Abstract
Previous studies have shown that children with acute myeloid leukemia (AML) who developed mixed chimerism (MC) were at high risk for relapse after allogeneic stem-cell transplantation (allo-SCT). We investigated the feasibility of intensified preemptive immunotherapy in children receiving allo-SCT for AML. Eighty-four children were registered in our trial from May 2005 to April 2009; of these, 71 fulfilled the inclusion criteria and were treated according to the study protocol. Serial and semiquantitative analyses of posttransplantation chimerism were performed. Defined immunotherapy approaches were considered in MC patients. Continuous complete chimerism (CC) was observed in 51 of 71 patients. MC was detected in 20 patients and was followed by immunotherapy in 13. Six of 13 MC patients returned to CC without toxicity and remained in long-term remission. Overall, the probability of event-free survival (pEFS) was 66% (95% confidence interval [95% CI] = 53%-76%) for all patients and 46% (95% CI = 19%-70%) in MC patients with intervention; however, this number increased to 71% (95% CI = 26%-92%) in 7 of 13 MC patients on immunotherapy who were in remission at the time of transplantation. All MC patients without intervention relapsed. These results suggest that MC is a prognostic factor for impending relapse in childhood AML, and that preemptive immunotherapy may improve the outcome in defined high-risk patients after transplantation.
Introduction
Allogeneic stem cell transplantation (allo-SCT) is an established method for treating high-risk acute myeloid leukemia (AML) in children1 even after reduced intensity conditioning (RIC).2 Nevertheless, therapeutic success is mainly affected by relapse.3,4 In cases of clinical relapse, further therapeutic options are limited; for example, a second allo-SCT may provide a small chance for cure but entails a high rate of morbidity and mortality.5 Donor lymphocyte infusion (DLI) is of limited value when initiated during frank hematologic relapse, and the high cell doses needed raise the risk of severe GVHD6 ; therefore, detection of impending relapse is desirable. It has been shown that impending relapse can be indicated by mixed chimerism (MC), and can in principle be prevented by preemptive immunotherapy (ie, discontinuation of immunosuppression and adoptive DLI).3,6-15
Based on an amendment to our previous study published in 2004,3 we performed a prospective multicenter trial both to confirm the clinical significance of MC and to assess whether frank hematologic relapse in childhood AML may be prevented in a higher number of patients by increasing the sensitivity for detecting impending relapse and intensifying preemptive immunotherapy, primarily by increasing cell doses for adoptive DLI compared with the initial study.3 Therefore, in addition to the peripheral blood (PB) samples (as used in our previous study3 ), we also analyzed BM samples for hematopoietic chimerism, including chimerism of CD33+ and CD34+ subpopulations. Analyses of CD33+ and CD34+ subpopulations within BM samples were performed to increase the sensitivity of detecting impending relapse, suggesting that relapse of AML would primarily occur within these BM subsets; therefore, impending relapse might be detectable earlier by chimerism analysis of CD33+ and CD34+ subpopulations than by whole BM chimerism. As in our previous study, patients with MC were offered preemptive immunotherapy.3,7,8 Immune intervention was also performed in patients with MC in either the whole BM or in CD33+ and CD34+ BM subsets. Because of the low incidence of GVHD in our previous study,3 the starting dose per kilogram of recipient body weight for adoptive DLI increased by 10-fold to 1 × 106 CD3+ cells in patients with a matched sibling donor (MSD) and by 2-fold to 1 × 105 CD3+ cells in cases with a matched unrelated donor (MUD). In the haploidentical setting (MMFD), the starting dose remained at 2.5 × 104 CD3+ cells.
Methods
Patients
Between May 1, 2005 and April 30, 2009, a total of 84 children from 11 pediatric transplantation centers in Germany who had received allo-SCT for AML were registered for this study. During the observation period from May 1, 2005 until October 31, 2009, patient data and PB and BM samples were gathered. In total, 2540 samples (mean, 30; range, 3-129) were referred to the study center in Frankfurt/Main, Germany.
This study was registered at the federal authority for the development of drugs and cellular products at the Paul Ehrlich Institute in Langen, Germany (number 1244/01). The study protocol was approved by the clinical ethics committees of the Universities of Tuebingen on September 1, 2004 (number 38/97) and Frankfurt/Main on March 16, 2005 (number 79/05) and was conducted according to the principles of the Declaration of Helsinki. Written informed consent was obtained from the patients and/or parents according to the institutional guidelines.
An independent data-monitoring committee (Claudia Rossig, Muenster, Germany; Christian Thiede, Dresden, Germany; and Arjan C. Lankester, Leiden, The Netherlands) analyzed data from every patient and retrospectively evaluated enclosure and grouping of patients according to their chimerism status. Weekly chimerism monitoring started with the first referred PB or BM sample at day +21 after transplantation. Because 3 centers sent in fewer samples than demanded by the protocol, the data-monitoring committee suggested that at least 7 PB or BM samples should be referred until day +100 and an additional 5 samples until day +200 to ensure continuous chimerism monitoring. This minimal requirement was not fulfilled for 9 of 84 patients, who therefore did not receive immunotherapy based on chimerism analysis and eventually were excluded from analysis. Excluding these patients resulted in an assumable selection bias toward patients with better outcome; however, according to the per-protocol analysis, 4 patients with favorable outcomes who received prophylactic DLIs based on their risk profiles before transplantation, but not on chimerism analysis as recommended by the study protocol, were also not included in the analysis. In total, 71 of 84 registered patients fulfilled the inclusion criteria and could be evaluated and, when indicated, were preemptively treated according to their chimerism status.
The patient characteristics of the study cohort are summarized in Table 1. Patients and donors received high-resolution HLA typing for HLA-A, HLA-B, HLA-C, HLA-DR, and HLA-DQ. MUDs were defined as having at least 9 of 10 HLA matches with the patients.
Chimerism assays and immunotherapy
Chimerism analyses were performed centrally in the study center in Frankfurt/Main, Germany. As described previously, a semiquantitative PCR approach based on the amplification of short tandem repeat markers was used for analyses.16-18 The sensitivity of this assay for detecting autologous cells was 1%.
Chimerism in PB was assayed weekly until day +200 and monthly thereafter (see supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Analyses of BM were suggested at days +30, +60, and +100, as well as 6, 9, 12, 15, and 18 months after transplantation. If patients had a MC > 1% of autologous cells in a PB or BM sample, another sample was referred to the study center within a period of 1 week. Patients with confirmed > 1% autologous cells in 2 consecutive samples after transplantation without exceeding grade I acute GVHD (aGVHD) and without life-threatening complications were offered immunotherapy. Immunotherapy for patients still receiving immunosuppression (IS) consisted of immediate discontinuation of IS. Subsequently, if MC remained > 1% of autologous signals in consecutive weekly BM or PB samples, DLI was given. Patients without IS received DLI as frontline treatment. The cell dose administered was based on the number and potential severity of HLA mismatches between the donor and recipient. The study design recommended starting doses of 1 × 106 T cells per kilogram body weight in cases of MSD, 1 × 105/kg in cases of MUD, and 2.5 × 104/kg in the haploidentical setting.
After DLI, chimerism was assayed weekly until complete chimerism (CC) status was restored. Patients with persisting autologous signals > 1% 21 days after the last DLI were offered another DLI 28 days after the previous one. In cases of MSD or MUD, the number of CD3+ cells infused was doubled compared with the previous DLI if no additional signs of GVHD had appeared.
Definition of chimerism status and response
Patients were classified on the basis of serial chimerism analysis by short tandem repeat-PCR. Patients who showed no evidence of autologous cells exceeding 1% at any time after transplantation were considered CC. Patients with perceptible autologous signals exceeding 1% at least twice after transplantation were defined as having MC. Response to immunotherapy was defined as restored CC status and continuous complete remission (CCR).
Statistical methods
The data are summarized as the median for quantitative data and the number or percentage for categorical data. The follow-up period was defined as the time period from the date of SCT until either a subsequent event or last observation (in the case of complete remission [CR]). The considered events were relapse or death in remission (treatment-related mortality [TRM]) but not graft failure (GF). The probability of event-free survival (pEFS) was calculated by Kaplan-Meier analysis, and the differences between time-to-event distribution functions were calculated with the log-rank test. The independence of categorical parameters was calculated using either the Pearson χ2 test or Fisher exact test. Multivariate Cox regression analysis was used to test the independence of factors predictive for pEFS. Cumulative incidences of relapse (CI-R) and TRM (CI-TRM) were calculated according to Kalbfleisch and Prentice, and the differences in cumulative incidence between subgroups were tested according to Gray. A 2-sided P < .05 was regarded as significant.
Results
Stratification
In total, 71 of 84 patients were included in the analysis. These patients exhibited either CC (72%, 51 of 71) or MC (28%, 20 of 71). Preemptive immunotherapy was recommended to all patients with MC without life-threatening complications and without exceeding grade I aGVHD. Adhering to these guidelines, interventions could be performed in 13 patients (65%), whereas in 7 patients, the clinical condition or progressive disease immediately after transplantation made intervention impossible. The final decision to administer immunotherapy was the responsibility of the treating physician. GF was seen in 5 patients who were regrafted. These patients did not receive DLI. Four of these patients remained in CR and 1 relapsed after GF and died. These patients were included in the analysis, but GF was not considered as an event.
Outcome of all patients according to chimerism status
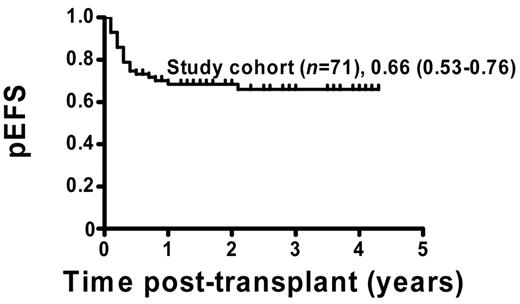
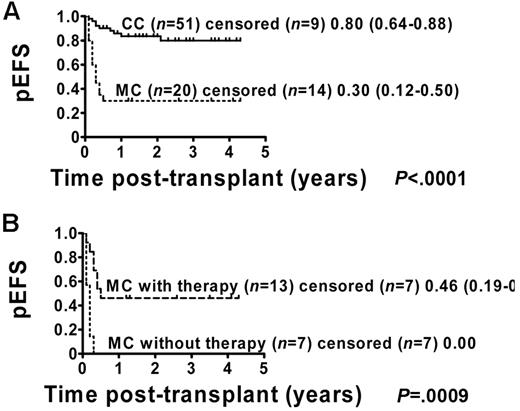
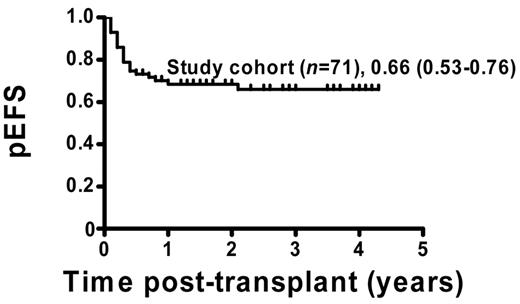
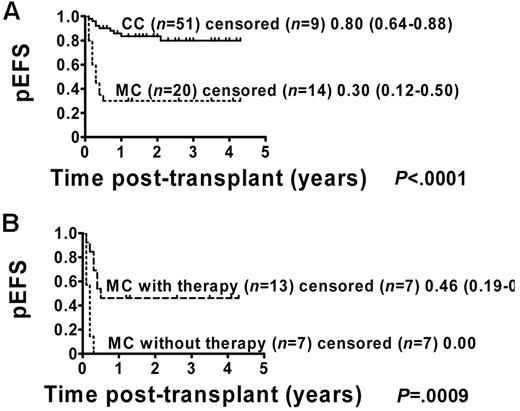
The pEFS for the 71 patients who were included in the analysis was estimated to be 66% (95% CI = 53%-76%; Figure 1). Patients with permanent CC (51 of 71) showed a pEFS of 80% (95% CI = 64%-88%), and patients with MC (20 of 71) had an estimated pEFS of 30% (95% CI = 12%-50%; pEFS, log-rank test, P < .0001; Figure 2A). Further analysis of the MC group revealed a significant difference regarding pEFS between the 13 patients receiving and the 7 patients not receiving immunotherapy (pEFS, log-rank test, P = .0009; Figure 2B). None of the patients in the intervention group died because of TRM; therefore, differences in pEFS between MC patients receiving or not receiving immunotherapy were caused by relapse. However, 7 of 20 MC patients could not receive immunotherapy. Of these 7 patients, 4 patients showed signs of aGVHD exceeding grade I and 3 patients had severe infections or were in a critical clinical condition. So as not to further increase risks, the study protocol did not consider immunotherapy in these patients.
pEFS of study patients. Kaplan-Meier survival plots for pEFS of study patients (n = 71). Study cohort: n = 71, pEFS = 0.66, 95% CI = 0.53-0.76.
pEFS of study patients. Kaplan-Meier survival plots for pEFS of study patients (n = 71). Study cohort: n = 71, pEFS = 0.66, 95% CI = 0.53-0.76.
pEFS according to chimerism status including MC patients with and without immunotherapy. Kaplan-Meier estimates of pEFS for patients with CC and MC (pEFS, log-rank test, P < .0001; Figure 2A). CC patients: n = 51; censored, n = 9. MC patients: n = 20; censored, n = 14. Kaplan-Meier analyses of pEFS of immunotherapy-treated MC patients and patients with MC who did not receive immunotherapy (pEFS, log-rank test, P = .0009; Figure 2B). MC patients with immunotherapy: n = 13; censored, n = 7, pEFS = 0.46, 95% CI = 0.19-0.70. MC patients without immunotherapy: n = 7; censored, n = 7, pEFS = 0.00.
pEFS according to chimerism status including MC patients with and without immunotherapy. Kaplan-Meier estimates of pEFS for patients with CC and MC (pEFS, log-rank test, P < .0001; Figure 2A). CC patients: n = 51; censored, n = 9. MC patients: n = 20; censored, n = 14. Kaplan-Meier analyses of pEFS of immunotherapy-treated MC patients and patients with MC who did not receive immunotherapy (pEFS, log-rank test, P = .0009; Figure 2B). MC patients with immunotherapy: n = 13; censored, n = 7, pEFS = 0.46, 95% CI = 0.19-0.70. MC patients without immunotherapy: n = 7; censored, n = 7, pEFS = 0.00.
Six of 13 patients with MC (46%) who received preemptive immunotherapy remained in CR. In 6 patients, CC was restored; however, despite continuing with DLIs, 4 of these patients developed MC again, relapsed, and died. One of these MC patients not in remission (NR) at day +31 after the first transplantation received a second transplantation (Table 2). Another MC patient did not respond to immunotherapy and died of progressive disease (Table 2).
In 4 of 13 MC patients who were treated by immunotherapy, detection of MC in CD33+ and CD34+ BM subsets was documented before identification of MC in the PB or the whole BM (mean, 32 days; range, 7-74 days; Table 2). Three of these patients relapsed and died, and 1 patient returned to complete donor chimerism and remained in CR.
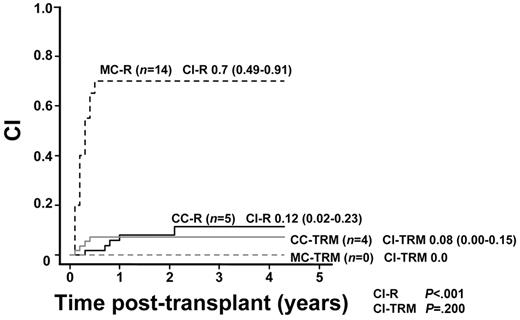
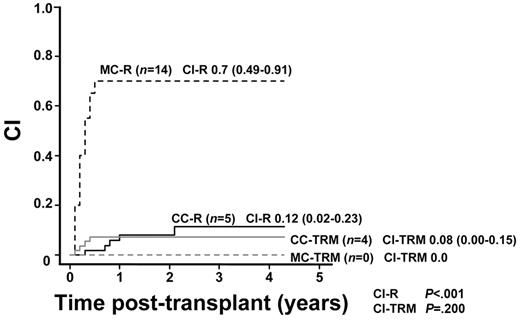
Of 71 patients, 19 showed hematologic relapse after transplantation (CI-R, 28%; 95% CI = 17%-40%). There was a significant difference in the CI-R between patients with CC (5 of 51 CI-R, 12%; 95% CI = 2%-23%) and those with MC (14 of 20 CI-R, 70%; 95% CI = 49%-91%; CI-R, Gray, P < .001; Figure 3).
CI-R in patients with MC (MC-R) or CC (CC-R) and CI-TRM in patients with MC (MC-TRM) or CC (CC-TRM). Cumulative incidences of relapse in patients with MC and in patients with CC were 0.70 (95% CI = 0.49-0.91) and 0.12 (95% CI = 0.02-0.23), respectively (CI-R, Gray, P < .001). Cumulative incidences of TRM in MC patients and in CC patients were 0.00 and 0.08, respectively (95% CI = 0.00-0.15; CI-TRM, Gray, P = .200).
CI-R in patients with MC (MC-R) or CC (CC-R) and CI-TRM in patients with MC (MC-TRM) or CC (CC-TRM). Cumulative incidences of relapse in patients with MC and in patients with CC were 0.70 (95% CI = 0.49-0.91) and 0.12 (95% CI = 0.02-0.23), respectively (CI-R, Gray, P < .001). Cumulative incidences of TRM in MC patients and in CC patients were 0.00 and 0.08, respectively (95% CI = 0.00-0.15; CI-TRM, Gray, P = .200).
Clinical relapse was not predicted based on chimerism monitoring in 5 patients, and relapse occurred at days +97, +251, +305, +371, and +772, respectively. Two of these relapsed patients could be rescued by additional allo-SCT and combined chemotherapy with intermittent DLIs. In 14 of 19 (74%) relapsed patients, MC was detected before relapse. In 9 of these patients, clinical relapse occurred before day +100 (range, days +19 to +93). Five patients relapsed beyond day +100 (range, days +120 to +180).
Outcome of patients in remission at the time of transplantation according to chimerism status
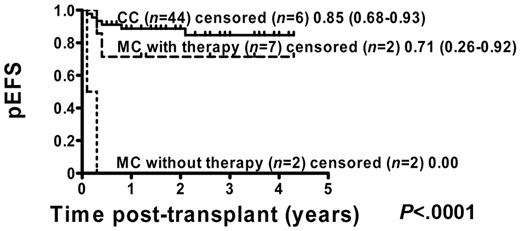
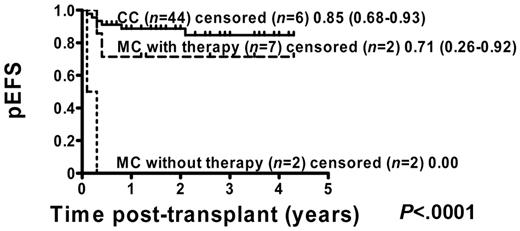
Of 71 patients, 54 (76%) were in remission before transplantation: 7 of 13 (54%) MC patients with intervention, 2 of 7 (29%) MC patients without intervention, and 45 of 51 (88%) CC patients. Kaplan-Meier analysis of these patients showed that pEFS was improved in CC patients from 80% (95% CI = 64%-88%) to 85% (95% CI = 68%-93%) compared with the total group of CC patients (Figure 4). The main improvement occurred in MC patients with immunotherapy, in whom pEFS increased from 46% (95% CI = 19%-70%) to 71% (95% CI = 26%-92%) compared with the total cohort of MC patients (Figure 4).
pEFS according to chimerism status including all patients who were in remission at the time of transplantation. Kaplan-Meier estimates of pEFS for patients in remission before transplantation who developed CC or MC and did or did not receive immunotherapy (pEFS, log-rank test, P < .0001). CC patients: n = 44; censored, n = 6, pEFS = 0.85, 95% CI = 0.68-0.93. MC patients with immunotherapy: n = 7; censored, n = 2, pEFS = 0.71, 95% CI = 0.26-0.92. MC patients without immunotherapy: n = 9; censored, n = 9, pEFS = 0.00.
pEFS according to chimerism status including all patients who were in remission at the time of transplantation. Kaplan-Meier estimates of pEFS for patients in remission before transplantation who developed CC or MC and did or did not receive immunotherapy (pEFS, log-rank test, P < .0001). CC patients: n = 44; censored, n = 6, pEFS = 0.85, 95% CI = 0.68-0.93. MC patients with immunotherapy: n = 7; censored, n = 2, pEFS = 0.71, 95% CI = 0.26-0.92. MC patients without immunotherapy: n = 9; censored, n = 9, pEFS = 0.00.
State of remission, donor source, and conditioning regimen are associated with outcome
In univariate analysis, the state of remission before transplantation was associated with the outcome. The pEFS estimates were 79% ± 10% for patients in the first CR (CR1), 81% ± 7% for patients in ≥ second CR (≥ CR2), and 21% ± 10% for patients who were NR before transplantation (log-rank test, P < .001, Table 3). Furthermore, 65% of patients who were NR before transplantation developed MC, compared with 26% of patients receiving transplantation in CR1 and 10% of patients in ≥ CR2 (Fisher exact test, P < .001, Table 3). In addition, pEFS estimates were 61% ± 10% and 91% ± 6% for patients with an MUD and an MSD and 40% ± 12% for patients with an MMFD (log-rank test, P = .004, Table 3). The pEFS was also affected by myeloablative (77% ± 7%) and RIC (43% ± 11%) regimens (log-rank test, P = .003). In contrast, the outcome was not affected by age, sex, stem-cell source, T-cell depletion, or aGVHD (Table 3).
A multivariate Cox proportional hazards analysis for pEFS was fitted to include the covariate remission status before transplantation, type of donor, graft source, T-cell depletion, myeloablative or RIC regimen, and status of chimerism. In this analysis, RIC and MC emerged as independent poor prognostic factors (RIC, P = .003, risk ratio, 3.78, 95% CI = 1.58-9.04, and MC, P < .001, risk ratio, 7.11, 95% CI = 2.93-17.24). The patients who received RIC allo-SCT for AML and those with MC were at risk of suffering an adverse subsequent event (relapse or TRM). RIC and MC were not correlated (P = .217), as determined by a 2-sided Fisher exact test; however, RIC and NR at the time of transplantation were associated (P = .004). It is therefore likely that NR at the time of transplantation influences the outcome rather than the RIC (odds ratio, RIC, 4.37 and NR, 14.3). Covariates of multivariate analysis not influencing pEFS included the following: type of donor (MUD, MSD, or MMFD; P = .543), graft source (BM, PBSCs, other); P = .230), T-cell depletion (no, yes; P = .124), and remission (CR1, CR2, > CR2, NR; P = .339).
Toxicity and GVHD
In total, aGVHD occurred in 53 of 71 (75%) patients after transplantation, and 13 of 53 (25%) aGVHD patients developed chronic GVHD. Most of the CC patients showed signs of aGVHD grade I-II (29 of 51, 57%), whereas severe aGVHD grade III-IV occurred in 8 of 51 (16%) CC patients. Severe aGVHD was highest in the MC patients (6 of 20, 30%); however, most of the MC patients developed aGVHD grade I-II (11 of 20, 55%).
In the intervention group, 11 of 13 (85%) MC patients developed aGVHD and 2 of 13 (15%) showed signs of chronic GVHD. Seven (54%) patients showed mild signs of aGVHD (grade I-II), 1 (8%) patient developed grade III aGVHD, and 3 patients (23%) developed grade IV aGVHD. All grade IV aGVHD patients relapsed and died. Two patients did not develop aGVHD after immunotherapy.
The TRM rate was 6% (4 of 71). CI-TRM was 6% (95% CI = 0%-11%) for study patients, 8% (4 of 51; 95% CI = 0%-15%) in CC patients, and 0% (0 of 20; CI-TRM, Gray, P = .200, Figure 3) in MC patients.
Discussion
The study presented here was conducted to investigate whether frank hematologic relapse in children receiving transplantations for AML can be prevented in defined patients by increasing the sensitivity for detection of impending relapse and by intensifying preemptive immunotherapy. The study design recommended immune intervention with increased T-cell doses in an early stage of relapse as indicated by MC in the PB and the BM, including the CD33+ and CD34+ BM subsets.
The effectiveness of immunotherapy has been demonstrated in high-risk AML patients relapsing after transplantation.19-22 In contrast to patients with chronic myeloid leukemia, in whom moderate doses of DLI were found to be effective, patients with AML required higher doses to achieve an antileukemic response6,23 ; therefore, immunotherapy seems to be more effective in stages of impending, rather than frank, hematologic relapse.6,23 Consequently, an early assessment of high-risk patients appears to be beneficial for successful immunotherapy after transplantation, allowing lower DLI doses than necessary to treat frank relapse.22
Because we previously demonstrated that the risk of inducing severe GVHD through preemptive immunotherapy in patients with MC was low,3 we decided to increase the starting dose of DLI by 10-fold in patients with an MSD and by 2-fold in patients with an MUD. In addition, immunotherapy was also offered to patients with MC in either the CD34+ or CD33+ BM-cell subsets, suggesting that MC in these compartments might reflect impending leukemic relapse rather than stromal cell elements and that MC in these BM compartments may indicate impending relapse earlier than MC in the whole BM. It has been shown that adult patients with increasing autologous signals in sorted CD34+ peripheral blood cells faced a significantly increased CI-R.24,25
In this study, the pEFS of patients with MC was 46% when our strategy of preemptive aggressive immunotherapy was applied. All patients with MC who did not receive immunotherapy ultimately relapsed and died. Because patients either recovered with leukemic blasts, developed aGVHD despite detection of MC, or were critically ill, this recommended preemptive immunotherapy approach could not be offered to all MC patients, thereby potentially biasing the differences in pEFS between MC patients with and without immunotherapy.
A high percentage of patients in the study cohort (17 of 71, 24%) were bad responders to induction and conditioning and therefore were NR at the time of transplantation. Most of these patients (11 of 17, 65%) developed MC, but could not be rescued by applying our study protocol. It is very likely that these patients would have relapsed irrespective of the immunotherapy approach; therefore, these patients with more aggressive disease may not benefit from our preemptive immunotherapy approach. However, our results might indicate a beneficial effect of immunotherapy in MC patients who were in remission before transplantation, because pEFS increased to 71% (95% CI = 26%-92%) in these patients. Although the small number of patients in this cohort precludes definitive conclusions, this may underline the value of our study design in MC patients with CR before transplantation.
In addition to the heterogeneity regarding disease status before transplantation and the limited number of patients in the intervention group, the assessment of responses to immunotherapy in our study was also limited because of multiple other confounding variables, such as donor type and conditioning regimens. However, such limitations may be inherent in any multicenter phase 2 trial, which is inevitable to study immunotherapy approaches in children with rare diseases.
Fatal, severe aGVHD may result from intensified immunotherapy approaches, especially in patients who receive higher DLI cell doses. The rate of aGVHD was relatively high in our study cohort (CC patients, 37 of 51, 73%; MC patients, 16 of 20, 80%). Most of the CC patients (29 of 51, 57%) showed signs of aGVHD grade I-II, whereas aGVHD grade IV was highest in MC patients (6 of 20, 30%). In our study, 4 of 13 (31%) patients developed aGVHD grade III-IV after preemptive immunotherapy, compared with 10 of 58 (17%) patients without intervention. However, the rate of aGVHD grade III-IV in our cohort was similar to that reported previously for patients receiving transplantations for AML who did not receive immunotherapy.26-28 All patients with grade III-IV aGVHD in the intervention group died from relapse, suggesting that this study design might not increase nonrelapse mortality and that severe aGVHD-required treatment might have aborted any GVL effect. TRM was unexpected low in this study, possibly because of bias resulting from no patients being enrolled before day +21.
In this study cohort, prediction of relapse by additional chimerism analyses of BM samples, including analyses of CD33+ and CD34+ BM subpopulations, improved compared with our former study,3 but impending relapse was not detected by MC in 5 patients. Remarkably, the majority of these undetected relapses occurred beyond day +200, after which time chimerism was monitored at longer intervals. It is therefore important to realize that chimerism development is a dynamic process and may change later in the course of follow-up. It would be desirable to continue to closely monitor beyond day +200 after transplantation; however, practical and psychosocial reasons might interfere with these attempts. Additional analysis of minimal residual disease (MRD) using methods for direct detection of the malignant clone could also improve the prediction of relapse in AML patients. Although this is not yet possible for all AML patients, new evolving techniques and methods may help to overcome this limitation.29-32 For example, analysis of the transcription factor Wilms' tumor 1 (WT1) gene, which is expressed in the majority of pediatric acute leukemia, has been evaluated as a marker for MRD detection in AML33-38 ; however, the predictive value of WT1 overexpression in monitoring MRD after allo-SCT has not yet been proven.39
Multivariate analysis indicated that RIC was an independent poor prognostic factor in this study cohort; however, in our analysis, most of the patients who were NR at the time of transplantation (13 of 17, 76%) received RIC. In contrast, patients with RIC and CR before transplantation had a good prognosis (pEFS, 82%; 95% CI = 41%-95%) compared with patients in NR at the time of transplantation (pEFS, 10%; 95% CI = 1%-36%; data not shown), indicating that RIC is not a negative predictive factor and that patients in NR before transplantation who received RIC were at the highest risk of relapse. Chimerism analysis was also suitable to guide preemptive treatment of these patients; therefore, other approaches are needed in these patients to improve the outcome. Such approaches could include adoptive immunotherapy at regular and fixed intervals or administration of natural killer cells.40-43 An important therapeutic option was also reported by Schmid et al, who recommended prophylactic DLIs.44 Furthermore, to improve outcome, one could consider a salvage treatment for relapse according to Pollyea et al.45
In conclusion, we show that serial monitoring of chimerism allows identification of patients with MC who are at the highest risk of subsequently relapsing. In these patients, intensified preemptive immunotherapy can be administered safely and successfully on the basis of MC, which may result in improved survival of children with AML after allo-SCT.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the data-monitoring committee of Claudia Rossig (Muenster, Germany), Christian Thiede (Dresden, Germany), and Arjan C. Lankester (Leiden, The Netherlands) for their accurate and critical evaluation and assessment of primary laboratory and clinical data and for their final approval.
This work was supported by grants from the Deutsche Krebshilfe (50-2737). E.R., T.K., and P.B. were also supported by the LOEWE Center for Cell and Gene Therapy Frankfurt/Main funded by Hessisches Ministerium für Wissenschaft und Kunst (III L 4-518/17.004, 2010).
Authorship
Contribution: E.R., A.M.W., H.K., and T.K. collected and analyzed the data and wrote the manuscript; A.B., W.H., B.K., B.S., W.W., C.M.-K., B.G., S.B., M.H.A., and P.-G.S. enrolled the patients, analyzed the data, and wrote the manuscript; and P.B. directed the research, analyzed and interpreted the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Prof Dr Peter Bader, Goethe-University Frankfurt/Main, Division for Stem Cell Transplantation, Hospital for Children and Adolescents II/III, Theodor-Stern-Kai 7, 60590 Frankfurt/Main, Germany; e-mail: peter.bader@kgu.de.