Abstract
The association of an IgM-Fc receptor (FcμR) with chronic lymphocytic leukemia (CLL) was suggested more than 30 years ago, but its authenticity has never been formally addressed. We examined the expression of the recently identified FcμR by B and T cells in CLL patients using receptor-specific monoclonal antibodies. CLL B cells (CD5+/CD19+) expressed much higher levels of FcμR on their cell surface than B cells from healthy donors. Such enhanced expression was more evident in immunoglobulin heavy chain variable region (IGHV)–mutated, CD38− or early Rai-stage CLL than in IGHV-unmutated, CD38+, or advanced Rai-stage CLL. Intriguingly, surface FcμR levels also were significantly elevated in the non-CLL B cells (CD5−/CD19+) and T cells (CD5+/CD19−), especially in IGHV-mutated CLL. CLL patients also had high serum titers of FcμR compared with healthy donors, and serum FcμR levels correlated significantly with circulating lymphocyte numbers but not with the IGHV mutation status or Rai stage. The serum FcμR was resolved as an ∼ 40-kDa protein, distinct from the cell surface FcμR of ∼ 60 kDa, and it was produced by both CLL B and non-CLL B cells. Mass spectrometric analysis revealed that the serum FcμR is a soluble form of the receptor encoded by an alternatively spliced FcμR transcript. These findings indicate enhanced levels of both membrane-bound and soluble forms of FcμR in CLL patients.
Introduction
Chronic lymphocytic leukemia (CLL) is the most common leukemia of adults in Western countries and accounts for ∼ 30% of all cases of leukemia in the United States. It is a heterogeneous leukemia that is thought to originate from antigen-stimulated B cells that escape from normal cell death mechanisms.1,2 Survival of CLL patients ranges from a few years to several decades. The most reliable marker thus far for predicting the prognosis of CLL is the mutation status in the immunoglobulin heavy chain variable region (IGHV). Unmutated (UM)–CLL is aggressive and mutated (MT)–CLL is more indolent.3-5 Because DNA sequencing is not practical for most clinical laboratories, various cell surface and intracellular proteins have been explored as potential surrogate markers. ZAP-70, a Syk family tyrosine kinase normally expressed in T cells, has proved to be a very good indicator for UM-CLL, but the required intracellular staining is technically challenging and results in diagnostic inconsistency among clinical laboratories.6-11 Other markers, including membrane proteins (eg, CD38, Fc receptor-like protein 2) and serum proteins (eg, thymidine kinase, soluble CD23, and β2-microglobulin), also have been studied as surrogate prognostic indicators.3,4,12-17
The association of the IgM Fc receptor (FcμR) with CLL has long been suggested based on the ability of CLL cells to form rosettes with IgM-coated erythrocytes.18-22 Unfortunately, this early intriguing suggestion was not pursued thereafter, probably because of uncertainties with such a crude detection procedure. During the course of analysis of B-cell activation antigens with the BAC-1 mouse IgM monoclonal antibody (mAb), we serendipitously found an IgM-binding protein of ∼ 60 kDa that was expressed on CLL B cells and activated normal B cells by immunofluorescence and biochemical analyses using various IgM ligands.23,24 The gene encoding an authentic FcμR has defied identification until our recent discovery of a bona fide FcμR cDNA in the B-lineage libraries, including one library derived from CLL B cells.25 Surprisingly, the corresponding FCMR gene had been already reported as TOSO or Fas apoptosis inhibitory molecule 3 (FAIM3).26 Notably, the reported inhibition of apoptosis was based on an assay in which apoptosis was induced by ligation of Fas on the Jurkat T-cell line with a mouse IgM mAb (CH11). Our results indicated that FcμR per se had no inhibitory activity in Fas-mediated apoptosis and that such inhibition was only achieved when anti-Fas mAb of an IgM but not IgG isotype was used for inducing apoptosis.25 This incorrect designation has led to the misconception that enhanced FAIM3/TOSO expression by CLL cells may be linked to their resistance to cell death mechanisms.27-29 Here, we examined the expression of FcμR in CLL patients using receptor-specific mAbs and have shown the enhanced expression of both the membrane-bound and soluble form of the receptor.
Methods
Recruitment of patients with CLL
CLL blood and serum samples were obtained from patients at the University of Alabama at Birmingham and Brookwood Medical Center in Birmingham, AL; all corresponding patients met National Cancer Institute criteria for the diagnosis of CLL.30 Blood and serum samples also were obtained from adult healthy volunteers. The study was approved by the institutional review boards of University of Alabama at Birmingham and Brookwood Hospital. Written informed consent was obtained from all participants before enrollment in the study in accordance with the Declaration of Helsinki, and all donor samples were made anonymous to maintain health information confidentiality. Serum samples were stored at −20°C until analysis.
Flow cytometric analysis of cell surface FcμR
PBMCs isolated by Ficoll-Hypaque gradient centrifugation were first incubated with an Fcγ receptor blocking mAb and then with biotin-labeled anti-FcμR mAb (HM14 clone; mouse γ1κ isotype) or biotin-labeled irrelevant mAb of γ1κ isotype as a control, along with FITC-labeled anti-CD5 mAb (HISM2 clone; mouse γ1κ) and allophycocyanin (APC)–labeled anti-CD19 mAb (HIB19 clone; mouse γ1κ).15,25 Anti-TOSO mAb (1E4 clone) that was used in a previous publication27 was purchased from Abnova, biotinylated, and used with the HM14 mAb in a side-by-side comparison of the expression of FcμR and TOSO in CLL patients. Phycoerythrin (PE)–labeled streptavidin (SA) was used for detection of biotin mAbs. The stained cells were analyzed using an FACSCalibur flow cytometer (BD Biosciences). Cells stained with the isotype-matched control mAbs labeled with FITC or APC were used to set up the background staining gates. Lymphoid cells with typical forward and side scatter characteristics were gated and examined for their expression of FcμR, CD5, and CD19, as described previously.15 The mean fluorescence intensity (MFI) indices of FcμR were determined as the ratio of the MFI of FcμR to the MFI of control mAb in the CD5+/CD19+ CLL B, CD5−/CD19+ non-CLL B, and CD5+/CD19− T-cell populations. In some experiments, these three cell populations in CLL patient blood samples were enriched by MoFlo cell sorter (Dako Cytomation) for RNA extraction or for cell culture where sorted cells were cultured at 106/mL in Hybridoma SFM medium (Invitrogen) for 7 days at 37°C under 5% CO2. The surface expression of CD38 by CLL B cells and the Rai stage were determined as previously described.15
ELISA for soluble FcμR
For quantitation of serum levels of soluble FcμR (solFcμR), a sandwich ELISA was performed. In brief, 96-well plates precoated with mouse anti–human FcμR mAb (clone HM6; γ2bκ isotype) at a protein concentration of 10 μg/mL (and then with BSA [1 mg/mL] to block any free sites) were incubated with 100 μL of serial dilutions of serum samples in triplicate. The bound FcμR was detected by the addition of biotin-labeled mouse anti–human FcμR mAb (HM14) and alkaline phosphatase–labeled SA before addition of the substrate p-nitrophenylphosphate. A biotin-labeled, isotype-matched irrelevant mAb was used as a control. The enzyme reaction was measured by the absorbance at 405 nm with a microplate spectrometer (BioTek Instruments). The serum of a CLL patient that contained high titers of solFcμR was used as an internal standard in each plate for all assays. The results were expressed as an arbitrary unit that was determined as follows. At a given optical density value in the linear range, the reciprocal dilutions of the serum samples were divided by the reciprocal dilution of the standard serum, and the lowest measurable value obtained for all the samples was arbitrarily set at 1 unit. When the linear range was not obtained with 10-fold diluted serum samples, the results were scored as undetectable. For determination of the effect of B-cell receptor cross-linkage on production of solFcμR, CLL cells were cultured in the presence or absence of a mouse anti–human μ mAb (SA-DA4.4 clone; IgG1κ) or an isotype-matched control mAb for 7 days at 37°C under 5% CO2 before assessment of solFcμR in culture supernatants by ELISA.25
Western blot analysis of membrane-bound and soluble FcμR
For membrane-bound FcμR, plasma membrane proteins on PBMCs (107 cells) from CLL patients were biotinylated, washed, quenched, and incubated with HM14 anti-FcμR or isotype-matched control mAb, before washes and solubilization in 1% nonidet P-40 lysis buffer containing protease inhibitors. The mAb-tagged cell surface proteins were precipitated with rat anti–mouse κ mAb-coupled beads, dissociated by 1M glycine-HCl buffer, pH 2.85, and resolved on SDS-PAGE under reducing conditions, followed by transfer onto membranes, blot with HRP-SA, and visualization by enhanced chemiluminescence (ECL; GE Healthcare) as described previously.25 For solFcμR, 50 μL of sera from 10 different CLL patients or 150 μL of culture supernatants from the sorted CLL B and non-CLL B-and T-cell populations were incubated with HM14 anti-FcμR or isotype-matched control mAb-coupled beads, and the bound materials were dissociated and separated on SDS-10% PAGE under reducing conditions, followed by transfer onto membranes. The membranes were sequentially blotted with biotin-labeled anti-FcμR mAbs (HM14 and HM7) and with HRP-SA, before visualization by ECL as described previously.25
Mass spectrometric analysis of solFcμR
Approximately 20 mL of sera pooled from 3 CLL patients with high titers of solFcμR were applied onto 1 mL of Sepharose 4B coupled with F(ab′)2 fragments of HM14 anti-FcμR mAb (3 mg/mL). After extensive washing, the bound materials were eluted by acid buffer, immediately neutralized, and separated on SDS-10% PAGE, followed by staining with Coomassie Brilliant Blue. The band migrating at ∼ 40 kDa was excised and subjected to mass spectrometric analysis. In brief, the destained gel piece was reduced, alkylated, and then digested with trypsin before extraction of peptides. An aliquot of extracted peptides was applied onto a C18 reverse-phase cartridge, and the bound peptides were flushed onto a C18 reverse-phase pulled tip analytical columns. The eluted peptides were passed into a modified MicroIonSpray interface of a 4000 Qtrap mass spectrometer (Applied Biosystems-MDS-Sciex). Possible FcμR sequences for monitoring scans were determined using in silico digestion software (MS-Digest, part of the Protein Prospector Version 5.9.2 suite of programs, http://prospector.ucsf.edu/prospector/cgi-bin/msform.cgi?form-msdigest) for possible parent–daughter transitions combination of the splice variant motifs. The multiple reaction monitoring technique with the triple quadrupole detection platform on the 4000 Qtrap mass spectrometer was used as a diagnostic tool for further sample analyses. The first of the 3 quadrupoles selected for the parent mass (parent ion) of the desired analyte. The second quadrupole dissociated the parent ions by collision with an inert gas (N2) into daughter ions (b and y ions). The third quadrupole selected for one of the daughter ions. The parent–daughter ion combination allowed for a highly specific and sensitive diagnostic tool for detection and quantification for specific protein(s) in complex solutions.
RT-PCR
Two micrograms of total RNAs isolated from the sorted cells from CLL patients' blood was converted to first-strand cDNA with an oligo(dT)18 primer, and the resultant first-strand cDNAs were used as a template DNAs for amplification of FcμR transcripts with a set of primers of 5′-TCTAGAAGGGACAATGGACT-3′ (forward) and 5′-TCAGGCAGGAACATTGATGT-3′ corresponding to the translation initiation and termination sites of the human FcμR cDNA as described previously.25 Amplification of glyceraldehydes-3-phosphate dehydrogenase (GAPDH) with a set of primers 5′-GGTCGGAGTCAACGGATTTGG-3′ (forward) and 5′-CCTCCGACGCCTGCTTCACCA-3′ (reverse) was performed as a control. Amplified products were resolved on 1% agarose gel electrophoresis containing ethidium bromide.
IGHV gene sequencing analysis
IGHV mutation status was determined according to established methods as described previously.15 Sequence data were analyzed in at least 2 databases (IMGT/V-QUEST, JOINSOLVER, National Center for Biotechnology Information IgBLAST), and sequences with a germ line homology of 98% or higher were considered as unmutated and those with a homology < 98% were considered as mutated.
Statistical analysis
All data comparison was performed by paired Student t test within the same individuals and by pooled Student t test for different groups, and a P value of < .05 was defined as statistically significant. The Spearman correlation coefficient analysis was used to analyze the relationship between soluble FcμR titers and Rai stage in CLL patients.
Results
Enhanced surface expression of FcμR in CLL patients
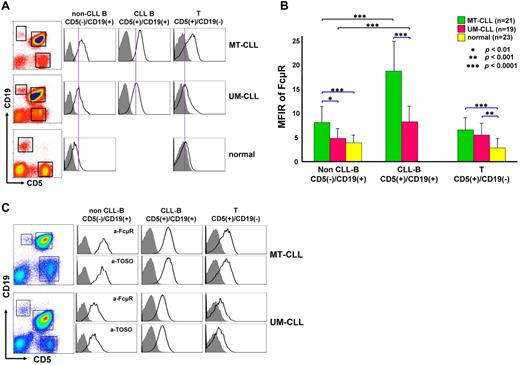
To determine whether the cell surface FcμR levels on CLL B cells have any clinical relevance, we conducted a 3-color immunofluorescence analysis of FcμR on blood lymphocytes from 40 CLL patients and from 23 healthy donors. The mutation status of IGHV of CLL B-cell receptors in these patients and donors was determined by RT-PCR and nucleotide sequence analyses, and 21 MT-CLL and 19 UM-CLL patients were enrolled in this study. Because FcμR is expressed by both B and T lymphocytes,25 FcμR expression was examined for 3 different cell populations in each patient (CD5+/CD19+ CLL B, CD5−/CD19+ non-CLL B and CD5+/CD19− T cells). For healthy donors, who have very few CD5+/CD19+ B cells, 2 populations (CD5−/CD19+ B and CD5+/CD19− T cells) were examined for their FcμR expression. As shown in Figure 1A, MT-CLL B cells express significantly higher surface levels of FcμR than do UM-CLL B cells (P < .0001). Moreover, the expression of FcμR by both types of CLL B cells was clearly enhanced compared with B cells from healthy donors (P ≤ .0001). Within the same individuals, the FcμR level of CLL B cells was higher than that of the corresponding non-CLL B cells in both MT-CLL and UM-CLL patients (P < .0001; Figure 1B). Remarkably, the FcμR levels of both non-CLL B- and T-cell populations in MT-CLL patients and of T cells in UM-CLL patients also were significantly elevated compared with those of normal B and T cells (P < .003). The surface FcμR level of CLL B cells was also negatively correlated with the Rai stage (P = .0317).
Surface FcμR expression by B and T cells in CLL patients and healthy donors. (A) A representative profile of each group. PBMCs from CLL patients with a mutated (MT-CLL; n = 21) or unmutated (UM-CLL; n = 19) genotype and from 23 healthy donors were stained with biotin–anti-FcμR (plus PE-SA), FITC–anti-CD5, and APC–anti-CD19 mAbs or with the corresponding, isotype-matched control mAbs. The CD5−/CD19+ non-CLL B, CD5+/CD19+ CLL B, and CD5+/CD19− T cells in patients and the CD5−/CD19+ B and CD5+/CD19− T cells in healthy donors were gated (boxes in left panels) and examined for their FcμR expression (open profiles) and background staining with control mAbs (shaded profiles). Purple lines drawn through the UM-CLL peak are included to allow comparison of the staining intensities in the different groups. (B) Cell surface FcμR levels on the indicated cell populations from MT-CLL (green), UM-CLL (red), and healthy (yellow) blood samples are shown as the mean fluorescence intensity ratio (MFIR; ± SD), calculated as the ratio of FcμR MFI/control MFI. Comparisons by paired and pooled t tests are indicated in black and blue lines, respectively. Other P values are listed in Table 1. (C) A representative profiles of comparative analysis of anti-FcμR (first and third rows) and anti-TOSO (second and fourth rows) mAbs in MT-CLL (top 2 panels) and UM-CLL (bottom 2 panels).
Surface FcμR expression by B and T cells in CLL patients and healthy donors. (A) A representative profile of each group. PBMCs from CLL patients with a mutated (MT-CLL; n = 21) or unmutated (UM-CLL; n = 19) genotype and from 23 healthy donors were stained with biotin–anti-FcμR (plus PE-SA), FITC–anti-CD5, and APC–anti-CD19 mAbs or with the corresponding, isotype-matched control mAbs. The CD5−/CD19+ non-CLL B, CD5+/CD19+ CLL B, and CD5+/CD19− T cells in patients and the CD5−/CD19+ B and CD5+/CD19− T cells in healthy donors were gated (boxes in left panels) and examined for their FcμR expression (open profiles) and background staining with control mAbs (shaded profiles). Purple lines drawn through the UM-CLL peak are included to allow comparison of the staining intensities in the different groups. (B) Cell surface FcμR levels on the indicated cell populations from MT-CLL (green), UM-CLL (red), and healthy (yellow) blood samples are shown as the mean fluorescence intensity ratio (MFIR; ± SD), calculated as the ratio of FcμR MFI/control MFI. Comparisons by paired and pooled t tests are indicated in black and blue lines, respectively. Other P values are listed in Table 1. (C) A representative profiles of comparative analysis of anti-FcμR (first and third rows) and anti-TOSO (second and fourth rows) mAbs in MT-CLL (top 2 panels) and UM-CLL (bottom 2 panels).
The fact that FcμR was originally incorrectly identified as TOSO/FAIM3 and reported to inhibit apoptosis has led to some confusion about the expression and role of this molecule in CLL. Therefore, we decided to perform a direct side-by-side comparison of its expression using our FcμR-specific HM14 mAb and the previously reported 1E4 TOSO mAb as detection antibodies. Six CLL blood samples (3 MT-CLL and 3 UM-CLL) were examined with essentially the same results for both mAbs (Figure 1C). Furthermore, both mAbs were found to recognize a similar or closely distributed epitope on the FcμR molecule, because preincubation of FcμR+ cells with unlabeled HM14 or 1E4 mAb inhibited subsequent reactivity with biotin-labeled 1E4 or HM14 mAb, respectively (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Notably, unlike T and B cells in healthy donors, preincubation in IgM-free media was not necessary for the detection of FcμR on CLL cells. In addition, patients with CD38− CLL were found to express higher levels of FcμR on CLL B cells (P < .008) and non-CLL B cells (P < .004), but not on T cells (P = .589) compared with patients with CD38+ CLL.
Collectively, these findings indicate that (1) CLL B cells express higher levels of FcμR on their cell surface than B cells of healthy donors as determined by 2 different receptor-specific mAbs; (2) surface FcμR levels on CLL B cells negatively correlate with the Rai stage; (3) FcμR expression is higher on mutated IGHV or CD38− CLL than unmutated IGHV and CD38+ CLL; and (4) enhanced FcμR expression is also evident on the non-CLL B- and T-cell populations, at least in mutated CLL.
High titers of FcμR in the sera from CLL patients
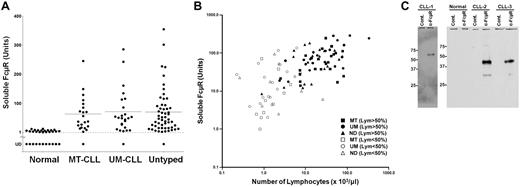
To determine whether CLL patients contain any soluble or extracellular form of FcμR in their sera, we performed a sandwich ELISA using one anti-FcμR mAb (HM6) for coating plates to capture FcμR and another biotin-labeled anti-FcμR mAb (HM14) as a developing reagent. Serum samples from 102 different CLL patients (22 MT-CLL, 25 UM-CLL, and 55 untyped) and from 31 healthy donors were analyzed. As shown in Figure 2A, serum titers of FcμR are clearly elevated, although there is a wide range, in many CLL patients in comparison with healthy donors (P < .001). Passage of sera through a 0.22-μm filter did not affect the serum titers of FcμR, ruling out the possibility of cellular contamination. There was no significant difference in serum FcμR titers between MT-CLL and UM-CLL patients. There also was no correlation between serum FcμR titers and the Rai stage in CLL (P = .4893). A strong correlation, however, was demonstrated between the serum titers of FcμR and the blood lymphocyte counts in CLL patients (r = 0.85; P < .001).
Levels and relative molecular mass estimation of the serum FcμR. (A) Serum levels in arbitrary units of FcμR in healthy donors (n = 31) and MT-CLL (n = 22), UM-CLL (n = 25), and untyped CLL patients (n = 55) were determined by a sandwich ELISA as described in “ELISA for soluble FcμR.” The horizontal line indicates arithmetic mean. UD indicates undetectable. (B) Correlation of soluble FcμR levels with blood lymphocyte (Lym) counts. The values of solFcμR level (units) and blood lymphocyte count (× 103/μL) in each patient or donor are plotted along the y-axis and x-axis, respectively. ●, ▴, and ■ indicate mutated (MT), unmutated (UM), and untyped (ND) CLL patients, respectively, whose lymphocytes constitute ≥ 50% of WBCs; and ™, Δ, and £ indicate MT, UM, and ND CLL patients, respectively, whose lymphocytes constitute < 50% of WBCs. (C) Western blot analysis of membrane-bound and serum FcμR. (Left) Plasma membrane proteins on CLL cells were labeled with biotin, incubated with isotype-matched control (cont.) or anti-FcμR mAb, immunoprecipitated, and immunoblotted as described in “Western blot analysis of membrane-bound and soluble FcμR.” (Right) Serum FcμR proteins from a healthy donor and 2 different CLL patients were immunoprecipitated and analyzed by protein blot using a mixture of biotin-labeled anti-FcμR mAbs (HM14 and HM7) plus HRP-SA, before visualization by ECL. Size markers (kilodaltons) are indicated. Essentially the same results were obtained with sera from 8 other CLL patients (data not shown).
Levels and relative molecular mass estimation of the serum FcμR. (A) Serum levels in arbitrary units of FcμR in healthy donors (n = 31) and MT-CLL (n = 22), UM-CLL (n = 25), and untyped CLL patients (n = 55) were determined by a sandwich ELISA as described in “ELISA for soluble FcμR.” The horizontal line indicates arithmetic mean. UD indicates undetectable. (B) Correlation of soluble FcμR levels with blood lymphocyte (Lym) counts. The values of solFcμR level (units) and blood lymphocyte count (× 103/μL) in each patient or donor are plotted along the y-axis and x-axis, respectively. ●, ▴, and ■ indicate mutated (MT), unmutated (UM), and untyped (ND) CLL patients, respectively, whose lymphocytes constitute ≥ 50% of WBCs; and ™, Δ, and £ indicate MT, UM, and ND CLL patients, respectively, whose lymphocytes constitute < 50% of WBCs. (C) Western blot analysis of membrane-bound and serum FcμR. (Left) Plasma membrane proteins on CLL cells were labeled with biotin, incubated with isotype-matched control (cont.) or anti-FcμR mAb, immunoprecipitated, and immunoblotted as described in “Western blot analysis of membrane-bound and soluble FcμR.” (Right) Serum FcμR proteins from a healthy donor and 2 different CLL patients were immunoprecipitated and analyzed by protein blot using a mixture of biotin-labeled anti-FcμR mAbs (HM14 and HM7) plus HRP-SA, before visualization by ECL. Size markers (kilodaltons) are indicated. Essentially the same results were obtained with sera from 8 other CLL patients (data not shown).
To determine the relative molecular mass (Mr) of serum FcμR in CLL patients, 10 different serum samples with relatively high titers of serum FcμR were incubated with anti-FcμR or control mAb-coupled beads, and the bound materials were analyzed by Western blot analysis with anti-FcμR mAbs. As shown in Figure 2C, all of the isolated serum FcμR proteins were resolved on SDS-10% PAGE as a single band of ∼ 40 kDa that is distinct in size from the membrane-bound FcμR of ∼ 60 kDa. No apparent size heterogeneity was observed with these randomly chosen samples. (A protein of ∼ 30 kDa was occasionally observed in the anti-FcμR precipitates, but its molecular identification remains unknown.) These findings thus indicate that many CLL patients' sera contain high levels of an ∼ 40-kDa FcμR species that is smaller than the ∼ 60-kDa membrane-bound receptor, and they exclude the possibility that the serum FcμR results from the extracellular release of the membrane-bound receptor, for example, by exosome-like vesicles.
Origin and cellular source of the solFcμR
In our previous RT-PCR analysis with a set of primers corresponding to the translation initiation and termination sites of the human FcμR cDNA, 2 smaller cDNA products of ∼ 1.0 and ∼ 0.9 kb, in addition to the full-length (membrane-bound form) FcμR cDNA of ∼ 1.2 kb, were identified in the phorbol myristate acetate (PMA)–activated human pre-B cell line 697. The nucleotide sequence analysis of the 1056-bp cDNA revealed that it was derived from an alternatively spliced FcμR transcript that resulted from the direct splicing of exon 4 (stalk 2) to exon 6 (cytoplasmic 1), skipping exon 5 (transmembrane; Figure 3 top panel). This splicing event results in a reading frame shift in exon 6 and generates a novel 70-amino acid hydrophilic carboxyl-terminal tail, suggesting that this FcμR splice variant encodes a soluble form of FcμR (gene accession HM480394). (We found that the ∼ 0.9-kb product was nonspecific.)
Amino acid sequence alignment of the full-length and alternatively spliced FcμR along with the exon organization and the mass spectrometric results of serum FcμR. (Top) Exon organization of FCMR is schematically drawn to the scale indicated. Exons (boxes) encoding particular regions of the receptor are denoted as follows: 5′ untranslated (5′UTR), signal peptide (SS), extracellular Ig-like domain (Ig), stalk (Stalk), transmembrane (TM), cytoplasmic (CY), and the 3′ untranslated (3′UTR) regions. Roman numbers in each intron (horizontal lines) indicate the phase occurring between 2 exons. The lines connecting exon 4 (stalk 2) with exon 6 (CY1) indicate the alternative splicing identified in the cDNA of PMA-activated 697 pre-B cell line (gene accession HM480394). (Bottom) Amino acid sequences (single-letter code) deduced from the full-length (membrane-bound form; top mem.) and the alternatively spliced (bottom sol.) FcμR are aligned. Amino acid identity is indicated by dots (·) and a deletion by dashes (-). The unique 70 carboxyl-terminal residues of the FcμR splice variant are purple, and 2 predicted hydrophobic regions (SS and TM) are underlined. The sequences identified by mass spectrometric analysis of the tryptic peptide of the ∼ 40-kDa FcμR in CLL patients' sera are framed and highlighted in yellow. (B) Multiple reaction monitoring scan profile of one of the serum FcμR-derived peptide sequences. Liquid chromatographytandem mass spectrometric analysis reveals that this peptide eluted at a retention time of 24.21 minutes corresponds with the tryptic peptide “Q236SPLQAGPPTGR247” of the splice variant FcμR with a mass of 1190.6 Da, which covers the boundary of splicing of exons 4 and 6.
Amino acid sequence alignment of the full-length and alternatively spliced FcμR along with the exon organization and the mass spectrometric results of serum FcμR. (Top) Exon organization of FCMR is schematically drawn to the scale indicated. Exons (boxes) encoding particular regions of the receptor are denoted as follows: 5′ untranslated (5′UTR), signal peptide (SS), extracellular Ig-like domain (Ig), stalk (Stalk), transmembrane (TM), cytoplasmic (CY), and the 3′ untranslated (3′UTR) regions. Roman numbers in each intron (horizontal lines) indicate the phase occurring between 2 exons. The lines connecting exon 4 (stalk 2) with exon 6 (CY1) indicate the alternative splicing identified in the cDNA of PMA-activated 697 pre-B cell line (gene accession HM480394). (Bottom) Amino acid sequences (single-letter code) deduced from the full-length (membrane-bound form; top mem.) and the alternatively spliced (bottom sol.) FcμR are aligned. Amino acid identity is indicated by dots (·) and a deletion by dashes (-). The unique 70 carboxyl-terminal residues of the FcμR splice variant are purple, and 2 predicted hydrophobic regions (SS and TM) are underlined. The sequences identified by mass spectrometric analysis of the tryptic peptide of the ∼ 40-kDa FcμR in CLL patients' sera are framed and highlighted in yellow. (B) Multiple reaction monitoring scan profile of one of the serum FcμR-derived peptide sequences. Liquid chromatographytandem mass spectrometric analysis reveals that this peptide eluted at a retention time of 24.21 minutes corresponds with the tryptic peptide “Q236SPLQAGPPTGR247” of the splice variant FcμR with a mass of 1190.6 Da, which covers the boundary of splicing of exons 4 and 6.
To determine whether the serum FcμR present in CLL patients is generated by this FcμR splice variant or by proteolytic cleavage of the membrane-bound receptor, the ∼ 40 kDa serum FcμR protein was purified by affinity columns, resolved on SDS-PAGE, and digested with trypsin before mass spectrometric analysis. Four tryptic fragments were found to match the amino-terminal sequences of both the membrane-bound and splice variant forms of FcμR, whereas the other 3 tryptic fragments matched only the carboxyl-terminal sequence of the FcμR splice variant (Figure 3A bottom panel). Importantly, one of these fragments (QSPLQAGPPTGR) was found to correspond to the junction formed by splicing exon 4 to exon 6, thereby defining the serum FcμR as a soluble form of the receptor encoded by an FcμR splice variant (Figure 3).
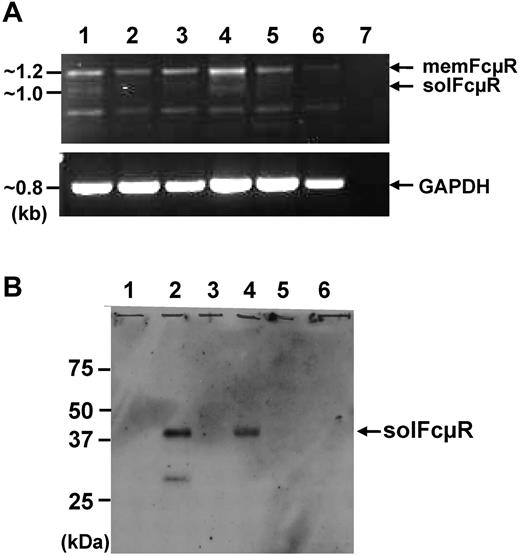
Next, to determine which cells produce the solFcμR, the CD5+/CD19+ CLL B, CD5−/CD19+ non-CLL B, and CD5+/CD19− T cells were purified from each mutated and unmutated CLL patient's blood by fluorescence activating cell sorter and were subjected to RT-PCR analysis. The solFcμR transcripts of ∼ 1 kb were detectable in CLL B cells and to a lesser extent in non-CLL B cells but undetectable in T cells (Figure 4A). The ∼ 40-kDa soluble FcμR protein also was demonstrated in the culture supernatants of both CLL B and non-CLL B cells, but not of T cells as determined by protein blot analysis (Figure 4B) and ELISA (data not shown). Ligation of membrane IgM on CLL B cells with a mitogenic anti-μ mAb did not enhance their secretion of solFcμR as determined by ELISA (percentage of solFcμR relative to media: 96.4 ± 6.4 for control mAb vs 98.7 ± 14.6 for anti-μ mAb).
Cellular origin of the soluble FcμR. (A) RT-PCR analysis of FCMR transcripts. The CD5+/CD19+ CLL B (lanes 1 and 4), CD5−/CD19+non-CLL B (lanes 2 and 5), and CD5+/CD19− T cells (lanes 3 and 6) in patients with MT-CLL (lanes 1-3) and UM-CLL (lanes 4-6) were enriched from PBMC by cell sorting. Total RNAs were extracted from the sorted cells and converted to first-strand cDNA before PCR amplification using a set of primers corresponding to the FCMR or GAPDH cDNA. An aliquot of the amplified FCMR (top panel) and GAPDH (bottom panel) products was electrophoresed on 1% agarose and stained with ethidium bromide. Lane 7 is a PCR control without a first-strand cDNA template. (B) Western blot analysis of soluble FcμR in culture supernatants. The above-sorted cells were cultured at 106 cells/mL for 7 days, and the culture supernatants from CLL B (lanes 1 and 2), non-CLL B (lanes 3 and 4), and T cells (lanes 5 and 6) were analyzed by Western blot as described in “Methods.”
Cellular origin of the soluble FcμR. (A) RT-PCR analysis of FCMR transcripts. The CD5+/CD19+ CLL B (lanes 1 and 4), CD5−/CD19+non-CLL B (lanes 2 and 5), and CD5+/CD19− T cells (lanes 3 and 6) in patients with MT-CLL (lanes 1-3) and UM-CLL (lanes 4-6) were enriched from PBMC by cell sorting. Total RNAs were extracted from the sorted cells and converted to first-strand cDNA before PCR amplification using a set of primers corresponding to the FCMR or GAPDH cDNA. An aliquot of the amplified FCMR (top panel) and GAPDH (bottom panel) products was electrophoresed on 1% agarose and stained with ethidium bromide. Lane 7 is a PCR control without a first-strand cDNA template. (B) Western blot analysis of soluble FcμR in culture supernatants. The above-sorted cells were cultured at 106 cells/mL for 7 days, and the culture supernatants from CLL B (lanes 1 and 2), non-CLL B (lanes 3 and 4), and T cells (lanes 5 and 6) were analyzed by Western blot as described in “Methods.”
Discussion
Using receptor-specific mAbs, we have shown enhanced levels of both the ∼ 60-kDa membrane-bound FcμR and the ∼ 40 kDa solFcμR in patients with CLL compared with healthy donors. Enhanced surface FcμR expression was more evident in IGHV mutated or CD38− CLL than IGHV unmutated or CD38+ CLL and was negatively correlated with the Rai stage. Surprisingly, the surface FcμR levels in both the CD5− non-CLL B-cell and the T-cell populations also were elevated at least in the mutated CLL patients. The serum levels of solFcμR in CLL patients strongly correlated with their circulating lymphocyte counts but not with either their IGHV mutation status or Rai stage. The ∼ 40-kDa solFcμR was found to be encoded by an alternatively spliced FcμR transcript and not by a cleavage of the membrane-bound receptor, and it was produced by both CD5+ CLL B and CD5− non-CLL B cells.
Our results thus confirm the constitutive expression of FcμR by CLL B cells that was suggested decades ago based on the ability of these cells to form rosettes with IgM-coated erythrocytes18-22 and to bind fluorochrome-labeled IgM-ligands.23,24 Unlike rosetting assays, the detection of FcμR on CLL cells by flow cytometry did not require their preincubation in IgM-free media. Several studies also have demonstrated enhanced expression of FAIM3/TOSO, the former designations of the FCMR gene, in CLL.6,27-29,31,32 Two studies found no correlation of TOSO expression levels with prognostic factors, including IGHV mutational status, cytogenetics, and previous treatment.6,27 Other studies found that high levels of TOSO expression were correlated with more aggressive leukemia associated with high white blood cell (WBC) counts, advanced Binet stage, unmutated IGHV, and the need for chemotherapy.28,29 Gene microarray studies also indicated selective expression of TOSO by naive and memory B cells in tonsils,33 the findings being consistent with our protein expression data.25 Our results indicate that surface FcμR levels on CLL B cells as determined by both HM14 anti-FcμR and 1E4 anti-TOSO mAbs are significantly higher in MT-CLL than UM-CLL and that the discrepancy in the different studies could be because of the detection methods used rather than the patient populations examined in different countries (England, Germany, and the United States). Our assessment was based on immunofluorescence with receptor-specific mAbs, whereas others have mainly relied on RNA expression, either by microarray6,27 or RT-PCR analysis.28,29 In fact, we have shown previously that the 697 pre-B cell line expressed FcμR transcripts constitutively but did not synthesize the cell surface receptor unless it was stimulated with PMA.24,25 Although the mechanism for enhanced FcμR expression on CLL cells is unclear, it may result from chronic antigenic stimulation as evidenced by (1) reduced levels of membrane IgM, IgD, and CD79/Igα/Igβ on CLL cells; and (2) reactivity of CLL-derived IgM antibodies with various autoantigens, including the membrane components of apoptotic cells, superantigens, or common antigens.1,2,32 Our previous finding that treatment of normal blood B cells with anti-μ mAb down-modulates membrane IgM and up-regulates FcμR cell surface expression25 is consistent with the hypothesis that CLL cells are being activated by certain common antigens and that antigen-driven stimulation may provide an alternative mode of survival for the leukemic cells.
The inducibility of FcRs by exposure to the corresponding Ig isotopes has been described for various cell types, but such Ig-binding molecules have defied biochemical characterization. For example, IgA-binding capacity of mouse T cells was induced by exposure to IgA in vivo and in vitro;34 however, this binding must be mediated by a non-FcαR/CD89 molecule, because mice lack the human FcαR/CD89 homolog gene.35,36 Similarly, overnight incubation of 697 pre-B cells with IgM led to IgM binding; however, this occurred without the cell surface expression of FcμR as determined by receptor-specific mAbs (our unpublished data).24 In CLL patients, serum Ig levels are typically depressed, but the sera often contain significant amounts of CLL-derived IgM.37-39 It will be of interest to determine whether this elevated CLL-derived IgM or the reduced total serum IgM levels contribute to the enhanced expression of surface FcμR on CLL cells. Conversely, it also would be interesting to know whether enhanced surface FcμR expression, soluble FcμR expression, or both contribute to the reduced serum IgM levels in CLL patients.
One of the remarkable findings in our study is the elevated FcμR levels on nonleukemic cell populations in MT-CLL patients: CD5− non-CLL B cells and T cells. Unlike chronic myelogenous leukemia, which is a stem cell disorder, there is no evidence for CLL being a disorder of a lymphoid committed stem cell. Thus, this enhanced expression could be a secondary effect associated with leukemia. In this regard, there are several precedents describing that nonleukemic cell populations in CLL patients apparently have some abnormal or CLL-induced reactive phenotypes.40,41 For example, serum levels of IL-6 and IL-10 (of both viral and human origin) were elevated in CLL patients and correlated with adverse disease features and short survival. Interestingly, the source of these cytokines seemed to be non-B cells for IL-6 and non-CLL B cells for IL-10.40 Another remarkable example was that T cells in CLL patients were incapable of forming immune synapses with antigen presenting cells because of impaired actin polymerization and that CLL B cells could induce this abnormality in normal allogenic T cells.41 It is thus conceivable that the surface phenotype and function of nonleukemic cells including non-CLL B cells and T cells from CLL patients are modulated by the bulk of the neoplastic clone. In our previous studies, both circulating B and T cells in healthy donors expressed FcμR, but there was a striking difference in their response after antigen receptor ligation or PMA stimulation. FcμR expression was up-regulated on B cells but down-modulated on T cells, suggesting differential regulation of FcμR expression during B- and T-cell activation.25 Thus, the elevated FcμR levels on both non-CLL B and T cells observed in MT-CLL patients are unique, and such enhancement in T cells may be mediated by signals through receptors other than their antigen receptors.
Another remarkable finding is the elevated serum levels of the ∼ 40-kDa solFcμR in CLL patients, but not in healthy donors. The solFcμR levels are closely correlated with circulating lymphocyte numbers but not with the Rai stage. The ex vivo production of solFcμR was not enhanced by anti-μ mAb treatment. There are precedents for the generation of other soluble receptors by various mechanisms (eg, cleavage of membrane-bound or glycosylphosphate inositol-anchored receptors, alternative splicing, and exosome-like vesicles) in CLL patients.42-48 The results from liquid chromatography-tandem mass spectrometry analysis unequivocally defined the ∼ 40-kDa serum FcμR as a soluble form of the receptor encoded by an FCMR splice variant. Intriguingly, not only CLL B cells but also non-CLL B cells produced the soluble FcμR, and its serum level correlated well with the number of lymphocytes in circulation. The molecular basis for such solFcμR production in CLL patients remains unclear as does the clinical effect of such high levels on the immune response of CLL patients. At least 70% of human genes have been shown to express multiple transcripts through alternative splicing of exons or exon segments.49 In addition, many soluble receptors either derived from neoplastic or non-neoplastic cell populations are elevated in sera from CLL patients, including CD23, CD14, CD44, intercellular adhesion molecule (ICAM)–1 and vascular adhesion molecule (VCAM)–1.
Our study provides new insight into the biology of CLL. The findings may have important clinical applications from 2 perspectives. First, cell surface expression of FcμR, which can be easily measured by flow cytometry, may be a valuable new marker for use in clinical laboratories to distinguish MT-CLL from UM-CLL. Second, levels of soluble FcμR in serum, which are even easier to measure by ELISA, may correlate with disease progression in CLL. Large patient studies will be required to validate these 2 potential applications.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Peter D. Burrows for valuable suggestions and critical reading of the manuscript, Dewitt Jones for technical assistance, Dr Alan Cantor for statistical analysis, and Jacquelin B. Bennett for preparation of the manuscript.
This work was supported in part by National Institutes of Health grants RR19231, DK79337, and AR50948 (to S.B.); AI67467 and CA131655 and CLL Global Research Foundation and the Cancer Research Institute (to R.S.D.); and AI42127 and AI82249 (to H.K.).
National Institutes of Health
Authorship
Contribution: F.J.L. and R.S.D. performed surface immunofluorescence analysis; Y.K. performed protein analysis; M.K.M. and T.M. conducted ELISA; L.W. and S.B. performed mass spectrometric analysis; L.F.B., J.C.B., and R.S.D. provided patient materials; H.K. designed research and wrote the manuscript; and all authors reviewed the manuscript and approved of its final form.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hiromi Kubagawa, Department of Pathology, Shelby 506, 1825 University Blvd, University of Alabama at Birmingham, Birmingham, AL 35294; e-mail: hiromikubagawa@uab.edu.
References
Author notes
F.J.L, Y.K., and M.K.M contributed equally to this paper.