Abstract
Abstract 3581
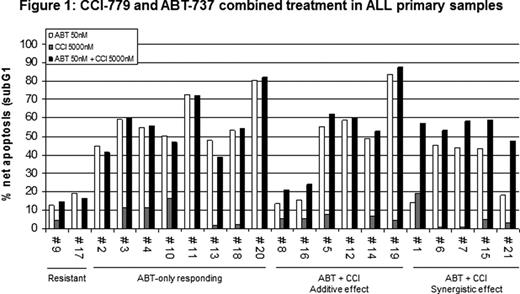
ALL cells are frequently characterized by deregulation of several intracellular signaling pathways involved in the control of proliferation and apoptosis. Among these, the constitutive activation of the mTOR signaling cascades has been frequently reported in the majority of ALL patients and molecular therapies targeting mTOR have been therefore proposed with variable preclinical efficacy, generally inhibiting proliferation in a large number of cell lines and only in some instances leading to apoptosis. To improve pro-apoptotic effects of the mTOR inhibition, especially in ALL cells, a combined Bcl-2 family inhibition approach can be explored, based on the pivotal role of Bcl-2 in lymphoproliferative disease. In addition, Mcl-1 protein levels may be controlled by the AKT/mTOR pathway, since the prosurvival AKT, negatively regulate Gsk-3. In fact, following AKT inactivation, Gsk-3 promotes apoptosis by Mcl-1 phosphorylation and its proteasome degradation. Moreover, Mcl-1 overexpression is a know resistance mechanism to the BH-3 mimetic, the ABT-737. Interestingly prolonged exposure to mTOR inhibitor rapamycin is reported to inactivate the AKT signaling pathway. Aim of this study was to evaluate the combined effects of mTOR (CCI-779) and Bcl-2/Bcl-XL (ABT-737) inhibition in ALL cell lines and primary samples (21). In MOLT-4 cell line exposure to CCI-779, up to 72 hours, induced a flat dose-response curve (35–55% growth inhibition) at concentrations ranging between 1 and 5000 nM and apoptosis induction was not seen until 5000 nM. In CEM-S, CEM-R and JURKAT cell lines, only minor cytostatic effects were observed until 20000 nM. In MOLT-4 cells, ABT-737 induced dose and time-dependent growth inhibition (IC-50= 198nM) followed at higher concentrations (250–500nM) by induction of apoptosis. In contrast, the CEM-S, CEM-R and JURKAT cells, proved resistant (IC-50 >5 μM), displaying Mcl-1 overexpression. We therefore investigated in these resistant cell lines the combined effects of CCI-779+ABT-737. The JURKAT cells showed a significantly higher (p< 0.01) induction of apoptosis following exposure to ABT-737 and CCI-779 (both at 1000nM), as compared to single agents. Similar effects were observed in CEM-R cells exposed to the drug combination (CCI-779 5000nM and ABT-737 1000nM) (p<0.01). In both cell lines, CCI-779 exposure was correlated with down-regulation of the Mcl-1 protein, mediated by proteasome degradation. This effect was associated with AKTS473 de-phosphorylation, due to CCI-779 prolonged exposure. The observed Mcl-1 decrease was, however, not seen in the resistant phenotype CEM-S cell line. Further RNA interference of Mcl-1 did not convert the resistant phenotype. Primary ALL cells exposed to CCI-779 (5000 nM), showed in a minority (4/21) of samples only a weak apoptosis induction (sub-G1 peak< 20%), while a stronger effect (> 40%) was observed in 15/21 treated with ABT-737 (50nM) (Fig.1). The remaining 6/21 samples, proved resistant to ABT-737; in two of these, interestingly, the combined treatment overcame resistance, inducing Mcl-1 down-regulation and apoptosis induction. In summary, the Bcl-2/Bcl-XL (ABT-737) inhibition is an active proapoptotic treatment of ALL, in addition the combined use of mTOR (CCI-779) inhibition may revert ABT-737 resistant phenotypes in a proportion of ALL, via AKT inactivation and Mcl-1 degradation. However, different resistant mechanisms involved in ALL cells (CEM S), prompt further investigation.
Disclosures:
Foà:Bristol-Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees.
Author notes
*
Asterisk with author names denotes non-ASH members.
© 2011 by The American Society of Hematology
2011