Abstract
We report the results of a prospective, randomized phase 3 trial evaluating autologous peripheral blood stem cell transplantation (ASCT) versus intensive consolidation chemotherapy in newly diagnosed AML patients in complete remission (CR1). Patients with AML (16-60 years) in CR1 after 2 cycles of intensive chemotherapy and not eligible for allogeneic SCT were randomized between intensive chemotherapy with etoposide and mitoxantrone or ASCT ater high-dose cyclophosphamide and busulfan. Of patients randomized (chemotherapy, n = 259; ASCT, n = 258), more than 90% received their assigned treatment. The 2 groups were comparable with regard to prognostic factors. The ASCT group showed a markedly reduced relapse rate (58% vs 70%, P = .02) and better relapse-free survival at 5 years (38% vs 29%, P = .065, hazard ratio = 0.82; 95% confidence interval, 0.66-1.1) with nonrelapse mortality of 4% versus 1% in the chemotherapy arm (P = .02). Overall survival was similar (44% vs 41% at 5 years, P = .86) because of more opportunities for salvage with second-line chemotherapy and stem cell transplantation in patients relapsing on the chemotherapy arm. This large study shows a relapse advantage for ASCT as postremission therapy but similar survival because more relapsing patients on the chemotherapy arm were salvaged with a late transplantation for relapse. This trial is registered at www.trialregister.nl as #NTR230 and #NTR291.
Introduction
Autologous bone marrow transplantation (ABMT) after marrow ablative chemotherapy or radiotherapy has originally been developed as an alternative to allogeneic stem cell transplants for patients with acute myeloid leukemia (AML) with no suitable donor. Several randomized studies in patients with AML in first complete remission (CR1) subsequently suggested reduced relapse rates after ABMT.1-6 However, ABMT has been associated with prolonged marrow aplasia and with an excess of nonrelapse mortality.2,3 As a result, the relapse advantage of an autologous transplant was offset by enhanced toxicity and mortality, which has precluded general acceptance of ABMT as postremission treatment in AML.1-6 In addition, these studies with marrow auto grafts were hampered by the fact that only a minority of the allocated patients actually underwent the transplantation.7,8
When hematopoietic growth factors provided the possibility to use peripheral blood stem cells as the source of stem cells, autologous peripheral blood stem cell transplants (ASCTs) offered the advantage of a markedly faster engraftment and accelerated hematologic recovery compared with marrow grafts.9-11 The switch to ASCT was also expected to enhance compliance to protocol treatment so that a greater fraction of patients assigned to ASCT would indeed receive their intended transplantation. However, critical prospective evaluations of ASCT have remained remarkably scarce and were performed in series with relatively small numbers of patients.11,12
Against this background, the Dutch-Belgian Hemato-Oncology Cooperative Group (HOVON) and Swiss Group for Clinical Cancer Research Collaborative Group (SAKK) leukemia cooperative groups set out to assess the clinical benefit of ASCT after high-dose cytotoxic therapy in a multicenter study in 517 patients with AML in CR1 after intensive anthracycline and cytarabine chemotherapy.13,14 ASCT was prospectively compared with intensive consolidation chemotherapy with etoposide and mitoxantrone, which have been reported to exert potent anti–leukemic effects.13-15
Methods
Study design and chemotherapy
Previously untreated patients with a confirmed diagnosis of AML were eligible for enrollment in the HOVON/SAKK AML-29 and AML-42 trials.13,15 The age range for the HOVON/SAKK AML-29 trial was 16 to 60 years and for the AML-42 trial 18 to 60 years. Patients with acute promyelocytic leukemia were eligible in the AML-29 trial but not in the AML-42 trial. Patients with another active cancer were not eligible, nor were patients with severe heart, lung, or neurologic disease (supplemental Methods, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Patients in CR1 after cycle 2 received consolidation with a third cycle of chemotherapy with etoposide (100 mg/m2 on days 1-5) and mitoxantrone (10 mg/m2 on days 1-5) in case of favorable cytogenetics and early CR after cycle 1. Unfavorable-risk patients were planned for an allogeneic stem cell transplantation and could be randomized in the study in case an allogeneic transplantation was not feasible. Intermediate-risk patients were candidates for an HLA matched allogeneic stem cell transplantation if a donor was available and the patient fulfilled the age criteria for an allograft in their center. If allogeneic stem cell transplantation appeared no realistic option, patients could be randomized between ASCT or the third cycle of chemotherapy with etoposide and mitoxantrone. Conditioning before ASCT consisted of high-dose chemotherapy with busulfan (4 mg/kg orally days −4 through −7 and cyclophosphamide (60 mg/kg intravenously, days −2 and −3) followed by autologous peripheral blood stem cell reinfusion (supplemental Methods).
This was an investigator-sponsored study with no pharmaceutical company involvement. The study was approved by ethics committees of the participating institutions and was conducted in accordance with the Declaration of Helsinki. All patients gave their written informed consent.
Prior remission induction chemotherapy
Remission induction chemotherapy according to the AML-29 and AML-42 protocols involved 2 cycles of combination chemotherapy.13,14 Cycle 1 consisted of cytarabine (200 mg/m2 on days 1-7) and idarubicin (12 mg/m2 on days 6-8). Cycle 2 consisted of cytarabine (1000 mg/m2 every 12 hours on days 1-6) and amsacrine (120 mg/m2 on days 4-6). In the AML-42 protocol, patients were also randomized between the aforementioned dose of cytarabine versus a more intensive cytarabine regimen (cycle 1, 1000 mg/m2 on days 1-5; and cycle 2, 2000 mg/m2 twice daily on days 1, 2, 4, and 6) as described.13,14 In the AML-29 and part of the AML-42 trial, patients were randomly assigned for induction to receive G-CSF or no G-CSF during cycles 1 and 2 as described.13
Criteria for response and end points
CR, relapse, and overall survival (OS) were previously defined.13,15 Relapse-free survival (RFS) refers to the interval from randomization to the date of death, or the date of relapse. Time to hematopoietic recovery was measured from the end of chemotherapy application both for patients treated according to chemotherapy cycle 3 or to the transplantation group to the time when the neutrophil and the platelet counts reached values of 0.5 × 109/L and 50 × 109/L, respectively.
Statistical methods
Design and randomization.
RFS was the primary end point. At the onset of this randomization in the AML-29 study, it was clear that the number of patients randomized between the third chemotherapy course and ASCT would not be sufficient to answer the question with sufficient power. Therefore, the randomization was planned to be continued in the successive AML-42 study. Randomization was closed in 2006. After an additional follow-up of 3.5 years, 343 events (relapse or death in CR1) have been observed in both groups together. This number of events gives a power of 71% for the detection of a significant difference if the true hazard ratio of failure in the ASCT group compared with the chemotherapy group would be 0.76, which corresponds to an increase in the 5-year RFS from 30% to 50%.
Randomized assignments to study groups were balanced with the use of a biased-coin minimization procedure as described (supplemental Methods).
Analysis.
All analyses were performed according to the intention-to-treat principle, irrespective of patient compliance with the protocol, but 12 ineligible patients randomized between cycle 3 (n = 5) and ASCT (n = 7) were excluded: patients with acute promyelocytic leukemia (n = 6), never reached CR1 (n = 2), relapsed before randomization (n = 2), and incorrect diagnosis (n = 2).
Cox regression analysis was used to estimate the effect of treatment group and covariates on RFS and OS (secondary endpoint). The possible heterogeneity of the treatment effects in subgroups was explored in post hoc analyses by estimation of the hazard ratios for each subgroup, together with 95% confidence intervals (CIs), and tests for interaction. A limited number of subgroup classifications were considered: cytogenetic risk category (favorable, intermediate, or unfavorable), age, World Health Organization performance status (0, or 1 or 2), presence of extramedullary disease and WBC count (≤ 20 × 109/L) at diagnosis, and early (CR after cycle 1) or late (CR after cycle 2) CR1. P values < .05 were considered statistically significant, except for the tests for interaction with subgroups, where P < .01 was used because of multiple tests performed.
Results
Between 1995 and 2006, 2017 patients at diagnosis were enrolled in the AML-29 and AML-42 trial for remission induction treatment. After 2 courses of chemotherapy induction therapy, 76% of the patients were in CR1 (Table 1). The recommended choice of consolidation treatment in the protocol according to the cytogenetic risk stratification (see “Study design and chemotherapy”) resulted in the randomization of 34% of patients, whereas 23% of patients went straight to consolidation chemotherapy (cycle 3) and 27% were consolidated in CR1 with an allogeneic SCT depending on the availability of an HLA-matched donor and clinical eligibility criteria (age, comorbidity). A total of 2% of patients received an ASCT without randomization and 15% did not receive further therapy in CR1 because of early relapse or prolonged hypoplasia (Table 1). Thus, of 517 randomized patients, 259 were assigned to consolidation chemotherapy cycle 3 and 258 patients to ASCT. Median follow-up of patients alive is 106 months (range, 13-177 months). Nine patients in the chemotherapy group and 7 patients in the ASCT have been lost to follow-up between 1 and 12 years. The 2 treatment groups were matched with respect to age, cytogenetic risk, and types of induction therapy (Table 2). A total of 93% of the patients randomized to consolidation chemotherapy received the planned chemotherapy according to protocol, and 91% of the patients assigned to ASCT actually received the autologous transplant (Table 3).
Outcome after chemotherapy or ASCT
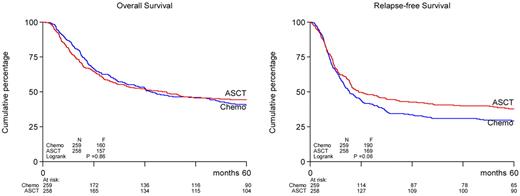
The ASCT treatment group showed a trend toward better RFS than the chemotherapy group (38% vs 29% at 5 years, P = .065, hazard ratio = 0.82; 95% CI, 0.66-1.1; Table 3; Figure 1). In the ASCT group, 156 patients had recurrence of AML, whereas 187 patients relapsed in the chemotherapy group, corresponding with an actuarial relapse rate at 5 years of 58% and 70%, respectively (P = .02, Table 3). Nonrelapse mortalities (a measure of treatment procedure-related deaths) were estimated at 4% and 1% (at 5 years) in the ASCT and chemotherapy groups (P = .02). OS did not differ between both treatment arms (44% vs 41%, P = .86). Second-line anti–leukemic treatment was applied to 116 (74%) of the 156 relapsing patients in the ASCT arm, which involved ASCT (n = 2, 1% of recurrences), allogeneic SCT (n = 27, 17%), and chemotherapy (n = 87, 55%). In contrast, 150 of 187 (80%) relapsing patients in the chemotherapy group were treated in second line with ASCT (n = 27, 14%), allogeneic SCT (n = 47, 25%), or chemotherapy (n = 76, 40%). Thus, a considerably greater proportion of patients after relapse in the consolidation chemotherapy group had the possibility for salvage with ASCT or allogeneic SCT (39% vs 18%). Second CRs were attained in 27% of the relapsed patients in the ASCT group and 47% in the chemotherapy group resulting in long-term survival of 7% and 15% for patients with relapse in the ASCT group and the chemotherapy group, respectively.
OS and RFS of patients with AML in CR1 randomized to ASCT or consolidation chemotherapy.
OS and RFS of patients with AML in CR1 randomized to ASCT or consolidation chemotherapy.
Hematologic recovery and treatment-related toxicity
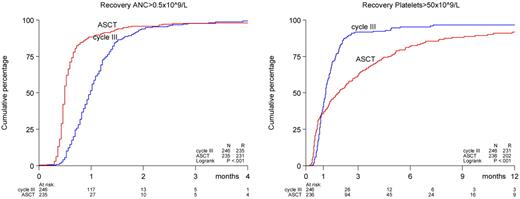
A significantly enhanced recovery of peripheral blood granulocyte count was seen after ASCT compared with consolidation chemotherapy (Figure 2). A total of 32% of ASCT patients reached neutrophil counts of more than 0.5 × 109/L at day 14 and 88% on day 28 after transplantation compared with 1% and 42%, respectively, for patients consolidated with chemotherapy (P < .001). Platelet recovery demonstrated a biphasic pattern; in the first month after end of treatment, the platelet recovery rate to more than 50 × 109/L was slightly faster in the ASCT group (P = .79). However, for the patients who had not recovered by that time, the platelet recovery proceeded at a slower rate in the ASCT group (P < .0001, Figure 2) A similar pattern was seen with respect to time to platelet transfusion independence; the median time to transfusion independence was comparable between both groups (24 days vs 23 days), but after that the duration was longer in the ASCT group (P = .003). In the ASCT group, the incidences of grade 3 and 4 bleedings and grade 3 and 4 infections were not different (supplemental Table 1). However, an increased incidence of fever of unknown origin (37% vs 21%, P < .001), gastrointestinal (72% vs 29%, P < .001), hepatic (18% vs 6%, P < .001), and neurologic (11% vs 4%, P = .004) grade 2 to 4 adverse events were noted in the ASCT group.
Recoveries of absolute neutrophil counts (ANC, 0.5 × 109/L) and platelet counts (50 × 109/L) after ASCT or consolidation chemotherapy. Recovery was measured from the date of transplantation in the ASCT group and for comparability from the last date of cycle 3 in the chemotherapy group. The calculations have been restricted to patients treated according to allocated treatment.
Recoveries of absolute neutrophil counts (ANC, 0.5 × 109/L) and platelet counts (50 × 109/L) after ASCT or consolidation chemotherapy. Recovery was measured from the date of transplantation in the ASCT group and for comparability from the last date of cycle 3 in the chemotherapy group. The calculations have been restricted to patients treated according to allocated treatment.
Prognostic factors and subgroup analysis
Table 4 shows the actuarial 5-year probabilities of RFS and OS and the hazard ratios in relation to clinical and hematologic factors and according to treatment group. Increasing age was associated with a reduced RFS (P = .01) and OS (P < .001). The presence of extramedullary disease at diagnosis also correlated with lower RFS (P = .016) and OS (P = .21). Cytogenetics showed particularly strong relationships with RFS and OS. The ASCT group showed better RFS than the chemotherapy group (at 5 years, 38% vs 29%), but this difference was not statistically significant (P = .065). However, if the patients of the monosomal karyotype with very poor RFS in both arms were excluded, the improvement in RFS for the ASCT arm was more pronounced (P = .014). Patients attaining late CR (ie, after induction cycle 2) had a considerably lower RFS and OS (P < .001) than those in CR already after cycle 1.
To explore for a possible differential effect of ASCT treatment on outcome in any of the subgroups defined by the aforementioned factors, the effect of treatment was estimated separately by hazard ratio for RFS and OS with associated CIs combined with tests for interaction. In none of the latter analyses, the test for interactions were significant (all P values for these tests > .10), including G-CSF priming and Ara-C dose applied.
Discussion
Randomized transplantation studies about ABMT in AML in CR1 had demonstrated reduced relapse rates in association with considerable procedural limitations, including low treatment compliance, delayed hematologic regeneration, and increased nonrelapse mortality.1-6 The present study demonstrates that these hurdles can largely be overcome. Autografts were successfully collected in a high number of patients, and a high proportion of randomized patients did indeed receive their assigned treatment, which enhanced the ability of evaluating the true value of ASCT according to intention to treat. The results show an advantage for ASCT as postremission therapy in terms of relapse rate (57% vs 70% at 5 years, P = .002) and with a higher RFS (39% vs 29% at 5 years, P = .065). The OS was only slightly better after ASCT (44% vs 41% at 5 years), but this difference was far from statistically significant (P = .86). It should be noted that the similar OS value in the 2 groups was the result of a higher proportion of successful salvage treatments, especially ASCT and allogeneic SCT, given after relapse in the chemotherapy group compared with the ASCT group.
Despite a marked accelerated granulocyte recovery in the ASCT arm, more side effects were noticed probably related to more intensive chemotherapy and resulting in a treatment-related mortality of 4% in the ASCT arm versus 1% in the chemotherapy arm.
An important question is whether the choice of the remission induction therapy and the third cycle of mitoxantrone-etoposide for remission consolidation furnishes a proper comparison regarding the value of ASCT. In the current study, ASCT was given after 2 cycles of induction therapy with cytarabine at conventional and intermediate-dose levels and compared with the same treatment plus a third cycle of mitoxantrone-etoposide. Does the latter treatment that served as a control represent a proper comparative reference? It has been thought for some time that a consolidation treatment with high-dose cytarabine (HD-Ara-C) is optimal for young and middle-aged adults with AML.18 However, although an earlier study had shown superiority of 3000 mg/m2 over 400 mg/m2 cytarabine, it did not furnish any direct evidence supporting the need of 4 cycles of HD-Ara-C.18 Accumulating data suggest that multiple cycles of HD-Ara-C and dose levels of cytarabine > 1000 mg/m2, whether applied in induction or consolidation, are of limited value. In one randomized trial, 2 postremission cycles of standard-dose Ara-C versus one HD-Ara-C schedule made no difference.19 In an additional study, 3 HD-Ara-C cycles applied after remission did not yield better outcome than one cycle.1 A large recent Japanese study has recently reinforced the notion that standard-dose levels of cytarabine applied as postremission therapy are not inferior to high-dose levels.20 Our group has previously used and applied in the standard arm of the current study an intensive treatment program involving a first induction cycle of standard-dose Ara-C, a second cycle of intermediate-dose Ara-C, and one third final consolidation cycle, and we have reported outcome in a range similar to that after 4 cycles of HD-Ara-C.13,15,21
Our study does not allow a critical analysis of the value of ASCT in cytogenetically defined favorable-risk and unfavorable-risk subsets of AML patients because of the limited numbers of patients studied. However, the results are in line with other studies demonstrating no advantage of ASCT in patients with monosomal karyotype.19,20 Excluding these patients from the analysis resulted in a significant advantage in RFS for the ASCT arm (P = .01).
Irrespective of the choice of postremission treatment (ie, ASCT or chemotherapy), relapse of leukemia remains the predominant cause of treatment failure. This is reflected by the profound impact of karyotype subtype on RFS and OS. During the time span of the study, a number of insights have evolved regarding the therapeutic management of AML, which may impact on the future enforcement of ASCT. For instance, within the cytogenetic defined intermediate-risk group, subgroups are defined with favorable and unfavorable outcome based on somatic gene mutations in CCATT enhancer binding protein-α, nucleophosmin-1 (NPM1), and Fms-like tyrosine kinase 3 (FLT3).23-25 One study has already demonstrated that the subset of patients with NPM1+ mutations without FLT3-internal tandem duplications (FLT3-ITD) derive no survival benefit from allogeneic SCT.25 Direct outcome data regarding ASCT in these and other genotypes are currently not available, but one might assume that the value of ASCT in these genotypic subsets will follow the cytogenetic prognostic analogies as has previously been demonstrated for allogeneic SCT.26 Allogeneic transplantation after reduced dose-intensive conditioning is nowadays quite commonly used in patients with AML because of reduced early toxicities, but it involves a greater relapse rate than myeloablative regimens.27-30 The ability of ASCT to suppress relapse in CR1 suggests that ASCT might also have merits in AML-CR1 as a an adjunct regimen before allogeneic SCT. ASCT appears to minimize the leukemic burden and stabilize remissions; thus, it might create better conditions and allow more time for graft-versus-leukemia control. Finally, the remarkably low procedural mortality after ASCT that we report here after prolonged follow-up makes ASCT also potentially attractive for other subgroups (eg, for favorable risk AML where it might contribute to preventing relapse).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the Leukemia Working Group of the HOVON/SAKK Cooperative Groups for conception and design; Ine Meulendijks, Jan van Tuijn, Martine Testroote, Christel van Hooije (HOVON), and Christina Biaggi (SAKK) for collection and assembly of data; and W.v.P. for data analysis and interpretation.
Authorship
Contribution: M.T. collected and assembled the data; W.v.P. analyzed and interpreted the data; and all authors wrote the manuscript and gave final approval of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Edo Vellenga, Department of Hematology, University Medical Center Groningen, PO Box 30.001, 9700 RB Groningen, The Netherlands; e-mail: e.vellenga@umcg.nl.