Abstract
Abstract 288
Chronic lymphocytic leukemia (CLL) patients with mutated IGHV genes (M-CLL) have better outcomes than patients with unmutated IGHV genes (U-CLL). It has been proposed that this difference reflects the fact that IGHV mutations alter the structure of the B-cell antigen receptor (BCR) such that it no longer binds stimulatory (auto) antigens and therefore cannot deliver trophic signals to the leukemic cells. For this theory to be correct, only replacement (R) mutations and in particular non-conservative R mutations that would more likely alter amino structure of the IGHV/D/J rearrangements would have relevance. Silent (S) mutations by definition do not change amino acid structure and could not alter antigen binding. We sought to investigate this hypothesis by analyzing the types (S, conservative R, non-conservative R) and distribution of mutations that occur in IGHVs of M-CLL clones and then comparing the time to first therapy (TTFT) in patients with different IGHV features. This analysis expanded an initial study of 1569 CLL cases in the US to include 1858 patients from Europe for a total of 3427 cases.
Using IGMT software and tools, we analyzed the rearranged IGHV sequences of 3427 cases and characterized their mutations in several respects: first, if IGHV mutations altered amino acid structure (S vs. R); second, if mutations occurred in CDRs (antigen binding domains) or FRs (scaffolds of the BCR); third, if R mutations were conservative or non-conservative as determined by charge, hydropathy, and size. TTFT for patients was examined with various combinations of the above parameters. Differences in TTFT were estimated by the method of Kaplan and Meier and assessed using the log rank test.
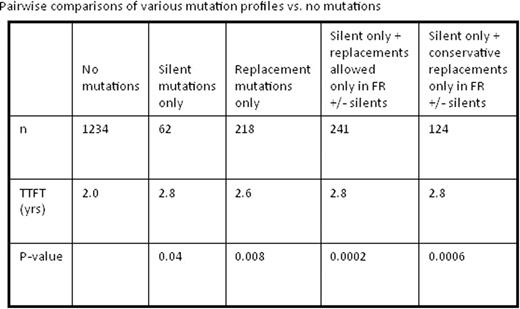
First, TTFT was compared for 4 groups of patients with the following mutation profiles: no mutations; only S mutations (median 1 per sample); only R mutations (median 1 per sample); and mixed S and R mutations (median 16 per sample). These 4 categories were significantly different (P<0.0001), with the median TTFT being 2.0 yrs for no mutations; 2.6 yrs for R only; 2.8 yrs for S only; and 7.3 yrs for mixed. All comparisons used patients with no mutations (n=1234) as the reference group.
We identified statistically longer TTFT (2.8 vs 2.0 yrs; P=0.04) in patients with only S mutations (n=62). Patients with only R mutations (n=218) also had superior outcomes (2.6 yrs vs. 2.0 yrs; P=0.008). There was no statistical significance in TTFT between patients with all S vs. all R mutations (2.8 yrs vs. 2.6 yrs; P=0.71).
Because exploring the relevance of BCR structural change on TTFT required us to focus on a small subset of patients with only silent IGHV mutations, our sample size was small. Therefore, we expanded the group to include IGHVs with R mutations limited to the FR region (S + R in FR only; n=241) since these mutations would be unlikely to have a major impact on BCR structure, particularly sparing the binding site. When compared to patients with no mutations, this group demonstrated a significantly longer TTFT (2.8 yrs vs. 2.0 yrs, P=0.0002). This finding was upheld when not permitting any non-conservative R mutations in this group, leaving only patients with S plus conservative R mutations in FRs only (n=124); TTFT in this case was 2.8 yrs vs. 2.0 yrs, respectively (P=0.0006).
Our findings show that patients with only S mutations have better outcomes than patients with no mutations, suggesting that a change in BCR structure that could lead to a loss of antigen binding is an unlikely reason for improved clinical course. This is further supported by the finding that the combination of only S plus conservative R mutations located solely in FRs, which probably would not result in major BCR structural changes and therefore antigen binding, is associated with a lengthened TTFT. Therefore, we suggest that the currently accepted paradigm to explain the enhanced survival of M-CLL patients needs to be re-evaluated.
Brown:Calistoga: Consultancy, Research Funding; Celgene: Honoraria, Research Funding; Genzyme: Research Funding; GSK: Research Funding. Kipps:Igenica: Equity Ownership, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Research Funding; Abbott Industries: Research Funding; Pharmacyclics: Membership on an entity's Board of Directors or advisory committees; Genentech: Research Funding; GSK: Research Funding; Gilead Sciences: Consultancy, Research Funding; Amgen: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.