Abstract
The outcomes in children with refractory/relapsed (R/R) acute lymphoblastic leukemia (ALL) are dismal. The efficacy and safety of intravenous clofarabine 40 mg/m2 per day, cyclophosphamide 440 mg/m2 per day, and etoposide 100 mg/m2 per day for 5 consecutive days in pediatric patients with R/R ALL was evaluated in this phase 2 study. The primary endpoint was overall response rate (complete remission [CR] plus CR without platelet recovery [CRp]). Among the 25 patients (median age, 14 years; pre-B cell ALL, 84%; ≥ 2 prior regimens: 84%; refractory to previous regimen: 60%), the overall response rate was 44% (7 CR, 4 CRp) with a 67.3-week median duration or remission censored at last follow-up. Most patients proceeded to alternative therapy, and 10 patients (40%) received hematopoietic stem cell transplantation. Six patients (24%) died because of treatment-related adverse events associated with infection, hepatotoxicity, and/or multiorgan failure. The study protocol was amended to exclude patients with prior hematopoietic stem cell transplantation after 4 of the first 8 patients developed severe hepatotoxicity suggestive of veno-occlusive disease. No additional cases of veno-occlusive disease occurred. The regimen offered encouraging response rates and sustained remission in R/R patients. Future investigation should include exploration of patient selection, dosing, and supportive care. This trial was registered at www.clinicaltrials.gov as #NCT00315705.
Introduction
Dramatic improvements have been made in the treatment of children with newly diagnosed acute lymphoblastic leukemia (ALL). However, poor outcomes are still observed in patients who have refractory or relapsed (R/R) disease after conventional chemotherapy. New therapeutic options are urgently needed.
Clofarabine is a second-generation purine nucleoside analog rationally designed to incorporate the best attributes of its predecessors, fludarabine and cladribine. The cytotoxicity of clofarabine is dependent on the intracellular accumulation of its triphosphate metabolite.1 Lymphocytes are particularly vulnerable to clofarabine because of high levels of deoxycytidine kinase and low levels of 5′-deoxynucleotidase.2 Studies have linked the expression of nucleoside transporters to sensitivity to nucleoside analogs and clinical responses.3-5 Although the uptake of clofarabine has been shown to be mediated by the nucleoside transporters hENT1, hENT2, hCNT2, and hCNT3,6 the potential relationship between the expression of these transporters and response to clofarabine is unknown.
Clofarabine has shown single-agent activity in previous phase 1 and 2 studies in pediatric patients with R/R ALL.7,8 In 2004, the United States Food and Drug Administration approved the use of clofarabine monotherapy in the treatment of pediatric patients (age, 1-21 years) with R/R ALL treated with at least 2 prior regimens.
Preclinical experiments have shown that clofarabine has synergistic activity with cyclophosphamide by inhibiting DNA repair.9 The combination of cyclophosphamide and etoposide has also demonstrated activity in relapsed pediatric ALL.10 These data provided the rationale for a phase 1 or 2 study evaluating the combination of clofarabine, etoposide, and cyclophosphamide.
The previously completed phase 1 study determined the recommended phase 2 doses of clofarabine (40 mg/m2), etoposide (100 mg/m2), and cyclophosphamide (440 mg/m2), each given daily for 5 consecutive days in induction and 4 consecutive days in consolidation.5 Twenty-five patients were enrolled (20 ALL; 5 acute myelogenous leukemia) with a median of 2 prior therapies; 28% were refractory to their immediate prior regimen, and 16% had undergone prior hematopoietic stem cell transplantation (HSCT). The phase 1 study produced an overall response rate (ORR, defined as complete remission [CR] plus CR without platelet recovery [CRp]) of 55% among 20 children with ALL and 100% among 5 children with acute myelogenous leukemia.11 The 16 responding patients had an 18.2-week median duration of remission (DOR; range, 6.6-105.1+ weeks, censored at last follow-up) and 37-week median overall survival (OS; range, 13.7-109.0+ weeks). Nine of 16 responders proceeded to HSCT.
The phase 2 portion of the study reported herein evaluated the efficacy and safety of this combination in the treatment of children with R/R ALL. The expression of nucleoside transporters was also evaluated during the phase 2 portion of the study to provide a potential basis for the identification of patients sensitive or resistant to therapy in future studies.
Methods
Study group eligibility
Patients eligible for study enrollment had a diagnosis of R/R ALL with ≥ 25% BM blasts and age between 1 and 21 years at the time of the initial leukemia diagnosis. Patients also had to have a Karnofsky performance status or Lansky performance status of ≥ 50, no overt central nervous system involvement, and ≤ 3 prior induction regimens. After 4 of the initial 8 patients developed severe hepatotoxicity, suggestive of veno-occlusive disease (VOD), the protocol was amended to exclude patients with prior HSCT, viral hepatitis, cirrhosis, or elevated conjugated bilirubin levels at study entry. Other eligibility criteria included adequate hepatic (conjugated serum bilirubin ≤ institutional upper limit of normal [ULN] for age if total bilirubin was elevated, aspartate aminotransferase and alanine aminotransferase ≤ 3.0 × ULN), renal (creatinine clearance ≥ 90 mL/min per 1.73 m2), pancreatic (serum amylase ≤ 2.0 × ULN for age, serum lipase ≤ 1.5 × ULN for age), and cardiac function (echocardiogram with shortening fraction ≥ 26% or with ejection fraction ≥ 46%). Exclusion criteria included prior clofarabine treatment, uncontrolled systemic infections, < 14 days since completion of previous cytotoxic therapy, < 7 days since completion of prior biological therapy, the presence of severe concurrent disease or a history of serious organ dysfunction, and positivity for hepatitis B or C infection.
The study protocol was approved by institutional review boards at the participating institutions. Parents or guardians provided informed consent, and patients 7 years or older provided assent. The study was conducted in accordance with the basic principles of the Declaration of Helsinki. This study was registered at www.clinicaltrials.gov as #NCT00315705.
Treatment
Patients were treated with clofarabine 40 mg/m2 (2-hour intravenous infusion), etoposide 100 mg/m2 (2-hour intravenous infusion), and cyclophosphamide 440 mg/m2 (30- to 60-minute intravenous infusion), all given daily for 5 consecutive days in induction and 4 consecutive days in consolidation. Patients underwent BM aspirate between days 14 and 21 of the first induction cycle to determine the next treatment course. Patients with ≥ 5% blasts in the absence of a hypocellular marrow received a second induction cycle, whereas those < 5% blasts could proceed to consolidation. Patients could receive up to 2 induction cycles, followed by consolidation for a maximum total of 8 cycles. Patients with > 5% blasts after the second induction cycle were determined to have treatment failure and were withdrawn from the study. Patients could come off study at any time to receive alternative therapy, including HSCT, at the discretion of the treating physician.
Intrathecal prophylaxis has been previously described.5 Infection prophylaxis was recommended per institutional guidelines.
Dose delays or reductions because of hematologic toxicity were not permitted during the induction cycles. Starting with the second cycle, drug doses were reduced or delayed for toxicity as previously described.11
Response and toxicity criteria
Response to treatment was determined by the investigator. A patient's best response after induction or reinduction was used to determine ORR (CR + CRp). CR was defined as M1 marrow (< 5% blasts) and recovery of peripheral counts (platelets ≥ 75 × 109/L and absolute neutrophil count ≥ 0.75 × 109/L). CRp was defined as CR, except for recovery of platelet count to ≥ 20 × 109/L but < 75 × 109/L. Partial remission was defined as complete disappearance of circulating blasts, appearance of normal hematopoietic progenitor cells, and either a BM with > 5% and < 25% blasts with recovery of peripheral counts or a BM with < 5% blasts with peripheral counts that did not qualify for CR or CRp.
At the completion of induction and/or reinduction therapy, minimal residual disease (MRD) in marrow samples was assessed by flow cytometry using a modification of a previously described method.12 MRD affecting > 1/10 000 cells (> 0.01%) was designated positive. Participation in the MRD studies was optional.
Adverse events (AEs) were graded by the investigator using the National Cancer Institute common Terminology Criteria for AE Version 3.0. An independent data safety monitoring board monitored safety.
Nucleoside transporter analysis
The expression of the nucleoside transporters hENT1, hENT2, hCNT2, and hCNT3 was determined by quantitative RT-PCR in RNA obtained from bone marrow blasts obtained at baseline and at time of relapse (if applicable). Quantitative RT-PCR was performed on first-strand cDNA prepared from each patient RNA sample. TaqMan primer-probe pairs (Applied Biosystems) specific to each nucleoside transporter and, as an internal control, for GAPDH were used. The expression levels of each nucleoside transporter normalized to GAPDH in individual patient samples were expressed relative to that in RNA from normal PBMCs obtained from pooled normal human blood (Clontech). The comparative cycle threshold method was used to express the relative levels of each transporter cDNA in PCR reactions.13
Statistical methods
The primary end point of this study was ORR. Secondary end points included safety, tolerability, rate of partial remission, DOR, event-free survival (EFS), 4-month EFS, and OS. MRD status and nucleoside transporter expression were exploratory endpoints. Using a Simon 2-stage optimal design,14 the phase 2 portion of this study was designed to test the null hypothesis that the ORR was ≤ 20% against the alternative hypothesis that the ORR was ≥ 40%. Assuming a one-sided α level of 10% and power of 80%, 25 patients were to be enrolled in this study. The therapy was to be considered effective if the cumulative number of responders (CR + CRp) was > 7 among 25 enrolled patients.
The primary efficacy analysis included all patients who received at least one dose of clofarabine. All patients who received any amount of study drug were included in the safety analysis. Descriptive statistics were used to describe response rates. Time-to-event outcomes, such as DOR and OS, were described using Kaplan-Meier estimates. DOR was calculated censoring patients known to be in remission at last follow-up, and separately with censoring at the time of alternative therapy or HSCT.
Results
Patients and treatment
A total of 25 patients were enrolled (Table 1). The majority had been previously treated with 2 or more regimens (84%). Most patients (60%) were refractory to their immediate prior regimen. Four patients who had a prior HSCT were enrolled before a protocol amendment, making such patients ineligible.
Patients received a median of one cumulative cycle of study drugs (range, 1-3 cycles), with a 28.5-day median duration between cycles (range, 19-59 days). None of the patients completed the maximum of 8 cycles and required post-study follow-up, and the majority went on to receive alternative therapy. Reasons for treatment discontinuation included HSCT (32%), death (28%), investigator decision (16%), failure to achieve a response after induction or reinduction (12%), AE or toxicity (4%), disease relapse (4%), and a decision to receive alternative therapy because of elevated bilirubin (4%).
Efficacy outcomes
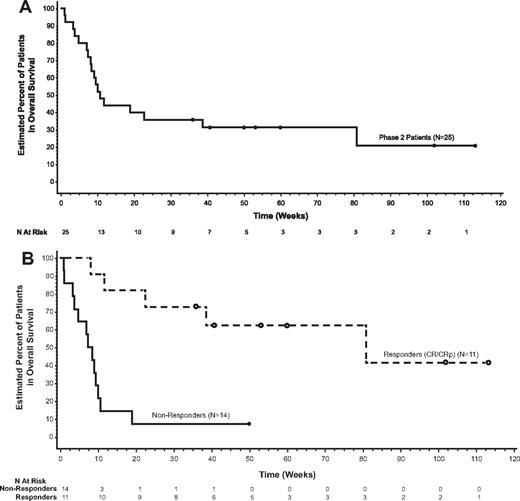
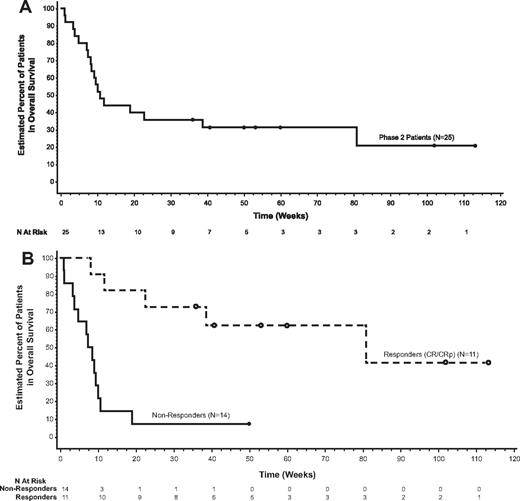
Seven patients achieved CR and 4 achieved CRp after induction, for a 44% ORR with a 67.3-week median DOR censored at last follow-up visit (Table 2). The majority of patients received alternative therapy following the study regimen. The median DOR censored at the time of alternative therapy was not estimable but ranged from 0.1+ to 10.9+ weeks. Three patients (12%) achieved a partial remission. For all patients, the median EFS and median OS were 10.7 weeks (Figure 1A). Patients with a CR or CRp had a median EFS of 73.9 weeks (range, 8.1-113.1+ weeks), and the median OS was 80.9 weeks (range, 8.1-113.1+ weeks; Figure 1B). Seven of 11 responders (64%) had been refractory to their immediate prior regimen (Table 3), 10 (91%) achieved their best response after 1 cycle, and 7 (64%) proceeded to HSCT.
Survival. Kaplan-Meier estimates for (A) OS and (B) OS by response to treatment.
Survival. Kaplan-Meier estimates for (A) OS and (B) OS by response to treatment.
Ten patients (40%) proceeded to HSCT. The median time to transplantation from last dose date was 6.8 weeks (range, 6.026.7 weeks). After a median post-HSCT follow-up of 53.7 weeks, 6 of the 10 patients who proceeded to HSCT were alive at last follow-up and still in remission. Post-HSCT complications included VOD in 2 patients (reversible in 1 patient, ongoing at time of death in the other) and GVHD, which was acute in 4 patients (1 mild, 2 moderate, 1 severe) and chronic in 2 (moderate).
Among the 4 responders evaluated for MRD, 3 were negative and 1 was positive. Two additional patients with partial remission (no evidence of leukemia but lacking absolute neutrophil count or platelet recovery criteria for CR or CRp) were also found to be MRD negative.
Safety
The most common all grade treatment-related AEs were vomiting (88%), nausea (72%), anemia (64%), thrombocytopenia (64%), and febrile neutropenia (60%). All patients experienced at least one more than grade 3 AE; 17 patients (68%) experienced at least one grade 4 AE, and 20 patients (80%) reported at least 1 related serious AE. Table 4 lists grade ≥ 3 treatment-related AEs reported for > 10% patients. The overall incidence of patients with infection was 76%.
Among the first 8 patients enrolled in this study, 4 experienced severe hepatotoxicity in the setting of infection, sepsis, and/or capillary leak syndrome. A clinical diagnosis of VOD was made in 3 of these 4 patients; one had radiographic evidence of VOD; another had evidence of VOD on autopsy. All 3 patients died with multiorgan failure. Two of the patients with clinical VOD had a prior HSCT < 12 months before study entry. The fourth patient, who had reversible grade 4 hepatotoxicity complicating an adenovirus infection, also had history of recent prior HSCT. No additional cases of VOD were observed in the 17 patients enrolled after the study protocol was amended to exclude patients with prior HSCT, viral hepatitis, cirrhosis, or elevated conjugated bilirubin. Four additional patients reported AEs of capillary leak syndrome in close temporal proximity to clofarabine administration. These patients had courses complicated by the effects of vascular leak and/or infection, multisystem involvement, and poor outcomes.
Three patients (12%) experienced a dose modification. One of these patients had a dose reduction during cycle 2 because of pancreatitis; 2 patients had infusion interruptions in cycle 1 (one because of vomiting, the other to capillary leak syndrome). One patient (4%) discontinued treatment because of a drug-related AE (fungal sinusitis).
Seven patients died because of AEs; 6 of these were considered to be drug-related. Causes of death were VOD (2 patients), septic shock (2 patients), acute renal failure (1 patient), pulmonary edema (1 patient), and infection (lung/fungal; 1 patient). Five of these deaths (all but the one resulting from renal failure and infection [lung/fungal]), 20% overall, occurred within 30 days from the last dose.
Nucleoside transporters
Sufficient RNA was available from 4 patients. No consistent pattern was found in the expression of hENT1, hENT2, and hENT3 transporters relative to that in normal PBMCs (Table 5). In contrast to these transporters, hCNT2 was not present in detectable amounts in any sample, including normal PBMCs.
Discussion
In this multi-institutional phase 2 study, the combination of clofarabine, etoposide, and cyclophosphamide showed efficacy (ORR = 44%) in refractory and heavily pretreated children with ALL. This response rate appears better than the previous study with clofarabine monotherapy (CR + CRp, 20%).8 The present efficacy results are similar to Locatelli et al,15 who reported a 56% ORR with a single course of clofarabine (40 mg/m2), cyclophosphamide (400 mg/m2), and etoposide (150 mg/m2), all given daily for 5 days, in children with refractory or multiple-relapsed ALL.
This clofarabine-containing regimen showed efficacy in patients with refractoriness to multiple conventional regimens. The majority of patients (60%) in this study had been refractory to their immediate previous therapy. One patient, who had been newly diagnosed and refractory to 3 prior regimens (vincristine/prednisone/ polyethylene glycosylated [PEG]-asparaginase/daunorubicin, etoposide/cyclophosphamide, and high-dose cytarabine/L-asparaginase), attained CR and negative MRD after only one cycle. He has remained disease-free for more than 2 years, after HSCT.
The majority of patients received this regimen as reinduction, receiving 1 or 2 cycles before coming off study to receive alternative therapy in consolidation and/or HSCT. Therefore, the overall length of remission may be attributable to the study regimen along with the subsequent therapies received. However, solid remission with negative MRD is imperative for successful HSCT16 or other subsequent therapy, and the negative MRD seen in 3 of 4 tested responding patients indicates solid remission. Remissions also tended to be durable (median DOR, > 60 weeks censored at last follow-up). Furthermore, 6 of 10 patients who underwent HSCT were alive at last follow-up (median follow-up, 53.7 weeks).
The early experience in this trial suggests that patients with prior HSCT are at higher risk of VOD when treated with this drug combination. This notion is supported by absence of VOD after the study eligibility criteria were amended to exclude this group of patients.
Compared with the phase 1 study,11 there were more deaths early in phase 2 patients (4 week, 0% vs 16%; and 8 week, 4% vs 28%, respectively; Genzyme data on file). In a multivariate post-hoc analysis exploring the apparent differences observed in OS and early deaths between the phase 1 and 2 portions of the study, baseline performance status was a statistically significant predictor of OS (data not shown). However, this nonrandomized study was not designed or sufficiently powered to compare phase 1 versus phase 2, and the ad hoc analyses had limited sensitivity to detect differences in survival.
The rate of early death seen in this phase 2 study falls within the range for similar populations published by others. For example, Saarinen-Pihkala et al reported a 21%-29% mortality rate during induction after second relapse in a large population study of Nordic children with ALL.17 The nature, frequency, and severity of AEs seen in the present study may reflect the susceptibility of these heavily pretreated patients to regimen-related toxicities. The high incidence of severe infections observed in this study raises the issue of whether routine use of antimicrobial prophylaxis should be considered as a risk-minimization strategy.
The difference in early mortality and overall survival observed between phase 1 and phase 2 patients suggests that the doses used in phase 2 may not represent the optimal doses for the patients studied. Responses were noted in all cohorts of the phase 1 study; thus, further exploration of the efficacy of lower doses of this combination chemotherapy in future studies may be a reasonable strategy to reduce the risk of toxicities in such high-risk patients.11
A number of ongoing and planned studies are further evaluating variations of this 3-drug combination in a variety of clinical settings. This combination was also evaluated by the Therapeutic Advances in Childhood Leukemia Consortium (www.tacl.us) in a phase 2 study in R/R AML. Excessive toxicity (grade 5 fungal infection, n = 1; grade 5 capillary leak/renal failure, n = 1) required a halt to accrual following the enrollment of 6 patients. Subsequently, the study was closed because of competing priorities. This 3-drug combination will also be evaluated as a part of consolidation regimen (along with vincristine and PEG-asparaginase) in an upcoming phase 3 Children's Oncology Group study in children with newly diagnosed very-high-risk ALL (#NCT01406756). To support this upcoming study, the 5-drug regimen was recently evaluated in a pilot study in pediatric ALL patients in first relapse (#NCT00991133). In this pilot study, 4 of 8 patients enrolled developed dose-limiting toxicities (bone marrow aplasia, n = 2; grade 4 lipase elevation, n = 1; and grade 4 hyperbilirubinemia, n = 1). Three of these dose-limiting toxicities occurred before the administration of vincristine and PEG-asparaginase and therefore were primarily attributable to the 3-drug backbone of clofarabine, cyclophosphamide, and etoposide (Genzyme data on file). These results have led to the reduction of the clofarabine dose to 30 mg/m2 in the upcoming Children's Oncology Group phase 3 study.
Cellular transmembrane transport systems are involved in the delivery of hydrophilic nucleosides and nucleoside analogs to their sites of action. King et al6 studied the transport of clofarabine using recombinant ENT and CNT transporters in model expression systems. In our study, we found that 3 (hENT1, hENT2, and hCNT3) of the 4 transporters implicated in clofarabine transport were expressed in patient blast samples and in normal PBMCs. Because of the small numbers of samples analyzed, no correlation between transporter expression and response could be made.
In conclusion, the combination of clofarabine, etoposide, and cyclophosphamide has shown efficacy in the treatment of children with relapsed or refractory ALL. The response rates and durability of remission observed with this regimen were encouraging given that these patients were highly refractory to prior therapies and heavily pretreated. Further investigation of this regimen should include careful consideration of drug doses, patient eligibility criteria, and the surveillance, prevention, and management of infections.
Presented in part in abstract form at the 52nd Annual Meeting of the American Society of Hematology, Orlando, FL, December 7, 2010.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs Rytting, Chu, Quezada, and Razzouk for their study participation and Dr Monica Nicosia (WordSmith Science) and Dr Angela Partisano (Genzyme) for editorial assistance.
This work was supported by Genzyme Corporation.
Authorship
Contribution: N.H. participated in study planning, contributed to patient accrual analyzed data, and wrote the manuscript; J.V.T. analyzed data and helped write the manuscript; E.B. participated in study planning, analyzed data, and helped write the manuscript; J.-A.P. was the study statistician, participated in study planning, and helped write the manuscript; P.G.S., W.L.C., and P.S.G. participated in study planning and contributed to patient accrual and manuscript editing; B.T., M.S.I., L.B.S., M.J.B., R.K., T.C., V.S., and G.D. contributed to patient accrual and manuscript editing; S.J. participated in study planning and contributed to manuscript editing; K.M. contributed to the interpretation of data and manuscript editing; and all authors provided final approval of the manuscript.
Conflict-of-interest disclosure: N.H. and P.G.S. have participated as members of a Genzyme advisory board and received honoraria and research support for clinical studies. S.J., P.S.G., and J.V.T. have received research support for clinical studies from Genzyme. B.T. has participated as a member of a Genzyme advisory board. E.B., J.-A.P., and R.K. are employees of Genzyme Corporation. The remaining authors declare no competing financial interests.
Correspondence: Nobuko Hijiya, Department of Pediatric Hematology-Oncology, Children's Memorial Hospital/Northwestern University Feinberg School of Medicine, 2300 Children's Plaza, Box 30, Chicago, IL 60614-3394; e-mail: nhijiya@childrensmemorial.org.