Abstract
T315I+ Philadelphia chromosome–positive leukemias are inherently resistant to all licensed tyrosine kinase inhibitors, and therapeutic options remain limited. We report the outcome of allogeneic stem cell transplantation in 64 patients with documented BCR-ABLT315I mutations. Median follow-up was 52 months from mutation detection and 26 months from transplantation. At transplantation, 51.5% of patients with chronic myeloid leukemia were in the chronic phase and 4.5% were in advanced phases. Median overall survival after transplantation was 10.3 months (range 5.7 months to not reached [ie, still alive]) for those with chronic myeloid leukemia in the blast phase and 7.4 months (range 1.4 months to not reached [ie, still alive]) for those with Philadelphia chromosome–positive acute lymphoblastic leukemia but has not yet been reached for those in the chronic and accelerated phases of chronic myeloid leukemia. The occurrence of chronic GVHD had a positive impact on overall survival (P = .047). Transplant-related mortality rates were low. Multivariate analysis identified only blast phase at transplantation (hazard ratio 3.68, P = .0011) and unrelated stem cell donor (hazard ratio 2.98, P = .011) as unfavorable factors. We conclude that allogeneic stem cell transplantation represents a valuable therapeutic tool for eligible patients with BCR-ABLT315I mutation, a tool that may or may not be replaced by third-generation tyrosine kinase inhibitors.
Introduction
The BCR-ABL T315I mutation confers in vitro resistance to all tyrosine kinase inhibitors (TKIs) approved for the treatment of chronic myelogenous leukemia (CML) and Philadelphia chromosome–positive (Ph+) acute lymphoblastic leukemia (ALL) to date.1 The survival of patients harboring a T315I mutation discovered by any available methodology, whether associated with other factors or not, is dependent on disease phase at the time of mutation detection,2 and prognosis remains particularly poor. However, allogeneic stem cell transplantation (SCT) presents a therapeutic alternative for several TKI-resistant patients.3-5 In the present study, we analyzed a series of 64 Ph+ leukemic patients (CML in all phases and Ph+ ALL patients) harboring a T315I BCR-ABL mutation who underwent allogeneic SCT to evaluate the impact of this procedure on survival.
Methods
Study population
Adult patients with CML and de novo Ph+ ALL whose disease was resistant to TKI according to the European LeukemiaNet guidelines6,7 or IRIS study (International Randomized Study of Interferon and ST1571) definitions8 and who harbored a T315I BCR-ABL mutation detected by any validated means between 1999 and 2010 were included in the analysis. Patients were identified from the European Blood and Marrow Transplantation (EBMT) registry and from a previously described updated international database that contained 222 T315I+ patients.2 They, or their legal representative, had provided written consent whenever possible. This retrospective analysis was approved by the institutional review board/ethics review committee in each participating site/country whenever necessary.
Data collection
Demographic, clinical, treatment, mutation, transplant, and survival data were collected and previously collected data were updated from each site from the EBMT registry and from the epidemiologic study database. Final data were combined in an ultimate database for analysis. The T315I mutation was detected by different techniques (predominantly direct sequencing, but also PCR–restriction fragment length polymorphisms and denaturing HPLC), including assaying banked material. Unfortunately, posttransplantation BCR-ABL data and cytogenetic and chimerism analyses were not available for the large majority of patients in this retrospective international study, and consequently, these data will not be presented.
Survival measurement
Overall survival (OS) was analyzed since diagnosis, since T315I detection, and since transplantation and was stratified according to disease phase. Progression-free survival could not be analyzed precisely because of missing data and is therefore not reported.
Statistical analysis
Survival was analyzed according to the Kaplan-Meier method and by log-rank tests for CML at different phases and for Ph+ ALL from the dates of T315I BCR-ABL mutation detection and transplantation. Multivariate analysis was performed with a Cox proportional hazard model adjusted for OS. Covariates included time from mutation detection to SCT, status at transplantation (chronic phase [CP], accelerated phase [AP], blast phase [BP], or Ph+ ALL), source of stem cells (peripheral blood stem cells versus BM), donor type (unrelated versus related), and reduced-intensity conditioning. P < .05 was considered significant.
Results and discussion
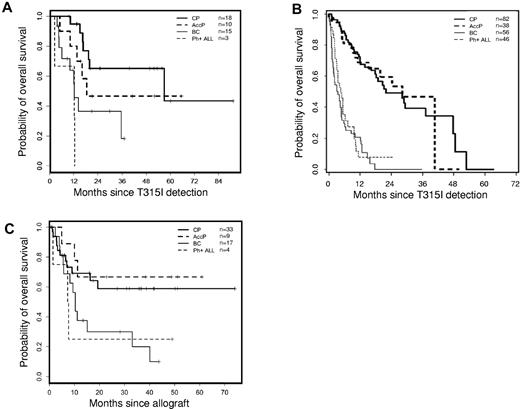
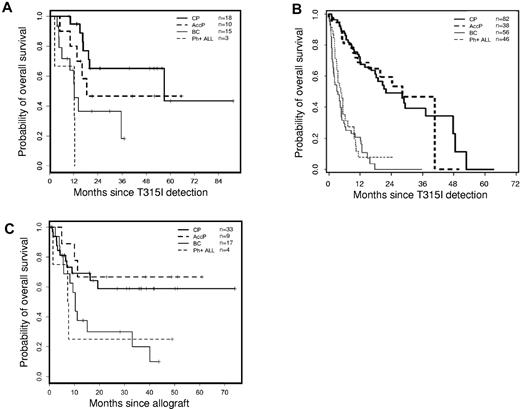
The 64 patients (who received 67 transplants) who harbored a T315I BCR-ABL mutation and who underwent transplantation were younger (median age 43 years) and had a relatively shorter disease history (median 36 months) before transplantation (Table 1) than those in the cohort of patients with T315I mutations from the previously published epidemiologic study (median age 54 years).2 The majority of patients were males (74%). Twenty-three percent of the patients were in BP at the time of detection of the T315I mutation, and 26.5% of these individuals remained in this phase at the start of conditioning. More than half of the patients were transplanted while in the CP (either first diagnosed in CP or when they returned to this phase after diverse treatments). The proportion of Ph+ ALL patients to CML patients was comparable at diagnosis, mutation detection, and transplantation. A significant proportion of patients in advanced phases at the time of T315I discovery returned to a second CP before transplantation (Table 1). At the time of transplantation, 37.5% of patients were in BP, and 6% presented with Ph+ ALL. The median interval between diagnosis and imatinib initiation was short (4 months; range 0-823 months) in contrast to earlier reports (12-20 months2,5,7 ) because of the presence of more patients (58%) receiving imatinib as first-line therapy in the present patient cohort. The median time from diagnosis and imatinib initiation to T315I detection was 51.6 and 37.8 months, respectively. This differed from what was observed in a previously described population of T315I patients2 containing a majority of nontransplanted patients as opposed to a minority of transplanted patients. The median time from T315I BCR-ABL mutation detection to SCT was 16 months. T315I status was not necessarily confirmed before transplantation. Sixty-one percent of the patients were transplanted with HLA-compatible unrelated donors, which may explain the relatively long gap between mutation detection and transplantation. The majority of the patients (51%) underwent transplantation with G-CSF mobilized peripheral blood stem cells as the source of cells (51%), and a myeloablative conditioning regimen was used in most cases (60%) in accordance with the young age of the patients in this population. The median follow-up from transplantation was 26 months (range 1.8-154.5 months). Three patients (4.5%) received > 1 transplant (2 patients received 2 SCTs, and 1 patient received 3 SCTs) and subsequently died of progressive disease. The median time to neutrophil engraftment was 17 days. Forty-three of the evaluable patients (71.7%) developed acute GVHD, which was grade I-II in 32 (53.3%) of 60 patients and grade III-IV in 11 (18.3%) of 60 patients. Twenty-four (48%) of the 50 evaluable patients developed chronic GVHD (12 limited, 12 extensive), and the occurrence of any chronic GVHD conferred a significant favorable impact on OS (log-rank P = .047). Only 5 (8%) of the evaluable patients received donor lymphocyte infusions, with 3 living and 2 deceased patients at latest follow-up. At latest follow-up, negative residual disease and full donor chimerism analyses could only be documented in 12 patients. Transplant-related mortality rates at 3 and 12 months for patients in CP were 9.1% and 18.2%, respectively, and for AP, they were 0% and 11.1%, respectively (Table 1). Survival probabilities 24 months after SCT were 59%, 67%, 30%, and 25% for CP, AP, BP, and Ph+ ALL, respectively (Figure 1A). These rates appear to be better than expected in the overall cohort of T315I patients,2 shown for comparison purposes in Figure 1B. The multivariate analysis did not identify any significant factors that adversely affected OS in T315I+ transplanted patients, with the exception of those patients who remained in BP at the time of SCT (hazard ratio 3.68, 95% confidence interval 1.34-10.09, P = .00011) and those who received transplants from an unrelated donor (hazard ratio 2.98, 95% confidence interval 1.28-6.93, P = .011). The interval between T315I detection and transplantation, the type of conditioning regimen used (reduced-intensity conditioning versus myeloablative), and the source of cells (peripheral blood stem cells versus BM) had no influence on OS.
Overall survival for patients harboring T315I mutated leukemias. (A) OS analysis for patients with CML in CP (bold solid line), AP (bold dashed line), or blast crisis (thin solid line) or with de novo Ph+ ALL (thin dashed line) since T315I ABL mutation detection who underwent allogeneic SCT. (B) OS analysis for patients with CML in CP (bold solid line), AP (AccP; bold dashed line), or blast crisis (BC; thin solid line) or with de novo Ph+ ALL (thin dashed line) since T315I ABL mutation detection in the overall cohort of patients with T315I mutation (Figure 2D in Nicolini et al2 ) compared with panel A. (C) OS analysis for patients with CML in CP (bold solid line), AP (bold dashed line), or blast crisis (thin solid line) or with de novo Ph+ ALL (thin dashed line) since allogeneic SCT according to disease status at transplantation. Median OS has not been reached for CP and AP and was 10.3 months for BP (range 5.7 months to not reached) and 7.4 months for Ph+ ALL (range 1.4 months to not reached).
Overall survival for patients harboring T315I mutated leukemias. (A) OS analysis for patients with CML in CP (bold solid line), AP (bold dashed line), or blast crisis (thin solid line) or with de novo Ph+ ALL (thin dashed line) since T315I ABL mutation detection who underwent allogeneic SCT. (B) OS analysis for patients with CML in CP (bold solid line), AP (AccP; bold dashed line), or blast crisis (BC; thin solid line) or with de novo Ph+ ALL (thin dashed line) since T315I ABL mutation detection in the overall cohort of patients with T315I mutation (Figure 2D in Nicolini et al2 ) compared with panel A. (C) OS analysis for patients with CML in CP (bold solid line), AP (bold dashed line), or blast crisis (thin solid line) or with de novo Ph+ ALL (thin dashed line) since allogeneic SCT according to disease status at transplantation. Median OS has not been reached for CP and AP and was 10.3 months for BP (range 5.7 months to not reached) and 7.4 months for Ph+ ALL (range 1.4 months to not reached).
The prognosis of T315I BCR-ABL+ leukemias in vivo has been uncertain despite in vitro evidence of the lack of efficacy of all TKIs, with both pessimistic9-11 and more optimistic12,13 studies. A recent larger worldwide epidemiologic study has demonstrated the severity and the heterogeneity of this disease.2 The therapeutic options for such patients remain few and are confined to untargeted therapies such as hydroxyurea, interferon-α,14 or omacetaxine mepesuccinate (formerly homoharringtonine),14-17 which provide only short-term and mostly hematologic responses with poor cytogenetic efficacy.17 Recently, a phase 1 trial with a third-generation TKI, ponatinib, provided very promising data.18 Whenever possible, allogeneic SCT has been proposed to eligible patients by physicians worldwide. Despite the limitations inherent in an observational study (eg, missing data, retrospective analysis), we have been able to provide some evidence that to date, allogeneic SCT appears to be the best treatment for T315I+ leukemias, providing acceptable OS rates and in some cases long-term control of the malignancy without detectable residual disease.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge the transplant teams in charge of these patients worldwide that have contributed to generate the data analyzed here. F.E.N., S.H., and M.M. thank Elodie Gadolet and Madeleine Etienne (CRAs, Unité de recherché clinique, Hematology Department, Hôpital Edouard Herriot, Lyon France, and Fi-LMC group) for their expertise and help in data collection and Mohamad Sobh (PharmD, CRA, same location) for editorial assistance. They thank Nicole Raus (data manager of the Société Française de greffe de moelle et de thérapie cellulaire, Bordeaux, France) for her help in transplantation data collection in France and Anja Henseler (Chronic Leukemia Working Party) of the EBMT, Leiden, The Netherlands, for monitoring and collecting some of the European data from the EBMT registry. They are grateful to Barbara Meunier-White for her kind and prompt editorial assistance. Finally, last but not least, the authors thank all the CML and Ph+ ALL patients for participating in this observational study.
J.F.A. is grateful for support from the NIHR Biomedical Research Center funding scheme. The authors thank the European LeukemiaNet (ELN, Mannheim, Germany) for supporting standardization methods for mutation screens and BCR-ABL quantification and some mutation data captures in European countries for the patients included in this study.
Authorship
Contribution: F.E.N. designed the research, collected data, performed the analysis, and wrote the paper; G.W.B. designed the research within the EBMT Chronic Leukemia Working Party; S.S. and G.M. supervised data collection from Italy; M.J.M., M.C.M., H.L., S.H., and G.E. collected data for analysis; A.H. collected the data, supervised data collection from Germany, and contributed to the writing of the manuscript and the interpretation of the data; C.C. collected data from Singapore; I.H.D. collected data from Denmark; G.R.-C. and G.S. collected data from Turin for analysis; M.M. collected data and contributed to data interpretation; S.M. performed the statistical analysis; E.O. supervised the data collection from the EBMT registry; W.Z. designed the research and contributed to the analysis; S.P. contributed to the design of the research and to the data analysis; J.F.A. supervised data collection from United Kingdom for analysis and contributed to the writing of the manuscript and to the interpretation of the data; and J.C. codesigned the research, supervised data collection, and proofread the paper. All the contributors proofread the paper and agree with the data presented.
Conflict-of-interest disclosure: A.H. received support from Novartis, Bristol-Myers Squibb, Ariad, Pfizer, and Merck, and S.P. and W.Z. are employees of Merck. The remaining authors declare no competing financial interests.
Correspondence: Franck E. Nicolini, MD, PhD, Hematology Department, Hôpital Edouard Herriot, 5, place d'Arsonval, 69437 Lyon, Cédex 03, France; e-mail: franck-emmanuel.nicolini@chu-lyon.fr.