Abstract
Abstract 3016 FN2
FN2
Hematopoietic stem cell transplantation from unrelated donors (UD) is an effective treatment for hematologic malignancies, but has been associated with relatively high rates of high non-relapsed mortality (NRM) in older and less physically fit patients. The development of reduced-intensity preparative regimens has allowed the extension of this form of treatment to older high risk patients. We hypothesized that a reduced–intensity preparative regimen with pre-transplant low-dose Alemtuzumab would reduce early NRM from regimen-related toxicity and GVHD respectively following UD transplantation, and the early use of donor lymphocyte infusion (DLI) without withdrawal of immunosuppression would promote complete donor T cell chimerism (DC) and thus improve control of the patient's malignancy. We tested this hypothesis in a prospective phase II study.
Thirty six patients were treated. Patient characteristics: median age, 59 years (range, 42–68 years), PBSC (n=34) or bone marrow (n=2); 10/10 HLA locus matched (n=33) or 9/10 matched (n=3); diagnoses AML= 14, CML= 3, MDS=4, MPS=2, NHL=8 CLL=3, and HD=1; CIBMTR disease risk high [n=7], intermediate [n=15], low [n=14]. The conditioning regimen was fludarabine (40mg/m2/d days -6, -5, -4, -3) and busulfan (16mg/kg or i.v. equivalent) for myeloid malignancies or fludarabine (30mg/m2/d days -5, -4, -3), cyclophosphamide (750mg/m2/d days -5, -4, -3), ± rituximab (days -13, -6, +1, +8) for lymphoid malignancies. Low dose subcutaneous alemtuzumab was administered to all patients at a total dose of 43mg over 3 days (-11, -10, -9). Post-grafting immunosuppression consisted of tacrolimus and methotrexate (5mg/m2 on days 1, 3, and 6).
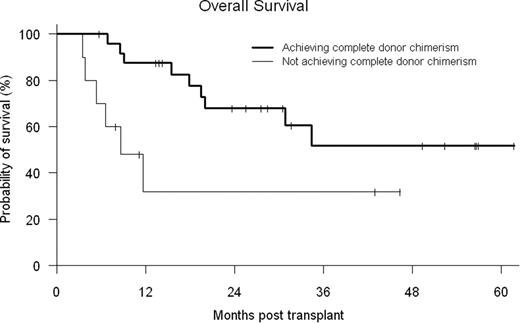
Donor engraftment occurred in 35/36 (97%) patients with median times to neutrophil and platelet recovery of 16 days (10–20) and 16 days (0–42), respectively. One patient expired prior to recovery secondary to a sagittal sinus thrombosis. Early mixed T cell chimerism generally occurred (median day +100 donor CD3 52% (0–100) and CD33 100% (0–100)), requiring donor lymphocyte infusion (DLI) (starting dose 1 × 107 CD3 cells/kg) in 30 of 36 patients (83%, median day 65 [range 36–576]), while 12 received ≥ 2 DLI. Complete DC (defined as ≥ 95% donor cells in peripheral blood CD3+ and CD33+ cells) was achieved at a median of 180 days (range, 33–426 days). Ten patients never achieved completed DC. Maximal cumulative incidence of grades II to IV acute graft-versus-host disease (GVHD) was 42% (14% grade III-IV) and that of chronic GVHD was 59% (12% severe) in patients who survived more than 100 days. Cumulative probability of non-relapse mortality at day 100 and one year was 3% and 14%, respectively. Estimated overall survival at day 100 and one year was 97% and 71%, respectively. CMV reactivation by quantitative PCR was observed in 18 of 24 (75%) at-risk patients. However, no CMV-related disease or mortality was observed. No fungal infections or infectious deaths were seen before day 100. The median hospital length of stay from start of preparative regimen through Day +30 and +100 was 9 days (1–31) and 13 days (1–52), respectively. With a median follow-up of 2.4 years, the probabilities of 2-yr overall (OS) and disease-free survival (DFS) are 57% and 38%, respectively. Patients who achieved complete DC had better OS (log-rank p-value=0.024) (see figure) than patients who failed to achieve this status. In Cox analysis, development of chronic GVHD had a protective effect, associated with decreased relapse (HR 0.150, p=0.089) and improved DFS (HR 0.342, p=0.081).
Low dose alemtuzumab based conditioning and preemptive DLI in order to promote complete donor T cell chimerism, results in low treatment related mortality and favorable survival outcomes in an older patient population with high-risk malignancies undergoing unrelated donor transplantation. The use of pre-emptive DLI while maintaining immunosuppressive therapy appears to control the incidence of severe GVHD and is not associated with an increased risk on infections.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract