Abstract
Abstract 1095
Beta-thalassemia is the most common monogenic diseases world-wide the pathophysiology of which results from beta-globin gene mutations which lead to ineffective erythropoiesis (IE) and anemia and manifest as transfusion-dependence in patients with beta-thalassemia major (TM) or the need for intermittent transfusions in patients with beta-thalassemia intermedia (TI). Many affected patients live in developing countries. As infrastructure improves – in the areas of sanitation, nutrition, and public health care – patients in these countries will live longer and pose an increasing global health problem unless easily implemented inexpensive therapies become available. No such therapies exist currently, with transfusion and chelation therapy still reaching a select group of affected individuals. A more complete understanding of the relationship between erythropoiesis and aberrant iron metabolism in this disease is central to the development of novel therapies. We previously demonstrated that apo-transferrin (Tf) treated beta-thalassemic (th1/th1) mice, a mouse model of TI, demonstrate an increase in hemoglobin (Hb).
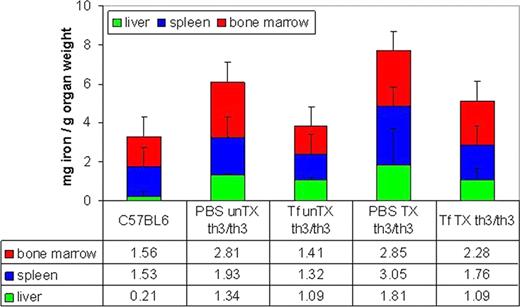
To test whether this approach could lead to amelioration of IE, extramedullary erythropoiesis (EMH), and organ iron overload also in mice affected by TM, fetal liver cells (E14 days) from th3/th3 embryos were injected into sub-lethally irradiated C57 BL/6J mice. Following transplant, mice were treated with 10 mg Tf (200 uL) or PBS IP. Because this mouse model is transfusion dependent, we hypothesized that Tf injections would increase Hb in th3/th3 mice receiving weekly transfusions (2–4 mice per group). Untransfused mice were also evaluated to determine if transfusion dependence can be mitigated using Tf injection. After 20 days of injections, end point evaluation included RBC parameters, alpha-globin precipitation on RBC membranes, erythroid precursor differentiation in the bone marrow, hepcidin expression, organ non-heme iron distribution, and liver EMH.
Our findings demonstrate that exogenous Tf decreases iron burden and EMH, results in a higher proportion of mature erythroid precursors in the bone marrow, and leads a larger increase in Hb following transfusion in Tf-treated th3/th3 mice. Taken together, these results support the development and use of Tf in patients with widely different clinical severities of beta-thalassemia syndromes, including those with TM.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.