasts as controls, in complete X-Vivo culture media in 5% human AB serum and in the absence of ILs. After 48 hours supernatants were collected and assayed for human cytokines on a Luminex IS100 instrument as per manufacturer's instructions (Millipore Corp). The cytokine levels were normalized to transduction efficiency. In addition, background levels measured on untransduced 3T3 fibroblasts were subtracted.
Other methods
Other methods, including manufacture of clinical grade PG13-19-28z vector stocks, serum cytokine analyses, cytotoxic T-lymphocyte assays, quantitative real-time PCR, Abs and reagents, flow cytometry, statistics, and IHC can be found in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Results
Patient characteristics
Eight patients with relapsed purine analog-refractory CLL have been enrolled and treated (Table 1). The median age of patients was 68 years with 7 of 8 being men. All patients were previously treated with 1-4 different chemotherapy regimens, with 7 of 8 patients having received ≥ 2 different regimens before enrollment. Chromosomal and genetic analyses of CLL tumor cells showed adverse prognostic features, including del17p with concomitant p53 gene deletion, del11q, and/or an unmutated Ig variable heavy chain domain. All patients exhibited advanced disease and tumor burden as evidenced by marked bulky lymphadenopathy.
Two patients with relapsed B-ALL have been enrolled and 1 patient has been treated (Table 1). The latter achieved an initial remission after induction therapy but subsequently relapsed after the third cycle of consolidation therapy at which point the patient was enrolled and T cells were obtained by leukapheresis. The patient achieved a second remission after reinduction chemotherapy and was treated on protocol. The second patient had a relapse of disease after initial induction therapy but became ineligible for treatment with modified T cells because of intervening complications.
Generation of patient-derived 19-28z–transduced T cells
Despite all patients having been heavily pretreated, we were able to obtain adequate numbers of T cells from the leukapheresis products in every case (Table 2). The mean time in culture to achieve the 19-28z+ T-cell dose was 16 days (range, 11-19 days) with a mean 120-fold expansion of T cells (range, 24- to 385-fold). The transduction efficiency in CD3+ T cells, as assessed by flow cytometry, ranged from 23% to 70% in CLL patient cells and from 4% to 8.6% in ALL patient cells. The average vector copy number per cell in these cell products ranged from 0.06 to 1.5 with a mean of 0.67.
Characterization of final 19-28z–transduced T cells
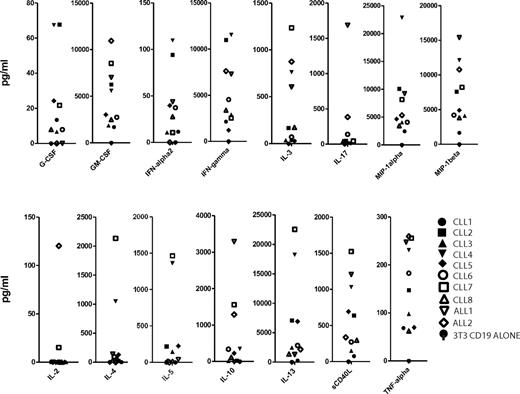
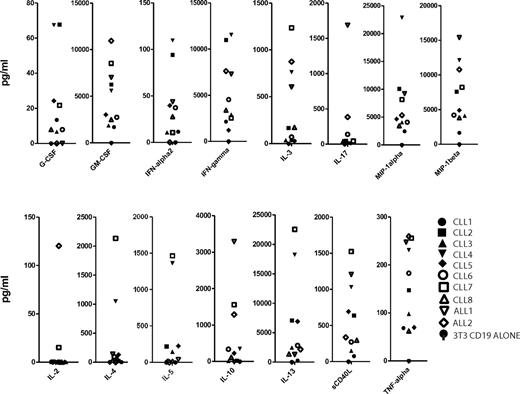
The final T-cell products exhibited a predominant CD4+ phenotype, particularly pronounced in the CLL EOP cells (mean CD4+ T-cell fraction of 88%, CD4/CD8 ratio of 10.5; Tables 3 and 4). The patients with B-ALL showed a mean CD4+ T-cell fraction of 63% (Table 3). Despite a marked prevalence of CD4+ T cells, all EOP T cells displayed robust in vitro cytotoxic activity against both autologous CLL tumor cells and the CD19+ Raji Burkitt lymphoma cell line (Table 2). Further analyses of final T-cell products showed minimal numbers of CD4+ FoxP3+ T regulatory cells and a significant retention of cell surface markers, including CD27, CD28, and CD62L. Although the percentage of these markers showed some variability (Table 3), the absolute numbers of infused T cells were overall similar in all patients (supplemental Figure 1) as well as their distribution in CAR– versus CAR+ T cells (Table 3; supplemental Figure 1). To further assess the infused cell products, all EOP cells were activated in Ag-specific fashion in vitro under the same conditions with the use of CD19+ AAPCs.11,30 The cytokine signatures induced by exposure to CD19 were overall similar, showing in particular robust secretion of GM-CSF, IFN-γ, TNF-α, MIP-1α, MIP-1β, and little or no IL-2, IL-4, and IL-10 in most patients (Figure 1).
Cytokine analysis of EOP 19-28z–transduced T cells stimulated on CD19+ AAPCs. EOP 19-28z–transduced T cells were plated on 3T3 fibroblast AAPCs expressing CD19. After 48 hours in culture, cell supernatants were assayed for cytokine levels. The cytokine levels were normalized to transduction efficiency. In addition, background cytokine levels measured on 3T3 fibroblasts that did not express CD19 were subtracted.
Cytokine analysis of EOP 19-28z–transduced T cells stimulated on CD19+ AAPCs. EOP 19-28z–transduced T cells were plated on 3T3 fibroblast AAPCs expressing CD19. After 48 hours in culture, cell supernatants were assayed for cytokine levels. The cytokine levels were normalized to transduction efficiency. In addition, background cytokine levels measured on 3T3 fibroblasts that did not express CD19 were subtracted.
Infusion of 19-28z–transduced T cells is well tolerated
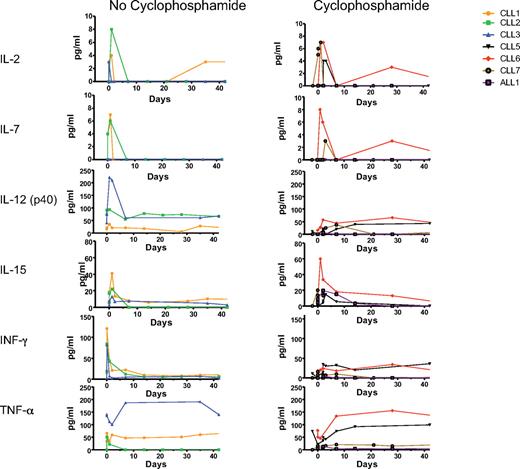
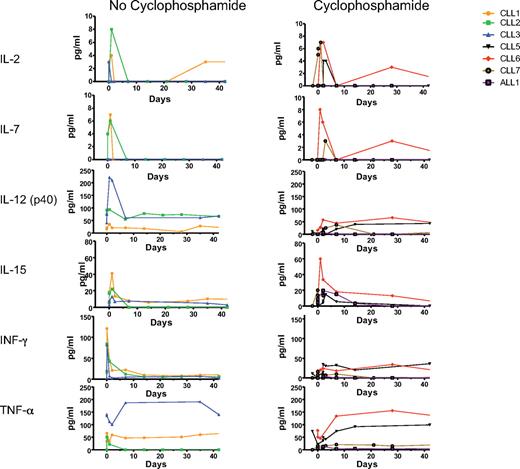
Nine patients have received intravenous infusion of 19-28z–transduced autologous T cells. Overall, patients tolerated therapy well with most patients experiencing rigors, chills, and transient fevers within 24 hours of modified T-cell infusions. In all cases, patient blood and urine cultures were obtained, and intravenous antibiotic therapy was initiated. However, with the exception of patient CLL-4, all patients readily recovered from these symptoms with negative blood and urine cultures and were released after an additional 48 hours of in-patient observation (Table 5). Patient CLL-4 experienced persistent fevers, developed a sepsis-like syndrome with hypotension and renal failure, and died within 48 hours of T-cell infusion. Previously published extensive analyses of this case point to an infectious cause as evidenced by marked serum cytokine abnormalities that preceded T-cell infusion, arguing against the 19-28z+ T cells being the primary cause of this adverse outcome.28 The subsequent cohort of 4 patients with CLL was treated at a 3-fold lower T-cell dose than CLL-4 and administered in a split-dose manner over 2 consecutive days. In addition, serum cytokine levels were assessed before both cyclophosphamide and T-cell infusions. Cytokine elevations, as noted in CLL-4, were not observed in the subsequently treated patients before or after cyclophosphamide therapy or after T-cell infusion. Significantly, serial serum analyses in these patients did not show evidence of cyclophosphamide-induced alterations in cytokine profiles compared with patients treated with T cells without cyclophosphamide conditioning (Figure 2).
Patient serum cytokine levels before and after cyclophosphamide and T-cell infusions. Serum cytokines were determined before and after 19-28z+ T-cell infusion. The first set of patients (CLL-1 to CLL-3) did not receive cyclophosphamide treatment before T-cell infusion, whereas the second set (CLL-5 to CLL-7 and ALL-1) received cyclophosphamide before T-cell infusion. The x-axis represents treatment time course with day 0 being the day of T-cell infusion and day −2 being the day of cyclophosphamide treatment.
Patient serum cytokine levels before and after cyclophosphamide and T-cell infusions. Serum cytokines were determined before and after 19-28z+ T-cell infusion. The first set of patients (CLL-1 to CLL-3) did not receive cyclophosphamide treatment before T-cell infusion, whereas the second set (CLL-5 to CLL-7 and ALL-1) received cyclophosphamide before T-cell infusion. The x-axis represents treatment time course with day 0 being the day of T-cell infusion and day −2 being the day of cyclophosphamide treatment.
Clinical responses to 19-28z+ T-cell infusion
Eight patients with CLL have been treated. The first cohort of 3 patients was treated without cyclophosphamide conditioning at the lowest planned T-cell dose of 1.2-3.0 × 107 19-28z+ T cells/kg with no objective disease responses (Table 6). Collectively, all 3 patients on this cohort exhibited further progressive disease and soon required additional salvage chemotherapy. All patients on this cohort have died of their disease. Patient CLL-4 died soon after T-cell infusion and therefore was not evaluable for clinical response.28
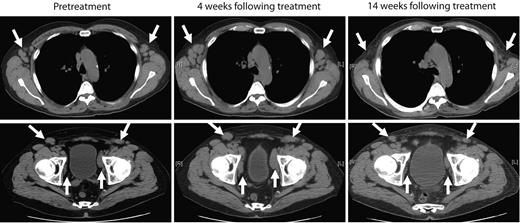
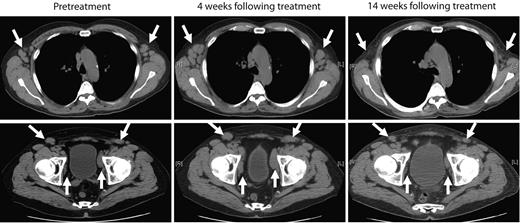
The next 4 patients (CLL-5 to -8) were treated with cyclophosphamide-conditioning chemotherapy, followed by infusion of 0.4-1.0 × 107 19-28z+ T cells/kg. Patient CLL-5 exhibited stable to progressive disease at 1 month after treatment as assessed by computed tomography scan but subsequently developed marked objective reduction of peripheral lymphadenopathy in the absence of any further therapeutic interventions, as assessed by physical examination and computed tomography scans over the subsequent 2 months (Figure 3). This marked reduction of lymphadenopathy remained stable over the subsequent 6 months, and thereafter the patient developed disease progression in the abdomen with associated ascites and worsening cytopenias, which required further chemotherapy. The patient died 15 months after T-cell therapy with progressive chemotherapy refractory disease. Patient CLL-6 exhibited progressive disease > 1 month after therapy requiring salvage chemotherapy and died of infectious complications 2 months later. Patients CLL-7 and CLL-8, both treated in the setting of rapidly progressive disease with increasing lymphadenopathy and cytopenias, exhibited stable disease with respect to lymphadenopathy over a 4- and > 2-month period of expectant management, respectively, after cyclophosphamide and T-cell infusions (data not shown).
Marked reduction of peripheral lymphadenopathy was observed after treatment with autologous 19-28z+ T cells. Representative computed tomography scan images of patient CLL-5 before treatment and from 4 weeks and 14 weeks after treatment show mild increase in axillary (top) and pelvic lymphadenopathy (bottom) at 4 weeks but regression of lymphadenopathy at 14 weeks after therapy with modified T cells.
Marked reduction of peripheral lymphadenopathy was observed after treatment with autologous 19-28z+ T cells. Representative computed tomography scan images of patient CLL-5 before treatment and from 4 weeks and 14 weeks after treatment show mild increase in axillary (top) and pelvic lymphadenopathy (bottom) at 4 weeks but regression of lymphadenopathy at 14 weeks after therapy with modified T cells.
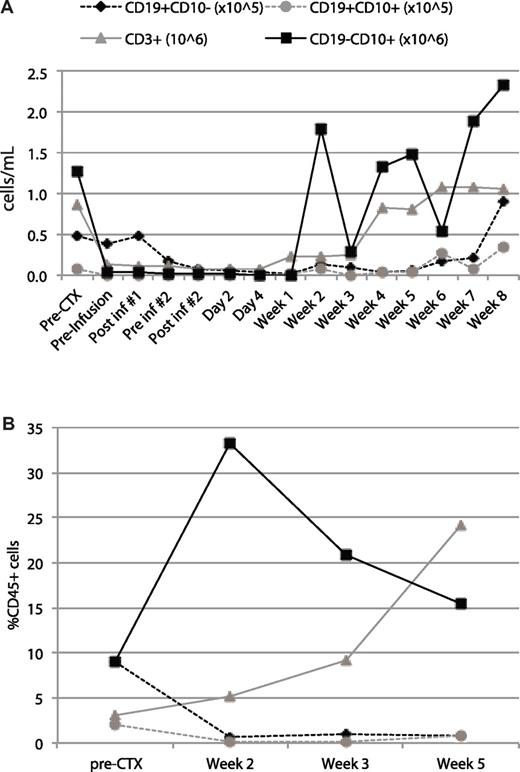
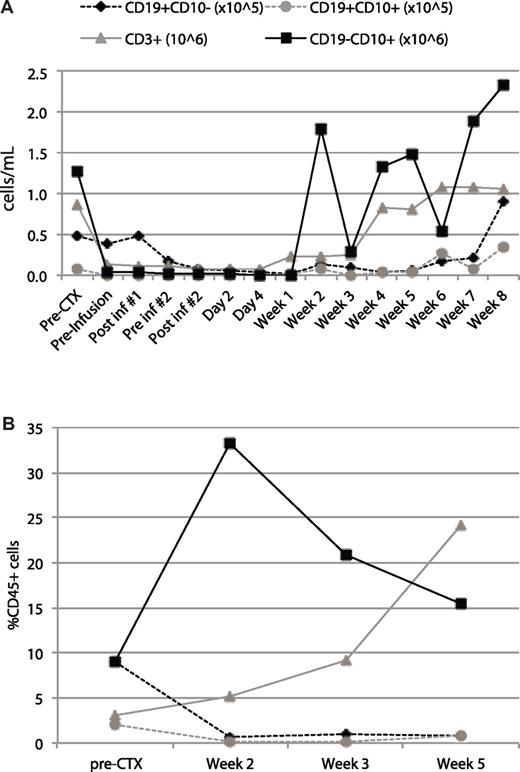
Patient ALL-1 was a 67-year-old male who relapsed during consolidation therapy, achieved a second remission with salvage chemotherapy, and received a single consolidation with high-dose cyclophosphamide followed 2 days later with T-cell infusion. This patient was followed over the next 8 weeks before undergoing an allogeneic BM transplantation from a related sibling. Despite T-cell and neutrophil recovery, the patient exhibited a persistent B-cell aplasia in the BM and peripheral blood with only very low level CD19+ B-cell recovery just before transplantation (Figure 4).
B-cell aplasia in the peripheral blood and BM of patient ALL-1 after infusion with autologous 19-28z+ T cells. Samples before and after infusion of blood (A) and BM (B) were obtained at the time points indicated on the x-axis and used for cell counts and immunophenotyping. Cell concentrations were measured with an AcT diff cell counter (Coulter). Immunophenotyping was performed by gating on CD45 and defining cell populations with the following cell surface markers: CD3+ (T cells), CD19+CD10− (mature B cells), CD19+CD10+ (progenitor B cells or tumor cells), CD19−CD10+ (granulocytes). (A) The plotted value for each cell population was derived as the product of the cell concentration by the frequency. (B) The plotted value for each cell population represents the percentage within the CD45 gate.
B-cell aplasia in the peripheral blood and BM of patient ALL-1 after infusion with autologous 19-28z+ T cells. Samples before and after infusion of blood (A) and BM (B) were obtained at the time points indicated on the x-axis and used for cell counts and immunophenotyping. Cell concentrations were measured with an AcT diff cell counter (Coulter). Immunophenotyping was performed by gating on CD45 and defining cell populations with the following cell surface markers: CD3+ (T cells), CD19+CD10− (mature B cells), CD19+CD10+ (progenitor B cells or tumor cells), CD19−CD10+ (granulocytes). (A) The plotted value for each cell population was derived as the product of the cell concentration by the frequency. (B) The plotted value for each cell population represents the percentage within the CD45 gate.
19-28z+ autologous T cells rapidly traffic to sites of CD19+ tumor
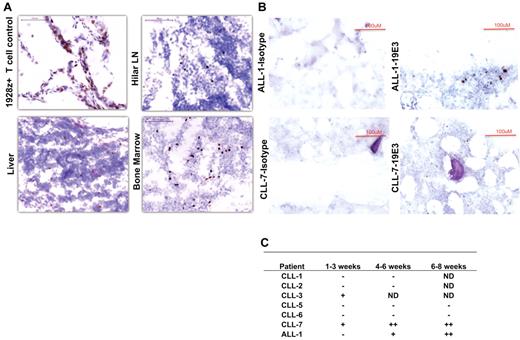
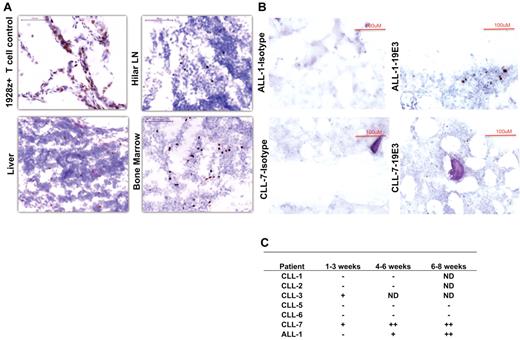
Cryopreserved autopsy tissue samples obtained from patient CLL-4 were analyzed by IHC for 19-28z+ T cells. IHC studies showed infiltration of targeted 19-28z+ T cells into CLL tumor beds in lymph nodes, liver, and BM (Figure 5A), reflecting rapid CAR-mediated trafficking of 19-28z+ T cells to sites of CD19+ tumor.
Immunohistochemistry staining of tissues with 19E3 anti-CAR Ab. (A) Trafficking of targeted T cells to tumor sites in CLL-4 44 hours after T-cell infusion. Images were acquired at room temperature using Nikon 90i Eclipse microscope system (Nikon) with a Nikon Plan Apo VC 20×/0.75 objective lens (magnification 200×), Nikon DigiSight Digital Camera Head and Nikon NSI-Elements Version 3.10 software. (B) Trafficking and homing of targeted T cells to BM. IHC staining on BM biopsies shows the presence of 19-28z+ T cells 5 and 6 weeks after infusion in patients ALL-1 and CLL-7, respectively (C) Trafficking and homing of targeted T cells to BM ≤ 8 weeks after infusion. ND indicates not done; (−) no positive 19-28z+ T cells found; (+) positive 19-28z+ T cells found occasionally, and (++) positive 19-28z+ T cells readily identifiable.
Immunohistochemistry staining of tissues with 19E3 anti-CAR Ab. (A) Trafficking of targeted T cells to tumor sites in CLL-4 44 hours after T-cell infusion. Images were acquired at room temperature using Nikon 90i Eclipse microscope system (Nikon) with a Nikon Plan Apo VC 20×/0.75 objective lens (magnification 200×), Nikon DigiSight Digital Camera Head and Nikon NSI-Elements Version 3.10 software. (B) Trafficking and homing of targeted T cells to BM. IHC staining on BM biopsies shows the presence of 19-28z+ T cells 5 and 6 weeks after infusion in patients ALL-1 and CLL-7, respectively (C) Trafficking and homing of targeted T cells to BM ≤ 8 weeks after infusion. ND indicates not done; (−) no positive 19-28z+ T cells found; (+) positive 19-28z+ T cells found occasionally, and (++) positive 19-28z+ T cells readily identifiable.
In vivo persistence of 19-28z+ autologous T cells
19-28z+ T-cell persistence was measured by IHC of BM samples and by RT-PCR and flow cytometry of serial peripheral blood and BM aspirate samples. With the use of an anti-idiotypic monoclonal Ab specific for the 19-28z CAR, IHC analyses of BM samples from the first cohort showed either discrete (CLL-3) or no evidence (CLL-1 and CLL-2) of 19-28z+ T cells at 1 month (Figure 5C). However, in the second cohort, further treated with cyclophosphamide conditioning, CLL-7 exhibited a significant number of retained 19-28z+ T cells within the BM ≤ 6 weeks after T-cell infusion (Figure 5B-C). Between 1 and 0.02 vector copies per 100 cells could also be detected by RT-PCR 2 and 3 weeks after infusion, respectively, in freshly obtained BM aspirates derived from the same patient. Similarly, patient ALL-1 exhibited 19-28z+ T cells in the BM at 5 weeks after therapy consistent with the B-cell aplasia observed after T-cell infusion (Figure 5B-C).
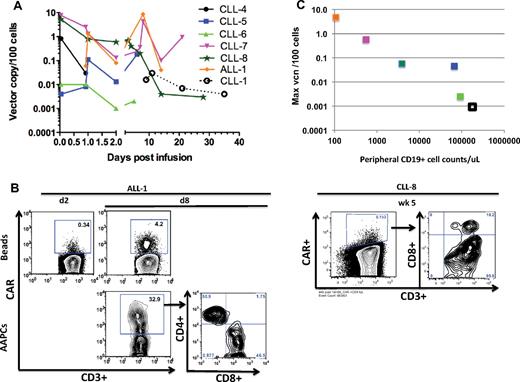
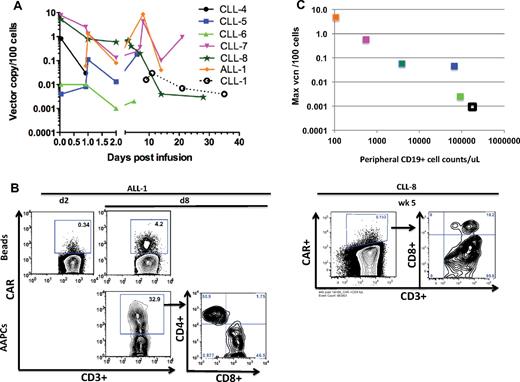
To overcome the high tumor burden background in most of the patients with CLL in postinfusion peripheral blood samples, T cells were selected and expanded with Dynabeads before analysis. RT-PCR (Figure 6A) and flow cytometry analyses (Figure 6B) of expanded peripheral blood samples showed an enhanced persistence of modified T cells in patients treated with prior cyclophosphamide (CLL-4 to CLL-8, ALL-1), despite a lower dose of infused 19-28z+ T cells, compared with the first cohort of patients treated with 19-28z+ T cells alone (CLL-1 to CLL-3).
In vivo persistence of modified T cells. (A) Persistence of 19-28z+ T cells after infusion in the peripheral blood. CD3+ T cells derived from the peripheral blood samples of patients with CLL and ALL at various time points after infusion were activated with Dynabeads. Quantitative PCR analysis was performed, and the average vector copy number (vcn) was determined per 100 cells. The average vcn was below the limit of detection for CLL-2 and CLL-3 patient samples at all available data points and, therefore, are not represented. Samples for ALL-1 were not available on day 0 (at 1 hour after infusion). (B) Expression of 19-28z+ CAR after infusion after restimulation with either AAPCs or Dynabeads. CD3+ T cells derived from ALL-1 peripheral blood 2 days (d2) and 8 days (d8) after infusion were selected and activated with either Dynabeads (top) or AAPCs (bottom). CD3+ T cells derived from CLL-8 peripheral blood 5 weeks (wk 5) after infusion were selected and activated with Dynabeads. Flow cytometric analysis was performed with anti-CD3 and anti-CAR Abs 7 days after restimulation. (C) Inverse correlation between peripheral CD19+ cell counts and maximum vcn detected in the peripheral blood. The peripheral blood CD19+ cell count (obtained by CD19 FACS analysis) is plotted on the x-axis (Table 1). The maximum vector copy number per 100 cells and normalized per 108 infused 19-28z+ T cells detected in the peripheral blood after infusion (day 1-8), as shown in panel A, are reported on the y-axis (supplemental Table 2).
In vivo persistence of modified T cells. (A) Persistence of 19-28z+ T cells after infusion in the peripheral blood. CD3+ T cells derived from the peripheral blood samples of patients with CLL and ALL at various time points after infusion were activated with Dynabeads. Quantitative PCR analysis was performed, and the average vector copy number (vcn) was determined per 100 cells. The average vcn was below the limit of detection for CLL-2 and CLL-3 patient samples at all available data points and, therefore, are not represented. Samples for ALL-1 were not available on day 0 (at 1 hour after infusion). (B) Expression of 19-28z+ CAR after infusion after restimulation with either AAPCs or Dynabeads. CD3+ T cells derived from ALL-1 peripheral blood 2 days (d2) and 8 days (d8) after infusion were selected and activated with either Dynabeads (top) or AAPCs (bottom). CD3+ T cells derived from CLL-8 peripheral blood 5 weeks (wk 5) after infusion were selected and activated with Dynabeads. Flow cytometric analysis was performed with anti-CD3 and anti-CAR Abs 7 days after restimulation. (C) Inverse correlation between peripheral CD19+ cell counts and maximum vcn detected in the peripheral blood. The peripheral blood CD19+ cell count (obtained by CD19 FACS analysis) is plotted on the x-axis (Table 1). The maximum vector copy number per 100 cells and normalized per 108 infused 19-28z+ T cells detected in the peripheral blood after infusion (day 1-8), as shown in panel A, are reported on the y-axis (supplemental Table 2).
Persistent 19-28z+ T cells retrieved from patient peripheral blood samples proliferate in response to CD19 and are cytotoxic
To assess the functional status of persisting 19-28z+ peripheral blood T cells, we investigated whether these cells could proliferate on coculture with CD19-expressing AAPCs (3T3-CD19-CD80).11,30 Peripheral blood T cells collected 8 days after T-cell infusion from patient ALL-1 indeed expanded on 3T3-CD19-CD80 fibroblasts, with 19-28z+ T cells reaching 32.9% of the CD3+ T-cell fraction after 7 days in culture (Figure 6B). In contrast, the same blood sample expanded by nonspecific activation with the use of anti-CD3/CD28 beads showed that 4.2% of the CD3+ T-cell fraction expressed the 19-28z+ CAR. Confirming and extending these findings, quantitative measurements of vector sequences showed 42.1 vector copies/100 cells in the whole blood and 8.6 vector copies/100 cells in the polyclonally activated sample (supplemental Table 2). Significantly, on a second restimulation on 3T3-CD19-CD80 AAPCs, the 19-28z+ T cells eradicated the monolayer of AAPCs within 24 hours, an outcome that is not seen with nontransduced T cells (data not shown).11,12,30 The CD4/CD8 ratios in the recovered and expanded CAR-modified T cells obtained ≤ 5 weeks after T-cell infusion were consistent with those measured in the infused EOP T cells (Table 4). Collectively, these data establish that 19-28z+ T cells isolated from a patient 8 days after infusion expand through CAR activation and retain their CD19-targeted cytotoxic potential.
In vivo 19-28z+ T-cell persistence is inversely associated with preexisting tumor burden
To assess whether initial in vivo T-cell persistence was related to the tumor burden found on infusion, we compared the peripheral blood tumor load to the persistence of modified T cells in the peripheral blood, as assessed by RT-PCR beyond 24 hours after T-cell infusion. We observed an inverse correlation (Kendall-Tau, −1, P = .003) between preexisting peripheral blood tumor burden and 19-28z+ T-cell levels (Figure 6C), suggesting that 19-28z+ T cells are more rapidly cleared from the circulation in the presence of greater target cell numbers.
Discussion
The number of phase 1/2 clinical protocols that use CAR-targeted T cells is rapidly expanding.31,32 However, despite promising preclinical data and intriguing case reports, evidence that this approach can yield consistent clinical responses is still lacking.33-40 There is currently limited insight into why this approach generally translates poorly to the clinical setting. It is therefore essential to use secondary end points to assess the in vivo expansion, persistence, trafficking to tumor, and in vivo function of CAR-modified T cells.
Herein, we report the initial results from 10 patients we enrolled for treatment with CD19-targeted T cells for either chemotherapy-refractory CLL or relapsed B-ALL. In heavily pretreated patients, we consistently and rapidly expanded CD19-targeted autologous T cells to sufficient numbers with the use of a validated protocol.27 Although CLL patient T cells tend to expand poorly in comparison to patients with ALL (Table 2), we found that overall gene transfer efficiency in CLL patient T cells was greater than in T cells derived from patients with B-ALL. The cause of this finding is unclear and may reflect stimulation of 19-28z–transduced T cells by residual CD19+ tumor cells, which are more abundant in the CLL patient leukapheresis samples. We further report a marked prevalence of CD4+ T cells in initial leukapheresis product and even more so in the EOP T cells from patients with CLL, a skewed phenotype much less evident in EOP T cells from patients with B-ALL (Table 4). The mechanisms underlying this skewing are unclear but may relate in part to the higher CD4/CD8 ratio in the CD28+ cells bound by the beads used for T-cell enrichment and activation (Table 4). Bonyhadi et al41 previously reported a starting CD4/CD8 ratio of 1.7 ± 1.2, similar to our starting ratio of 2.0 ± 1.5, reaching 2.7 ± 2.8 after a 2-week expansion, whereas the ratio measured in our EOP cells was higher, averaging 10.5 ± 6.6. The reasons for this skewing in our CLL patient EOP cells, which is greater than that found in bead-expanded T cells from subjects with nonhematologic malignancies,42 are unclear, but they may relate to the severe disease status of the patients enrolled in the present study or to their substantial prior chemotherapies. Consequently, the T-cell therapy administered to patients with CLL practically consisted of a CD4+ T-cell therapy. The EOP T cells from patients with CLL nonetheless exhibited a robust in vitro cytotoxic activity against both autologous tumor cells and the CD19+ Raji tumor cell line (Table 2).
We found substantial albeit variable levels of CD27, CD28, and CD62L markers in all patients' EOP T cells (Table 3) consistent with findings on ex vivo–expanded CLL patient T cells reported by Bonyhadi et al.41 Phenotypic analyses of the EOP T cells showed measurable but consistently low levels of T regulatory cells. In addition, comparative analysis of all EOP T-cell products did not show major disparities in their cytokine profiles after exposure to CD19 in standardized fashion with the use of HLA-negative, CD19-positive AAPCs (Figure 1). Overall, the absolute numbers of infused T-cell subsets and their cytokine profiles were similar between patients and did not appear to correlate with clinical response or T-cell persistence.
Infusion of T cells was well tolerated at the T-cell doses of 0.2-1.1 × 107 19-28z+ T cells/kg in cyclophosphamide-conditioned recipients. As previously reported, 1 patient developed persistent fevers with a subsequent sepsis-like syndrome of hypotension and renal failure, in the context of a markedly aberrant serum cytokine profile existing in this patient before the T-cell infusion, consistent with a preexisting subacute infection.28 In light of this finding, serum cytokine analyses were conducted on all subsequent patients before treatment on either protocol. These studies failed to show evidence of serum cytokine abnormalities before or subsequent to cyclophosphamide chemotherapy or T-cell infusions. Although other investigators have reported increased levels of IL-15 or IL-7 or both after high-dose cyclophosphamide/fludarabine conditioning,43,44 suggesting that this induced cytokine production may enhance adoptive cell therapy, we found no significant differences in IL-2, IL-7, IL-12, IL-15, IFN-γ, or TNF-α levels in patients treated with or without cyclophosphamide.
The trafficking of targeted effector T cells to sites of tumor is a prerequisite for their antitumor activity.45 Prior attempts to assess T-cell trafficking to tumor have involved whole-body imaging of radiolabeled gene-modified T cells in patients with ovarian cancer or measuring the relative vector ratio in autopsy samples from a patient infused with T cells targeting ERBB2.34,37 Both studies failed to show clear evidence of preferential trafficking to the known sites of tumor. On the basis of IHC analysis of autopsy specimens obtained from patient CLL-4, we demonstrate rapid trafficking of modified T cells to multiple sites of tumor involvement, including the lymph nodes, liver, and BM, consistent with efficient trafficking of modified T cells to tumor within 44 hours after infusion. Furthermore, we were able to detect 19-28z+ T cells by IHC in postinfusion BM aspirates from patients with CLL and ALL ≤ 2 months after T-cell infusion, further supporting that the infused 19-28z+ T cells may be functional in vivo.
To more directly address this key question, we attempted to demonstrate whether 19-28z+ T cells retain their proliferative and cytotoxic potential after adoptive transfer. To this end, we first demonstrate the ability to nonspecifically expand T cells that retain expression of the 19-28z CAR with the use of anti-CD3/CD28 beads in postinfusion samples derived from all patients who received cyclophosphamide. Further, we show that CAR-modified T cells recovered in this manner from patients ≤ 5 weeks from infusion, generally retain the CD4/CD8 ratio from the EOP-infused product, suggesting equivalent ability of 19-28z–transduced CD4+ and CD8+ T cells to persist in vivo in this time window. Most significantly, we demonstrate, for the first time to the best of our knowledge, that T cells recovered after infusion retain Ag-specific CAR-mediated expansion as well as 19-28z CAR-mediated cytotoxicity on expansion with CD19-expressing AAPCs. These data suggest that T cells bearing a second-generation CAR persist and retain cytotoxic function ranging from 8 to ≥ 42 days after infusion in vivo. Furthermore, these data, based on the use of AAPCs, validate this approach as a robust assay to study the function of infused CAR-transduced T cells retrieved from patient tissues in future trials.
In contrast to patients treated with modified T cells without prior cyclophosphamide (CLL-1 to -3), wherein T cells were detected at very low frequency in the peripheral blood of only one patient (CLL-1), T cells were more readily detected over time in the blood and BM of patients CLL-5, CLL-7, CLL-8, and ALL-1, who received prior cyclophosphamide conditioning, as shown by FACS, RT-PCR, or IHC. These data are consistent with previously published preclinical and clinical trial reports showing enhanced persistence and efficacy of adoptively transferred tumor-specific T cells after prior conditioning chemotherapy.43,46
Significantly, in contrast to the lack of clinically relevant responses seen in patients treated with CAR-modified T cells without prior cyclophosphamide, 3 of 4 evaluable patients with CLL treated with prior cyclophosphamide exhibited either stable disease or a marked reduction of peripheral lymphadenopathy, whereas the patients with B-ALL, treated in second remission, exhibited B-cell aplasia. Patient CLL-5, who presented with significant bulky disease and peripheral disease burden, exhibited a pronounced, slowly progressive tumor response. This patient developed over the 3 months after T-cell infusion a dramatic and diffuse reduction of lymphadenopathy, accompanied by little hematopoietic amelioration. Although tissue analyses failed to show the presence of modified T cells in the BM of this patient, it is probable that the 19-28z+ T cells predominantly trafficked to disease in the lymph nodes. The CLL clinical trial at the time of this antitumor response did not allow for biopsy or fine needle aspirates of peripheral lymph nodes. Further, these data show that, similar to previously published data about adoptive therapy with donor T-cell infusions,47,48 assessment of disease response to modified T-cell adoptive therapy, especially in the setting of bulky preexisting disease, requires observation over a significant period of time measured in weeks to months to avoid removing patients from these clinical trials prematurely and thereby missing potential clinical benefit from this therapeutic approach. While CLL-5 had a delayed response, the B-cell aplasia in patient ALL-1 was evident within 48 hours of treatment (Figure 4). Before infusion of gene-modified T cells, the patient had low levels of T cells, B cells, and granulocytes, probably secondary to multiple chemotherapeutic regimens. After treatment with cyclophosphamide and CD19-targeted T cells, the patient's T cells and granulocytes recovered, but the B cells did not. We hypothesize that the high tumor burden in patients with CLL prevents B-cell aplasia, which is therefore expected to occur only in patients with low tumor burdens. Significantly, the CLL subjects enrolled here were relatively resistant of the cyclophosphamide conditioning. On close examination of T-cell persistence and pretreatment tumor burden, we identified an inverse association between tumor load at the time of infusion of 19-28z–transduced T cells and 19-28z+ T-cell persistence according to RT-PCR analyses of patient peripheral blood samples. Although a larger patient series is needed to confirm this finding, these data are consistent with a clinically enhanced antitumor efficacy mediated by CAR-modified T cells in the setting of lower disease burdens. This is consistent with preclinical mouse studies by James et al,49 who demonstrated that the function of T cells targeted to CD20 was enhanced when leukemia-bearing mice were depleted of B cells before T-cell infusion.
In light of these observations, the role of the preinfusion conditioning regimens needs to be carefully evaluated. Our findings suggest a potentially greater effect of conditioning regimens through tumor burden reduction than the induction of a supportive cytokine response in patients with refractory disease. Despite the well-documented benefit of conditioning chemotherapy in enhancing in vivo persistence and antitumor efficacy of adoptively transferred tumor-specific T cells,32,35,43,50 its mechanism remains to be fully elucidated. Our data further establish that CD19-targeted T cells are more rapidly cleared from the circulation in the presence of a higher peripheral blood tumor burden. The mechanism underlying this phenomenon remains to be determined, although our studies establish that CD19-targeted T cells can rapidly infiltrate tumor and be undetectable in blood at the same time.
On the basis of our findings reported here, we are planning an additional clinical trial wherein we will treat patients with CLL after initial frontline chemotherapy. Patients eligible for this clinical trial are those who, after initial therapy, have either overt persistent disease in the setting of a partial response or those who have molecular evidence of minimal residual disease (NCT01416974).
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by National Institutes of Health grants P01 CA059350 (M.S., I.R., and R.J.B.), 3R01CA138738-02S1 (R.J.B.), P30 CA08748 (I.R.), and K08 CA148821 (M.L.D.); the Alliance for Cancer Gene Therapy (M.S.); Mr William H. Goodwin and Mrs Alice Goodwin of the Commonwealth Cancer Foundation for Research and the Experimental Therapeutics Center of Memorial Sloan-Kettering Cancer Center (M.S., R.J.B., and I.R.); Damon Runyon Clinical Investigator Award (R.J.B.); The Annual Terry Fox Run for Cancer Research (New York, NY) organized by the Canada Club of New York; Kate's Team (R.J.B.); ASCO Conquer Cancer Foundation Young Investigator Awards (M.L.D. and J.H.P.); and AACR Postdoctoral Fellows Award (J.H.P.).
National Institutes of Health
Authorship
Contribution: R.J.B., I.R., and M.S. designed the research plan; R.J.B., I.R., J.H.P., M.L.D., D.S., M.F., and M.S. designed the clinical studies; X.W., J.S., R.Y., O.B.-O., C.T., M.O., M.P., D.H., H.P., E.S., and Y.U. performed research; Y.B., S.B., D.P., and J.H. contributed to quality control testing; R.J.B., J.H.P., M.L.D., J.J., M.H., P.M., E.H., and M.F. enrolled patients on study and took care of patients; G.H. oversaw statistical analyses; I.R. directed cell processing and molecular monitoring studies; R.J.B., I.R., and M.S. wrote the manuscript; R.J.B., I.R., J.H.P., M.L.D., and M.S. contributed to the revisions of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Renier Brentjens, Memorial Sloan-Kettering Cancer Center, 1275 York Ave, Box 242, New York, NY 10065; e-mail: brentjer@mskcc.org.
References
Author notes
R.J.B. and I.R. contributed equally to this study.