Abstract
Abstract 1512
The introduction of tyrosine Kinase Inhibitors (TKI) has significantly improved the outcome of patients (pts) with Ph+ ALL. Dasatinib (Db) is a second generation dual SRC/ABL TKI with greater potency compared to Imatinib in inhibiting BCR/ABL.
To determine the outcome of pts with Ph+ ALL treated with hCVAD + Db.
Between 9/06 and 7/09, pts with newly diagnosed Ph+ ALL received Db 50mg oral (PO) twice daily (BID) or 100mg PO daily for the first 14 days of each of 8 cycles of alternating hCVAD, and high dose cytarabine and methotrexate. Pts in complete remission (CR) continued to receive maintenance Db 50mg PO BID or 100mg PO daily, as well as monthly prednisone and vincristine for 2 years, followed by Db indefinitely. From 8/09 protocol was amended and pts received 100mg Db for the first 14 days of cycle #1 and then 70mg daily continuously for the next 7 cycles, as well as 2 doses of rituximab 375 mg/m2 during each of the first 4 cycles. Maintenance was with Db, vincristine and prednisone.
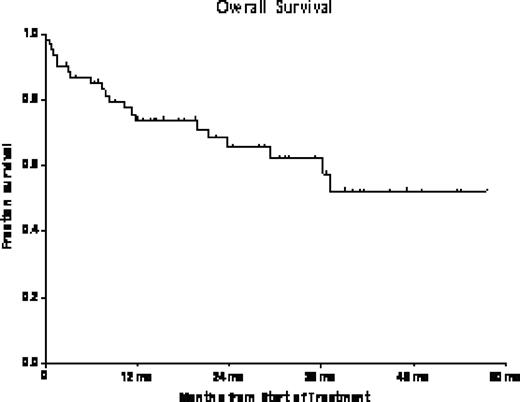
Sixty-one pts with newly diagnosed Ph+ ALL have been treated to date. Median age was 56 years (yrs) (range (r), 22–80) and 41 (67%) pts were >50 yrs. The median follow up is 26.1 months (mo) (r, 4–58). Central nervous system (CNS) involvement was noted in 9 (14%) pts at diagnosis. Sixteen (26%) pts had Ph+ alone, 38 (62%) pts had Ph+ with additional abnormalities, and 7 (12%) pts were Ph negative, and BCR/ABL positive. Median white blood cell count (WBC) at diagnosis was 13.4 × 109/L (r: 0.4–658), and 22 (36%) pts had WBC >30 × 109/L at diagnosis. BCR/ABL transcript was identified in 60 (98.3%) pts at diagnosis, including e1a2 in 46 (76%) pts, b2a2 in 10 (17%) pts, b2a2+b3a2 in 2 (3%) pts, b3a2 and e1a3 in 1 (1.6%) pt each. One pt had a variant transcript that was not detectable with the standard primers. The median number of induction and maintenance cycles received were 6 cycles (r: 1–8) and 13.5 cycles (r: 1–24), respectively. Fifty seven (94%) pts achieved CR1 and 1 (1.5%) pts achieved CR with incomplete platelet recovery with first induction cycle of chemotherapy. Three (4.5%) pts died before response assessment could be performed due to infections. Thirty-nine (64%) pts received maintenance, 3 (5%) pts are currently receiving induction and 19 (31%) pts had no maintenance [9 pts received allogeneic stem cell transplant (ASCT) prior to maintenance, 10 pts had progression of disease]. To date, twelve (19%) pts have relapsed and Abl kinase domain mutations were analyzed in 7 pts; mutations were noted in 4 pts. These included T315I in 2 pts, and F359V and V299L in 1 pt each. CNS relapse occurred in 5 pts. Salvage (S1) regimens included [hCVAD + another TKI in 7 pts, single agent TKI in 2 pts, single agent monoclonal antibody in 1 pt, methotrexate, vincristine, asparginase, dexamethasone (MOAD) in 1pt, intrathecal cytarabine/methotrexate plus CNS radiation and Db in 1 pt]. Eight pts achieved CR2, 3 pts were refractory (2 pts with T315I and 1 pt with F359V) and one is still undergoing salvage treatment. Median DFS and OS after S1 were 5.3 mo (r: 0.7–17.3) and 6.7 mo (r: 0.6–24.4), respectively. ASCT was performed in 15 (24%) pts, including 10 pts in CR1 and 5 pts in CR2. Donors were related in 8 (53%) and unrelated in 7 (47%) transplants. Sixteen pts have died 11 (68%) pts from infectious complications, 2 (13%) pts from multi-organ failure, 1 (6%) pt with graft versus host disease, and 2 (13%) pts from unknown causes. Three-year disease free survival (DFS) and overall survival (OS) (n=61) were 49% and 62%, respectively.
Db plus hCVAD is an effective regimen with durable responses in pts with newly diagnosed Ph+ ALL.
Kantarjian:BMS: Research Funding. Jabbour:Pfizer: Honoraria; BMS: Honoraria; Novartis: Honoraria. Cortes:Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol-Myers Squibb: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees, Research Funding; Ariad: Membership on an entity's Board of Directors or advisory committees, Research Funding; Chemgenex: Membership on an entity's Board of Directors or advisory committees, Research Funding. Ravandi:Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.