Abstract
Effective vaccines consist of 2 components: immunodominant antigens and effective adjuvants. Whereas it has been demonstrated that targeted delivery of antigens to dendritic cells (DCs) improves vaccine efficacy, we report here that co-targeting of TLR ligands (TLRLs) to DCs strongly enhances adjuvanticity and immunity. We encapsulated ligands for intracellular TLRs within biodegradable nanoparticles coated with Abs recognizing DC-specific receptors. Targeted delivery of TLRLs to human DCs enhanced the maturation and production of immune stimulatory cytokines and the Ag-specific activation of naive CD8+ T cells. In vivo studies demonstrated that nanoparticles carrying Ag induced cytotoxic T-lymphocyte responses at 100-fold lower adjuvant dose when TLRLs were co-encapsulated instead of administered in soluble form. Moreover, the efficacy of these targeted TLRLs reduced the serum cytokine storm and related toxicity that is associated with administration of soluble TLRLs. We conclude that the targeted delivery of adjuvants may improve the efficacy and safety of DC-based vaccines.
Introduction
Most vaccines currently on the market are based on the induction of long-lived Ab responses. A major challenge is the generation of vaccines that, next to Abs, also induce Ag-specific killing of pathogen-infected cells or tumor cells by cytotoxic T lymphocytes (CTLs). This might open new opportunities for the treatment of cancer or persistent viral infections, for which induction of strong cellular immunity seems to be essential.1 Dendritic cells (DCs) are professional APCs that play a key role in regulating adaptive immunity, and both preclinical and clinical studies have exploited DCs in an attempt to induce antiviral or antitumor CTL responses. Most of these studies explored ex vivo Ag loading of autologous monocyte-derived DCs that were readministered to the patient, a laborious and costly procedure. The discovery of pattern recognition receptors, such as C-type lectin receptors (CLRs) that mediate Ag uptake, allow for a more direct strategy by targeting Ags to DCs in vivo. Some of these CLRs are restricted to APCs, such as macrophages or DCs. Therefore, targeted delivery exploiting CLRs has already been demonstrated to enhance Ag presentation and results in immunity when DC maturation stimuli such as TLR ligands (TLRLs) are co-administered.2
TLRs constitute a family of pattern recognition receptors that recognize pathogen-associated molecular patterns and trigger immune cell activation. Consequently, natural TLRLs and their mimetics are increasingly being applied in immunotherapeutic strategies.3 TLRLs are potent inducers of innate immune responses and have been evaluated as monotherapies to treat infection and cancer.4,5 In addition, TLRLs have been applied as adjuvants to stimulate adaptive immune responses.3 We hypothesized that targeted delivery of both Ag and TLRLs directly to DCs may greatly enhance immune responses. This approach would guarantee that the APCs that take up Ag are the ones being activated. It also ensures that Ag and TLRL are delivered to the same cellular compartment, a prerequisite for efficient Ag presentation.6-9 Targeted delivery of TLRLs might have several advantages over systemic TLRL administration, which has been demonstrated to be associated with high serum cytokine levels and toxicity caused by systemic TLR activation in humans.10,11 Such systemic TLRL administration has also been shown to induce a state of immunosuppression and may consequently hamper responses against subsequent infections.12
To test our hypothesis, we encapsulated TLRLs within targeted biodegradable poly(lactic-co-glycolic acid) (PLGA) nanoparticles (NPs) that effectively deliver Ag to human DCs in vitro, as described previously.13 These NPs were surface-coated with lipid-PEG to prevent nonspecific interactions with cells or proteins. The NPs were loaded with a combination of the TLR3 ligand polyinosinic:polycytidylic acid (poly I:C) and the TLR7/8 ligand resiquimod (R848) as adjuvants, because they synergistically trigger a Th1-polarizing program in DCs, which enhances the induction of CTL responses.14 In addition to TLR3, poly I:C activates cells through MDA-5, a pathogen recognition receptor located in the cytosol.15 A further advantage of these TLRLs is that their receptors are located intracellularly within the endosomal compartment and require their ligands to be internalized by the cell, thus preventing accidental activation of nontargeted cells. The NP surface was coated with DC-SIGN–specific humanized Abs to determine whether TLRLs display enhanced adjuvant properties when targeted to human DCs. DC-SIGN is a CLR that is predominantly expressed by DCs in humans and internalizes Ag for presentation to T cells.2,16 In addition, the safety and efficacy of targeted TLRLs for vaccination purposes was evaluated in mice in vivo using NPs loaded with Ag and TLRLs. These NPs were targeted to the DC-specific CLR DEC-205, because DC-SIGN is differentially expressed in mice and humans and is mainly located intracellularly in mice.2,17 Conversely, DEC-205 has been harnessed for DC-targeted vaccination strategies in numerous studies in mice.18-21 The results of our studies show that targeted delivery of TLRLs to surface receptors on human and mouse DCs significantly enhances adjuvanticity. In combination with targeted Ag, it allows induction of cellular immune responses at low doses of TLRLs, thereby reducing toxicity.
Methods
Mice
C57Bl/6J mice (Charles River Laboratories) were maintained under specific pathogen-free conditions at the Central Animal Laboratory (Nijmegen, the Netherlands). OT-I mice were bred at the same facility. Drinking water and food were provided ad libitum. The experiments were performed according to guidelines for animal care of the Nijmegen Animal Experiments Committee.
Reagents and Abs
R848 was from Axorra, poly I:C from Sigma-Aldrich, and endotoxin-free ovalbumin (OVA) from Hyglos. Abs used were: anti–human CD80 (BD Biosciences), anti–human CD83 (Beckman Coulter), anti–human CD86 (BD Biosciences), anti–human perforin (Invitrogen), anti–human CD69 (BD Biosciences), anti–human granzyme B-PE (Sanquin), rat IgG F(ab′)2 fragment (Jackson ImmunoResearch), anti–mouse CD4-APC (BD Biosciences), anti–mouse CD8-PerCP (BioLegend), and anti–mouse Va2-PE (BD Biosciences). The composite IgG2/IgG4 humanized anti–human DC-SIGN Ab hD1 and its control 5g1.1 were described previously.16,22,23 F(ab′)2 fragments derived from a rat Ab-recognizing mouse DEC-205 were purified from NLDC-145 hybridoma.
Generation of targeted NP vaccine carriers
NP vaccine carriers coated with lipid-PEG and carrying Abs or F(ab′)2 fragments were generated using the copolymer PLGA essentially as described previously.13 For experiments with human DCs, TLRLs were encapsulated by adding poly I:C (4 mg in 100 μL of PBS) and R848 (0.8 mg in 50 μL of H2O/DMSO, 9:1) to 50 mg of PLGA. For mouse experiments, TLRLs were encapsulated by adding R848 (0.4 mg) and poly I:C (1.6 mg) and, to part of the samples, endotoxin-free OVA (4 mg in 100 μL of PBS) to 50 mg of PLGA during the first step of the production process. The encapsulation efficiency of poly I:C and R848 was determined by reverse-phase HPLC, essentially as described previously.24 The αDC-SIGN Ab hD1, its isotype control 5g1.1, or F(ab′)2 fragments of the αDEC-205 Ab or isotype control Ab were attached to the lipid-PEG layer as described previously.13
Cells
Buffy coats were obtained from healthy volunteers after confirmed consent in accordance with the Declaration of Helsinki and according to institutional guidelines. Human immature DCs (iDCs) were cultured from PBMCs isolated from the blood of HLA-A2.1+ individuals, as reported previously.16 Isolation and TCR transfection of human naive CD45RA+CD8+ gp100-specific T cells was performed as described previously.25 Mouse BM-derived DCs (BMDCs) were obtained from C57Bl/6J femurs and tibias and cultured in IMDM (GIBCO-BRL) containing 10% FCS and 30% GM-CSF–containing J558 conditioned medium, prepared as described previously.26 BMDCs were routinely checked for purity and maturation markers by flow cytometry. OT-1 CD8+ T cells were isolated from lymph node and spleen suspensions of OT-1 mice. The OT-I cells were sorted using the Miltenyi Biotec CD8+ magnetic cell-sorting kit. Phenotypic characterization of OT-I cells was performed using a CyAn flow cytometer (Beckman Coulter).
Binding and uptake of NPs by human DCs
Particle uptake and binding was studied by incubating 105 human iDCs with 100 μg/mL of NPs carrying TLRLs and the fluorescent dye FITC. Binding was assessed after incubation on ice for 1 hour at 4°C, and uptake was studied by incubation at 37°C. At the indicated time points, some of the cells were washed and cell-associated fluorescence was quantified by flow cytometry on a FACSCalibur (BD Biosciences).
DC maturation and activation
Human day 6 iDCs were plated and incubated with or without TLRLs (encapsulated or soluble) at various concentrations for 48 hours in a 24-well plate. After 2 days, the supernatant was harvested and IL-12, TNF-α (both from Pierce), and IL-6 (Sanquin) levels were determined by ELISA. DCs were isolated, stained for the maturation markers CD80 and CD83, and analyzed by flow cytometry. Relative expression levels were determined by dividing mean fluorescent intensities of experimental samples by those of iDCs.
Human naive CD8+ T-cell activation
DCs matured with soluble or encapsulated poly I:C and R848 or iDCs were plated and pulsed with gp100280-88 or gp100154-62 peptide. Subsequently, DCs were washed and cocultured with TCR-transfected naive CD8+ T cells. Cytokine production was measured in supernatant collected at days 1 and 5 by cytometric bead array (Th1/Th2 kit; BD Biosciences). T cells were harvested on day 1 to determine CD69 expression or on day 5 to determine granzyme B and perforin expression by flow cytometry. Relative expression levels were determined by dividing mean fluorescent intensities of T cells exposed to gp100280-88-pulsed DCs by those of T cells exposed to DCs pulsed with irrelevant peptide.
In vitro Ag presentation by mouse BMDCs
Day 8 mouse BMDCs were cultured at 5000 cells/well with 50 000 OT-1 cells/well and various amounts of targeted and nontargeted NPs carrying OVA or OVA together with TLRLs. To test for T-cell activation, the culture supernatant was harvested after 3 days to determine IFN-γ production by ELISA (Thermo Scientific). To determine T-cell proliferation, tritiated thymidine (1 μCi [0.037 MBq]/well; MP Biomedicals) was added to the cell cultures. Tritiated thymidine incorporation was measured after 16 hours in a β-scintillation counter.
Mouse in vivo T-cell proliferation assay
Targeted or nontargeted NPs were intravenously injected into the tail veins of C57Bl/6J mice. Soluble TLRLs were injected 15 minutes after NP administration in the other tail vein to prevent binding of TLRLs to the NP surface because of electrostatic interactions. Three days later, 2-3 × 106 CFSE-labeled, OVA-specific CD8+ T cells derived from OT-1 mice were injected intravenously. At day 6, the mice were killed and spleens were isolated. Single cell suspensions were made and analyzed by flow cytometry after staining with fluorophore-coupled Abs to CD4 and the TCRVα2 (BD Pharmingen).
Mouse in vivo cytotoxicity assay and cytokine analysis
Targeted or nontargeted NPs were IV injected into the tail veins of C57Bl/6J mice. Body temperature was measured and serum was obtained 3 hours after injection. TNF-α, IL-6, IL-8, and IL-10 were determined using the BioPlex system (Bio-Rad) in combination with a Milliplex cytokine kit (Millipore). IFN-α and IFN-β were levels were determined by ELISA (PBL Interferon Source). Seven days after IV injection splenocytes were prepared from wild-type C57Bl/6J mice and divided into 2 groups. Cells were pulsed with relevant OVA SIINFEKL peptide or irrelevant E1A peptide and labeled with either 1μM (irrelevant peptide) or 10μM (relevant OVA peptide) CFSE. The cells were mixed and 5-10 × 106 total cells were injected intravenously into the mice. As a positive control for Ag-dependent killing of target cells, splenocytes were also injected into an OT-1 mouse for each experiment, which specifically eliminated the CFSEhigh cells loaded with the relevant OVA peptide (data not shown). The following day, the mice were killed, spleens were isolated, and single cell suspensions made. Killing was measured by flow cytometry using the following formula: (1 − [(peakrelevant/peakirrelevant)vaccinated × (peakrelevant/peakirrelevant)naive]) × 100%.
Statistical analysis
Data were analyzed by 1- or 2-way ANOVA, followed by a Bonferroni posttest. In experiments comprising 2 groups, data were analyzed with an unpaired 2-tailed t test.
Results
NPs carrying TLRLs and DC-SIGN Abs target human DCs
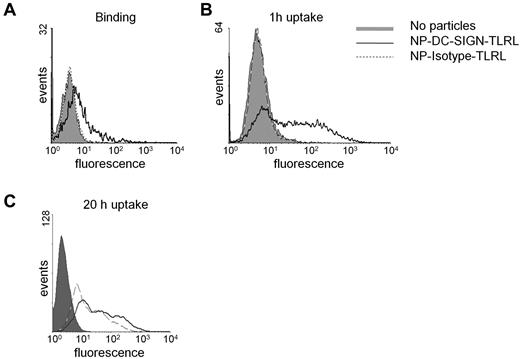
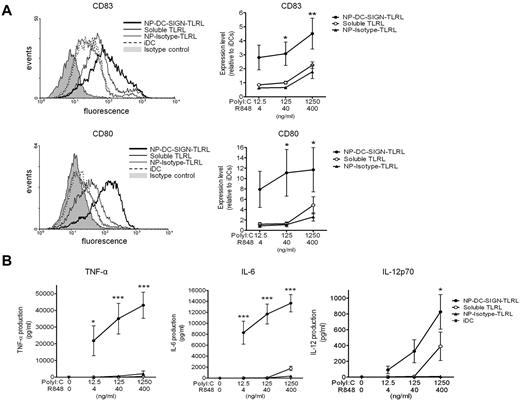
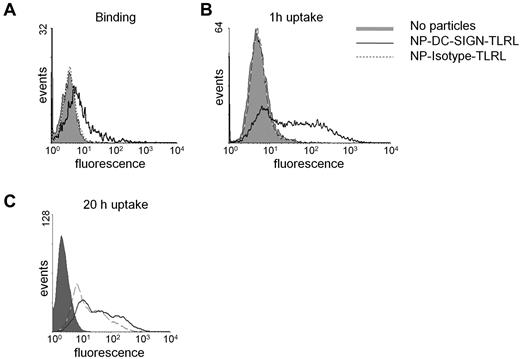
Poly I:C and R848 were encapsulated within 200-nm NPs carrying the humanized anti–DC-SIGN Ab hD1 (for the physical and biochemical characteristics of the NPs used in this study, see supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The hD1 Ab is a composite IgG2/IgG4 Ab, which should prevent interactions with complement or Fc receptors.16,23 Human DCs readily bound and internalized NPs carrying TLRLs targeted to DC-SIGN (NP-DC-SIGN-TLRL, Figure 1A-C). Within 1 hour, most DCs had taken up targeted NPs, as shown by flow cytometry (Figure 1B). In contrast, uptake of isotype control Ab NPs (NP-Isotype-TLRL) was only detected after 20 hours of incubation, demonstrating that nonspecific particle uptake was negligible (Figure 1C). Internalization of NP-DC-SIGN-TLRL was confirmed by confocal microscopy (supplemental Figure 1).
Binding and uptake of NPs by human monocyte–derived DCs. NP uptake and binding was studied by incubating immature human monocyte–derived DCs with 100 μg/mL of targeted (NP-DC-SIGN-TLRL) and nontargeted (NP-Isotype-TLRL) NPs carrying TLRLs and a fluorescent dye. Binding was assessed after incubation on ice for 1 hour at 4°C (A), whereas uptake was studied by incubation for 1 hour (B) or 20 hours (C) at 37°C. Data from 1 representative experiment of 3 are shown.
Binding and uptake of NPs by human monocyte–derived DCs. NP uptake and binding was studied by incubating immature human monocyte–derived DCs with 100 μg/mL of targeted (NP-DC-SIGN-TLRL) and nontargeted (NP-Isotype-TLRL) NPs carrying TLRLs and a fluorescent dye. Binding was assessed after incubation on ice for 1 hour at 4°C (A), whereas uptake was studied by incubation for 1 hour (B) or 20 hours (C) at 37°C. Data from 1 representative experiment of 3 are shown.
Targeting TLRLs to human DC-SIGN enhances DC maturation and activation
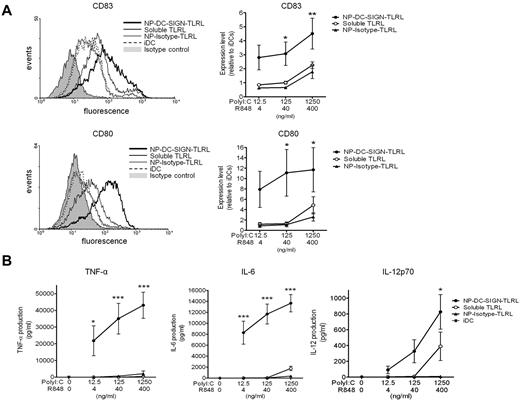
To demonstrate the efficacy of targeted TLRLs, we compared the expression of DC-maturation markers by human DCs exposed to soluble TLRLs, NP-DC-SIGN-TLRL, or NP-Isotype-TLRL. Note that the highest concentrations of poly I:C (1250 ng/mL) and R848 (400 ng/mL) used in our studies were 10-fold lower than what is generally used to obtain full-blown activation of moDCs in vitro, and is around the threshold level for the induction of IL-12p70 production.14 Soluble TLRLs induced DC maturation marker expression to the same level as NP-Isotype-TLRL. In contrast, NP-DC-SIGN-TLRL significantly enhanced DC maturation compared with soluble TLRLs and NP-Isotype-TLRL. DC-SIGN targeting resulted in a > 100-fold more effective induction of CD80 and CD83, indicative of DC maturation and activation, as determined with DCs from 6 independent donors (Figure 2A). To further support these findings, we studied DC activation as determined by measuring cytokine production. We observed that soluble TLRLs and NP-Isotype-TLRL minimally induced secretion of the proinflammatory cytokines TNF-α and IL-6, whereas NP-DC-SIGN-TLRL induced high levels of TNF-α and IL-6 expression by DCs (Figure 2B). Moreover, IL-12p70, a cytokine of key importance in initiating CTL responses, was expressed by DCs treated with NP-DC-SIGN-TLRL at all concentrations tested, whereas it was only expressed by DCs treated with the highest concentration of soluble TLRLs. We could not detect IL-12p70 production by DCs treated with NP-Isotype-TLRL at any of the concentrations tested (Figure 2B). These data strongly support the notion that only targeted TLRLs can induce DC activation at low concentrations.
DC-targeted TLRLs enhance maturation and activation of human DCs in vitro. Human monocyte–derived DCs were cultured in the presence of various concentrations of poly I:C and R848, either in soluble form (soluble TLRL) or encapsulated within NPs carrying αDC-SIGN (NP-DC-SIGN-TLRL) or isotype control (NP-Isotype-TLRL) Abs for 48 hours. DCs cultured in medium without TLRLs were included as iDCs. (A) DC maturation was checked by analyzing CD80 and CD83 expression by flow cytometry. Representative flow cytometric histograms of a single donor are shown in the left panels. Relative expression levels were determined for each donor by dividing mean fluorescent intensities of experimental samples by those of iDCs. Right panels show mean relative expression levels of 6 independent donors ± SEM, with the x-axis representing the concentration of the respective soluble or encapsulated TLRLs present in the culture medium. (B) TNF-α, IL-6, and IL-12 production by the DCs were determined in the culture supernatant. Values represent mean cytokine production levels of DCs from 6 independent donors ± SEM. The x-axis represents the concentration of the respective soluble or encapsulated TLRLs present in the culture medium. *P < .05, **P < .01, and ***P < .001 for the significant difference between soluble TLRL and NP-Isotype-TLRL.
DC-targeted TLRLs enhance maturation and activation of human DCs in vitro. Human monocyte–derived DCs were cultured in the presence of various concentrations of poly I:C and R848, either in soluble form (soluble TLRL) or encapsulated within NPs carrying αDC-SIGN (NP-DC-SIGN-TLRL) or isotype control (NP-Isotype-TLRL) Abs for 48 hours. DCs cultured in medium without TLRLs were included as iDCs. (A) DC maturation was checked by analyzing CD80 and CD83 expression by flow cytometry. Representative flow cytometric histograms of a single donor are shown in the left panels. Relative expression levels were determined for each donor by dividing mean fluorescent intensities of experimental samples by those of iDCs. Right panels show mean relative expression levels of 6 independent donors ± SEM, with the x-axis representing the concentration of the respective soluble or encapsulated TLRLs present in the culture medium. (B) TNF-α, IL-6, and IL-12 production by the DCs were determined in the culture supernatant. Values represent mean cytokine production levels of DCs from 6 independent donors ± SEM. The x-axis represents the concentration of the respective soluble or encapsulated TLRLs present in the culture medium. *P < .05, **P < .01, and ***P < .001 for the significant difference between soluble TLRL and NP-Isotype-TLRL.
Targeting TLRLs to human DC-SIGN enhances CD8+ T-cell responses
Activation of naive CD8+ T cells by Ag-presenting DCs is essential to inducing bona fide CTL responses. Therefore, we determined whether targeting TLRLs to DC-SIGN would, in addition to enhancing DC maturation and activation, result in Ag-specific activation of naive CD8+ T cells. DCs, matured and activated with NP-DC-SIGN-TLRL and NP-Isotype-TLRL or soluble TLRLs for 2 days, were pulsed with melanoma-associated antigenic gp100 peptides and cocultured with naive CD8+ T cells transfected with mRNA encoding a gp100 specific TCR.25,27 DCs induced expression of the early activation marker CD69 on the TCR-transfected CD8+ T cells in an Ag-dependent, but maturation status independent, fashion (supplemental Figure 2). Only T cells exposed to DCs treated with both peptide Ag and TLRLs produced cytokines, and the highest amounts of IL-2 and IFN-γ were detected when T cells were cocultured with DCs treated with NP-DC-SIGN-TLRL. Control NP-Isotype-TLRL induced only low levels of IFN-γ (< 600 pg/mL) and no IL-2. Moreover, soluble TLRLs were 100-fold less effective in inducing IL-2 and IFN-γ production compared with NP-DC-SIGN-TLRL (Figure 3A). After 5 days of coculturing DCs and T cells, we clearly observed enhanced expression of the CTL marker perforin in T cells cultured with DCs treated with the highest concentration of NP-DC-SIGN-TLRL compared with those cultured with DCs exposed to soluble TLRLs and NP-Isotype-TLRL (Figure 3B). In addition, a second CTL marker, granzyme B, was induced in T cells when the relevant Ag was present. As expected, granzyme B expression levels were highest in T cells exposed to DCs treated with the highest concentration of TLRLs. Moreover, granzyme B expression was most pronounced in T cells exposed to DCs treated with NP-DC-SIGN-TLRL (Figure 3C). These data indicate that targeting encapsulated TLRLs to DC-SIGN is very effective in maturing and activating human DCs to prime CD8+ T cells for Ag-specific CTL responses.
DC-targeted TLRLs enhance CD8+ T-cell stimulation potential of human DCs in vitro. DCs matured and activated by the various TLRL preparations (NP-DC-SIGN-TLRL, soluble TLRL, NP-Isotype-TLRL) and untreated controls (iDC, irrelevant [irr] peptide) were pulsed with the tumor-associated gp100280-88 peptide Ag (NP-DC-SIGN-TLRL, soluble TLRL, NP-Isotype-TLRL, and iDC) or irrelevant peptide and cocultured with gp100280-88-specific naive CD8+ T cells. (A) IFN-γ and IL-2 levels were determined in supernatants after overnight culture of DCs and T cells. Part of the T cells were cultured for an additional 4 days to determine the expression levels of the CTL markers (B) perforin and (C) granzyme B by flow cytometry. Three experiments were performed using cells from independent donors, giving similar results. Data represent mean values of one experiment performed in triplicate ± SEM. The x-axis in panels A and C represents the concentration of the respective soluble or encapsulated TLRLs present in the culture medium. Bar graph (B) only shows data obtained at the highest TLRL concentrations tested, 1250 ng/mL of poly I:C in combination with 400 ng/mL of R848. *P < .05 and **P < .01 for the significant difference from soluble TLRL.
DC-targeted TLRLs enhance CD8+ T-cell stimulation potential of human DCs in vitro. DCs matured and activated by the various TLRL preparations (NP-DC-SIGN-TLRL, soluble TLRL, NP-Isotype-TLRL) and untreated controls (iDC, irrelevant [irr] peptide) were pulsed with the tumor-associated gp100280-88 peptide Ag (NP-DC-SIGN-TLRL, soluble TLRL, NP-Isotype-TLRL, and iDC) or irrelevant peptide and cocultured with gp100280-88-specific naive CD8+ T cells. (A) IFN-γ and IL-2 levels were determined in supernatants after overnight culture of DCs and T cells. Part of the T cells were cultured for an additional 4 days to determine the expression levels of the CTL markers (B) perforin and (C) granzyme B by flow cytometry. Three experiments were performed using cells from independent donors, giving similar results. Data represent mean values of one experiment performed in triplicate ± SEM. The x-axis in panels A and C represents the concentration of the respective soluble or encapsulated TLRLs present in the culture medium. Bar graph (B) only shows data obtained at the highest TLRL concentrations tested, 1250 ng/mL of poly I:C in combination with 400 ng/mL of R848. *P < .05 and **P < .01 for the significant difference from soluble TLRL.
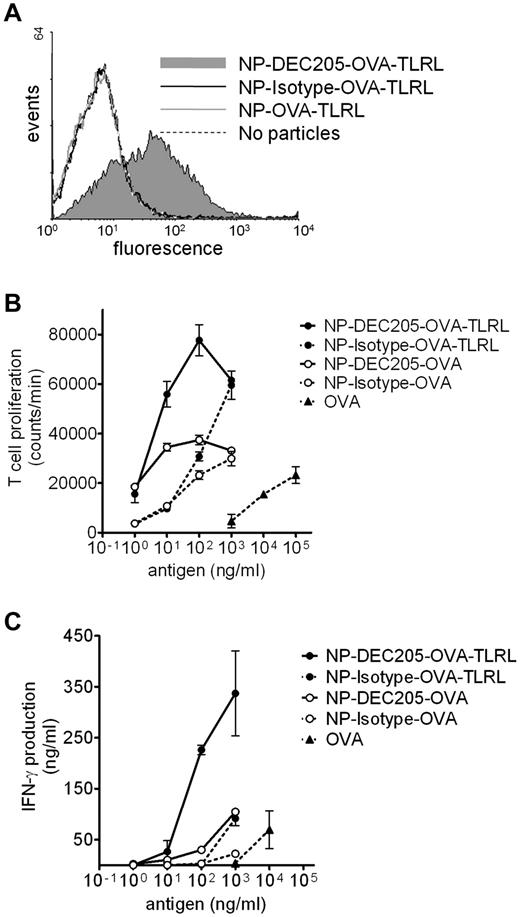
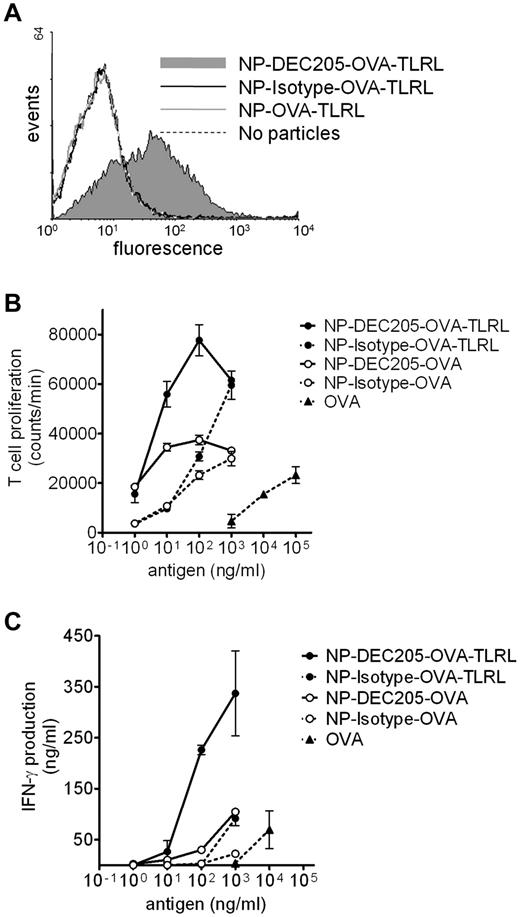
Targeting TLRLs and Ag to mouse DEC-205 enhances CD8+ T-cell activation in vitro
We also evaluated whether targeted delivery of a complete vaccine harboring targeting component, Ag, and TLRLs also enhanced vaccine efficacy in vivo. The model Ag OVA was co-encapsulated with or without the TLRLs poly I:C and R848 (NP-OVA-TLRL and NP-OVA, respectively). F(ab′)2 fragments of an Ab recognizing DEC-205 or control F(ab′)2 fragments were coated on the NPs for targeting purposes, which should prevent internalization via Fc receptors. We verified that only DEC-205–targeted NPs bound to mouse DCs (Figure 4A). Targeted delivery of Ag significantly enhanced OVA presentation and subsequent proliferation of murine CD8+ T cells in vitro compared with nontargeted controls. Furthermore, T-cell proliferation was significantly enhanced by co-encapsulation of TLRLs and OVA for targeted and nontargeted particles (Figure 4B). To determine Ag-specific activation of T cells, we measured IFN-γ secretion. As is the case with human data, high levels of IFN-γ were only detected when TLRLs were specifically targeted to DCs (Figure 4C). Targeted delivery of NP vaccines also enhanced CD4+ T-cell proliferation and activation (supplemental Figure 3A-B). The effect of targeting on T-cell activation was more pronounced for CD8+ than for CD4+ T cells. This is in agreement with earlier reports showing that targeting DEC-205 with Ag-Ab fusion proteins preferentially induces CD8+ T-cell responses.20
Binding, uptake, and Ag presentation of NPs by mouse DCs. (A) Mouse BMDCs were incubated with NPs harboring OVA and TLRLs for 1 hour on ice. Particles carried no (NP-OVA-TLRL), rat anti–mouse DEC205 (NP-DEC205-OVA-TLRL), or control (NP-Isotype-OVA-TLRL) F(ab′)2 fragments. NP binding was analyzed by staining with fluorescently labeled secondary Abs recognizing rat IgG and FACS analyses. (B-C) CD8+ T-cell proliferation and activation was determined after incubation of mouse BMDCs and OT-1 T cells with various concentrations of OVA, either in soluble form targeted or nontargeted within NPs harboring both OVA and TLRLs (NP-DEC205-OVA-TLRL and NP-Isotype-OVA-TLRL, respectively) or targeted or nontargeted within NPs harboring only OVA (NP-DEC205-OVA and NP-Isotype-OVA, respectively). Note that NPs carrying TLRLs contain 0.06 ng of R848 and 0.2 ng of poly I:C per nanogram of OVA. After 3 days, CD8+ T-cell proliferation was measured by tritium thymidine incorporation assay (B) and CD8+ T-cell activation was determined by measuring IFN-γ levels in the supernatant (C). Two independent experiments were performed, yielding similar results. Data represent mean values of 1 experiment performed in duplicate ± SEM.
Binding, uptake, and Ag presentation of NPs by mouse DCs. (A) Mouse BMDCs were incubated with NPs harboring OVA and TLRLs for 1 hour on ice. Particles carried no (NP-OVA-TLRL), rat anti–mouse DEC205 (NP-DEC205-OVA-TLRL), or control (NP-Isotype-OVA-TLRL) F(ab′)2 fragments. NP binding was analyzed by staining with fluorescently labeled secondary Abs recognizing rat IgG and FACS analyses. (B-C) CD8+ T-cell proliferation and activation was determined after incubation of mouse BMDCs and OT-1 T cells with various concentrations of OVA, either in soluble form targeted or nontargeted within NPs harboring both OVA and TLRLs (NP-DEC205-OVA-TLRL and NP-Isotype-OVA-TLRL, respectively) or targeted or nontargeted within NPs harboring only OVA (NP-DEC205-OVA and NP-Isotype-OVA, respectively). Note that NPs carrying TLRLs contain 0.06 ng of R848 and 0.2 ng of poly I:C per nanogram of OVA. After 3 days, CD8+ T-cell proliferation was measured by tritium thymidine incorporation assay (B) and CD8+ T-cell activation was determined by measuring IFN-γ levels in the supernatant (C). Two independent experiments were performed, yielding similar results. Data represent mean values of 1 experiment performed in duplicate ± SEM.
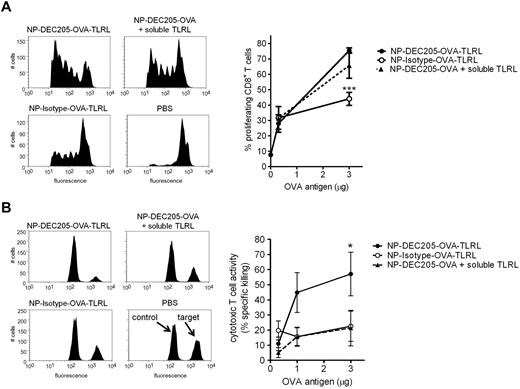
Targeting TLRLs and Ag to mouse DEC-205 enhances CTL responses in vivo
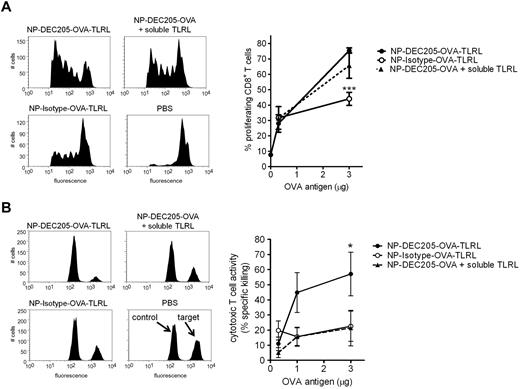
Vaccination of mice with NP-DEC205-OVA-TLRL enhanced CD8+ T-cell proliferation levels compared with NP-Isotype-OVA-TLRL (Figure 5). Encapsulation of Ag within NPs by itself was very effective for the induction of CD4+ T-cell proliferation. At the Ag dose at which differences in CD8+ T-cell proliferation became apparent, CD4+ T-cell proliferation was not further enhanced by targeted delivery to DEC-205 (supplemental Figure 3C). Vaccination with NP-DEC205-OVA-TLRL resulted in only a slight, but not significant, increase in proliferative CD8+ T-cell responses compared with NP-DEC205-OVA combined with soluble, nontargeted TLRL administration (Figure 5A). However, only CD8+ T cells isolated from mice vaccinated with NP-DEC205-OVA-TLRL produced high levels of IFN-γ on restimulation (supplemental Figure 4). This is in agreement with earlier reports showing that Ag targeted to DEC-205 induces T-cell proliferation in mice irrespective of DC-maturation stimuli. However, in the absence of TLR stimuli, T cells fail to differentiate into proper CTLs, resulting in tolerance.18 To study the effect of DC-targeted TLRLs on Ag-dependent CTL responses, we performed an in vivo cytotoxicity assay 1 week after vaccination by evaluating the sensitivity of adoptively transferred congeneic, Ag-pulsed splenocytes for lysis by CTLs. In contrast to the T-cell proliferation assay, the cytotoxicity assay was conducted without adoptive transfer of Ag-specific T cells and determined the CTL activity induced in the endogenous T-cell pool. The results show that only NP-DEC205-OVA-TLRL induced significant CTL responses in an Ag-dependent manner (Figure 5B).
Enhanced CTL induction by co-targeting of Ags and TLRLs to DCs in mice. Mice were vaccinated intravenously with various amounts OVA Ag encapsulated in targeted NPs containing TLRLs (NP-DEC205-OVA-TLRL), nontargeted NPs containing TLRLs (NP-Isotype-OVA-TLRL), targeted NPs combined with soluble TLRLs (NP-DEC205-OVA + soluble TLRL), or PBS. Note that per microgram of OVA, 0.06 μg of R848 and 0.20 μg of poly I:C were injected, either co-encapsulated with the OVA in NPs or in soluble form. (A) To determine CD8+ T-cell proliferation, CFSE-labeled OVA-specific OT-1 CD8+ T cells were injected and 3 days later lymph nodes were harvested. OT-1 T-cell proliferation was assessed by FACS analysis. Histograms show proliferation as measured by CFSE dye dilution in OT-I T cells for mice receiving NPs carrying 3 μg of OVA. Data in graph represent means ± SEM of 3 independent experiments with 2 mice per group. ***P < .001 for the significant difference from NP-DEC205-OVA-TLRL. (B) Ag-dependent cytotoxic T-cell activity of the endogenous T-cell pool was assessed by an in vivo cytotoxicity assay, determining killing of differentially labeled target and control cells pulsed with OVA peptide and a high CFSE concentration or irrelevant peptide and a low CFSE concentration, respectively. Seven days after vaccination, the CFSEhigh target and CFSElow control cells were injected and the next day, spleens were harvested and specific killing was determined by flow cytometry. Representative histograms of CFSEhigh target and CFSElow control cells from mice receiving the highest Ag dose (3 μg of OVA) or PBS are shown. Data in graph represent means ± SEM of 3 independent experiments with 2 mice per group. *P < .05 for the significant difference from NP-Isotype-OVA-TLRL and NP-DEC205-OVA + soluble TLRL
Enhanced CTL induction by co-targeting of Ags and TLRLs to DCs in mice. Mice were vaccinated intravenously with various amounts OVA Ag encapsulated in targeted NPs containing TLRLs (NP-DEC205-OVA-TLRL), nontargeted NPs containing TLRLs (NP-Isotype-OVA-TLRL), targeted NPs combined with soluble TLRLs (NP-DEC205-OVA + soluble TLRL), or PBS. Note that per microgram of OVA, 0.06 μg of R848 and 0.20 μg of poly I:C were injected, either co-encapsulated with the OVA in NPs or in soluble form. (A) To determine CD8+ T-cell proliferation, CFSE-labeled OVA-specific OT-1 CD8+ T cells were injected and 3 days later lymph nodes were harvested. OT-1 T-cell proliferation was assessed by FACS analysis. Histograms show proliferation as measured by CFSE dye dilution in OT-I T cells for mice receiving NPs carrying 3 μg of OVA. Data in graph represent means ± SEM of 3 independent experiments with 2 mice per group. ***P < .001 for the significant difference from NP-DEC205-OVA-TLRL. (B) Ag-dependent cytotoxic T-cell activity of the endogenous T-cell pool was assessed by an in vivo cytotoxicity assay, determining killing of differentially labeled target and control cells pulsed with OVA peptide and a high CFSE concentration or irrelevant peptide and a low CFSE concentration, respectively. Seven days after vaccination, the CFSEhigh target and CFSElow control cells were injected and the next day, spleens were harvested and specific killing was determined by flow cytometry. Representative histograms of CFSEhigh target and CFSElow control cells from mice receiving the highest Ag dose (3 μg of OVA) or PBS are shown. Data in graph represent means ± SEM of 3 independent experiments with 2 mice per group. *P < .05 for the significant difference from NP-Isotype-OVA-TLRL and NP-DEC205-OVA + soluble TLRL
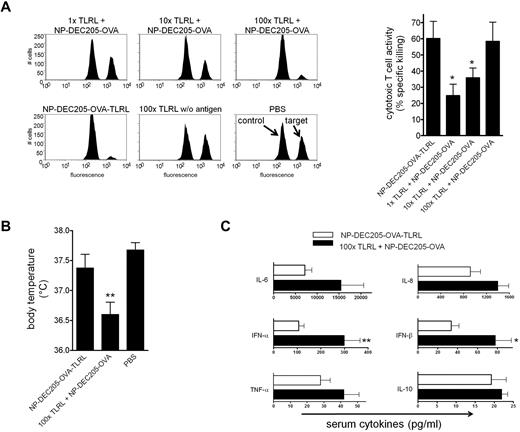
Targeted delivery of TLRLs enhances adjuvanticity and reduces toxicity
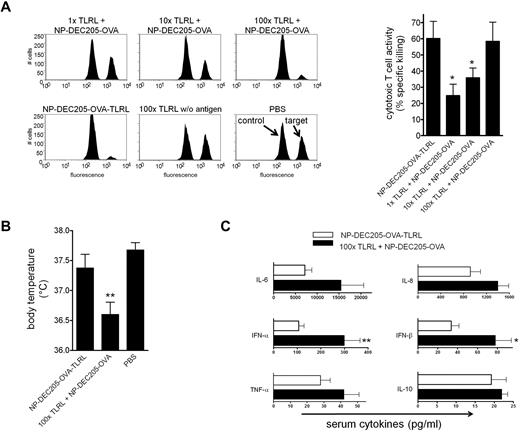
To further substantiate the importance of targeting adjuvants to DCs, we determined the dose of soluble TLRLs that is required to induce similar CTL responses as induced by DEC-205-targeted TLRLs. Mice were vaccinated with NP-DEC205-OVA-TLRL or NP-DEC205-OVA together with increasing doses of soluble TLRLs. We observed that soluble TLRLs required a 100-fold higher dose to induce CTL responses comparable to those induced by targeted TLRLs (Figure 6A). Within hours after administration of these high doses of soluble TLRLs, mice were hypothermic (Figure 6B) and showed severe signs of discomfort such as hunched backs and reduced mobility, resulting in death of 20% of the animals within 4 hours (data not shown). There was full-blown DC maturation in spleen and peripheral lymph nodes, evidenced by strong up-regulation of the maturation markers CD80 and CD86 by CD11c+ cells (supplemental Figure 5). In contrast, mice receiving NP-DEC205-OVA-TLRL showed no signs of hypothermia or discomfort and an overall less-mature DC phenotype (Figure 6B and supplemental Figure 5). The symptoms displayed by mice receiving high-dose soluble TLRLs resemble shock-like symptoms caused by cytokine storms after strong immune activation.28 One of the key cytokines induced by poly I:C and R848 is IFN-α, which is a potent DC activator but is also thought to be responsible for many of the adverse effects observed in human subjects treated with these TLRLs.10,11,29,30 Indeed, in analyzing serum cytokine levels, we observed significantly increased IFN-α and IFN-β levels in mice treated with the high-dose soluble TLRLs compared with those in mice receiving low-dose targeted TLRLs. Low-dose targeted TLRLs (NP-DEC205-OVA-TLRL) also tended to induce lower serum levels of TNF-α, IL-6, IL-8, and IL-10 than the 100-fold higher dose of nontargeted, soluble TLRLs, but the differences were not significant (Figure 6C). Serum cytokine levels of mice receiving NP-Isotype-OVA-TLRL or NP-DEC205-OVA alone or in combination with low-dose soluble TLRLs were not elevated compared with control mice receiving no TLRLs (supplemental Figure 6). This shows that the NP vaccines were effectively targeted to specific DCs that were responsible for inducing serum cytokines to a level that supports CTL induction without concurrent toxicity.
Targeted TLRLs are effective at low doses, preventing high serum IFN-α and IFN-β levels and hypothermia. (A) Mice were vaccinated intravenously with targeted NPs carrying 3 μg of OVA, 0.18 μg of R848, and 0.61 μg of poly I:C (NP-DEC205-OVA-TLRL) or soluble R848 and poly I:C at 1, 10, or 100 times the dose in combination with targeted NPs carrying 3 μg of OVA (1×, 10×, and 100× TLRL + NP-DEC205-OVA). Control mice received IV injections with PBS or the highest dose of soluble TLRLs in the absence of Ag (100× TLRL without Ag). Seven days after vaccination, Ag-dependent CTL activity was assessed by in vivo cytotoxicity assay. Representative histograms of CFSEhigh target and CFSElow control cells isolated from mice injected with NPs, 100× TLRLs without Ag, or PBS are shown. Data in bar graph represent means ± SEM for mice receiving NP-DEC205-OVA-TLRL and NP-DEC205-OVA combined with soluble TLRLs. *P < .05 for the significant difference from NP-DEC205-OVA-TLRL. (B) Mice were vaccinated as in panel A with NP-DEC205-OVA-TLRL, 100× TLRL + NP-DEC205-OVA, or PBS. Body temperature was measured 3 hours after vaccination. **P < .01 for the significant difference from PBS control. (C) Mice were vaccinated with NP-DEC205-OVA-TLRL or 100× TLRL + NP-DEC205-OVA as in panel A. Blood was drawn 3 hours after vaccination and serum cytokine levels determined. *P < .05 and **P < .01 for the significant difference from NP-DEC205-OVA-TLRL. Data in graphs represent means ± SEM from pooled data of 2 independent experiments with 2 mice per group.
Targeted TLRLs are effective at low doses, preventing high serum IFN-α and IFN-β levels and hypothermia. (A) Mice were vaccinated intravenously with targeted NPs carrying 3 μg of OVA, 0.18 μg of R848, and 0.61 μg of poly I:C (NP-DEC205-OVA-TLRL) or soluble R848 and poly I:C at 1, 10, or 100 times the dose in combination with targeted NPs carrying 3 μg of OVA (1×, 10×, and 100× TLRL + NP-DEC205-OVA). Control mice received IV injections with PBS or the highest dose of soluble TLRLs in the absence of Ag (100× TLRL without Ag). Seven days after vaccination, Ag-dependent CTL activity was assessed by in vivo cytotoxicity assay. Representative histograms of CFSEhigh target and CFSElow control cells isolated from mice injected with NPs, 100× TLRLs without Ag, or PBS are shown. Data in bar graph represent means ± SEM for mice receiving NP-DEC205-OVA-TLRL and NP-DEC205-OVA combined with soluble TLRLs. *P < .05 for the significant difference from NP-DEC205-OVA-TLRL. (B) Mice were vaccinated as in panel A with NP-DEC205-OVA-TLRL, 100× TLRL + NP-DEC205-OVA, or PBS. Body temperature was measured 3 hours after vaccination. **P < .01 for the significant difference from PBS control. (C) Mice were vaccinated with NP-DEC205-OVA-TLRL or 100× TLRL + NP-DEC205-OVA as in panel A. Blood was drawn 3 hours after vaccination and serum cytokine levels determined. *P < .05 and **P < .01 for the significant difference from NP-DEC205-OVA-TLRL. Data in graphs represent means ± SEM from pooled data of 2 independent experiments with 2 mice per group.
Discussion
A variety of strategies have been used to facilitate uptake of Ags by APCs. Supplying Ags in particulate form, such as virus-like particles, PLGA particles, liposomes, and immune stimulating complexes facilitates passive targeting to DCs, thereby enhancing antigenicity.31 The first vaccines actively targeting Ags to DCs using fusion proteins of Ag and mannose receptor- or DEC-205-specific Abs are currently being evaluated in clinical trials and focus on determining the optimal route of administration.32,33 So far, these Ab/Ag fusion proteins have been administered intradermally and intravenously to compare local versus systemic vaccine effects. In contrast, for safety reasons, the adjuvants R848 and polyICLC are only being applied locally in these trials.33 Consequently, most of the targeted DCs will not be properly activated on IV vaccine administration. Our findings now clearly show that in vivo DC-vaccination strategies benefit from actively co-targeting Ag and adjuvant via nanometer sized particulate vaccine carriers.
Previous findings indicated that the specific delivery of immune stimulators to DCs boosts immune responses. Targeted delivery of the strongly immunogenic carrier protein keyhole limpet hemocyanin to DC-SIGN in Rag2−/− γC−/− mice reconstituted with human immune cells has been reported to reduce the growth of Burkitt lymphoma–derived RAJI cells.34 Therefore, we believe that targeted delivery of TLRLs to specific immune cells with nanocarriers may significantly improve monotherapies. With respect to vaccination, there is a striking lack of studies on targeted delivery of immune stimulators to APCs, whereas the first reports on Ab-mediated targeting of Ags to APCs already date back more than 20 years.2 Because many immune stimulators such as TLRLs interact with cell-surface molecules themselves, this significantly complicates specific targeting to DCs. In fact, vaccines have been generated consisting of conjugates between TLRLs and Ags that make use of this principle of TLRLs binding to surface receptors. Conjugates comprising the TLR2 ligand Pam3CysSK4 or the TLR9 ligand CpG are effectively internalized by cells in a TLR-independent fashion by as-yet-undefined receptors, whereas TLR-dependent signaling facilitates cross-priming.35,36 Shielding TLRLs by encapsulation within polymeric carriers prevents such nonspecific interactions. However, specifically targeting polymer particles to DCs is challenging because it requires effective shielding to reduce nonspecific uptake of particles while simultaneously promoting specific uptake. To the best of our knowledge, there is only one report on Ab-mediated delivery of a polymer vaccine carrier in vivo. It describes targeting of OVA Ag encapsulated within acid-degradable microparticles coated with Abs recognizing mouse DEC-205 in the absence of additional adjuvants.37 Spleen cells isolated from mice vaccinated with these targeted microparticles show twice as many IFN-γ–producing CD8+ T cells and stronger Ag-dependent CTL activity ex vivo compared with those isolated from mice treated with particles carrying isotype control Abs; it was speculated that their particles possess an inherent capacity to drive the maturation and activation of DCs essential to induce CTL responses.37 Our previous in vitro study showed that NPs can be more effectively targeted to human DCs than microparticles because they can be effectively shielded to prevent nonspecific uptake.13 This is supported by our current findings showing that TLRLs encapsulated in nontargeted PLGA NPs shielded with a lipid-PEG layer are poor inducers of DC maturation and activation in vitro and fail to induce potent CTL responses in vivo. However, incorporation of Abs or F(ab′)2 fragments recognizing CLRs as DC-specific portals transforms these NPs into highly effective inducers of immune responses. These small, 200-nm NPs likely penetrate tissues much better than microparticles, including entry to the lymph nodes along the conduits, which allow only passage of structures up to a few hundred nanometers.38 Another major advantage of PLGA is that it has already been used in patients; nontargeted PLGA particles have already been evaluated as a vaccine carrier in numerous preclinical studies.39 In addition, nontargeted PLGA NP vaccines carrying Ag and TLRLs have recently been shown to induce robust humoral responses against various influenza strains in mice and nonhuman primates.40 Another recent study showed that nontargeted PLGA NPs loaded with a TLR4 agonist and melanoma-associated antigenic peptides delays growth of B16 melanoma cells in a prophylactic setting in mice.41 These studies support the use of PLGA NPs as carriers of antigens and adjuvants, whereas the use of agents (or derivatives thereof) that are already being evaluated in clinical trials, such as PLGA, poly I:C, R848, and humanized composite Abs, will facilitate translation of our targeted approach to clinical studies.
Systemic application of high-dose TLRLs to induce DC maturation were accompanied by the release of high levels of IL-6, IL-8, and type I IFNs into the serum. Interestingly, IL-6 is a known marker for lethality in endotoxin-treated mice, which correlates to the mortality we observed with high-dose TLRL treatment.42-44 A wide variety of cell types could be responsible for this proinflammatory cytokine release, because TLRs are broadly expressed. The extent to which such proinflammatory cytokines produced by bystander cells transactivate DCs to induce protective immune responses is not entirely clear. The adjuvant effect of poly I:C is severely reduced when either BM-derived or radioresistant cells lack expression of the poly I:C receptors TLR3 and MDA-5, showing that bystander cells can contribute significantly to the immune response. These bystander cells are the main source of type I IFNs, which are essential for the induction of DC maturation and effective CD4+ T-cell responses.29 However, inflammatory mediators produced by bystander cells after TLRL administration are insufficient to induce IL-12 production by DCs or to promote functional T-cell responses in TCR transgenic mouse models, although they do induce up-regulation of DC maturation markers.45,46 This suggests that DCs require direct recognition of TLRLs to become properly activated. In our experiments, type I IFNs in serum and expression levels of maturation markers by splenic and lymph node DCs were significantly higher after high-dose systemic than after low-dose targeted TLRL treatment, whereas both treatments generated comparable CTL responses. This supports the notion that a strong and potentially dangerous systemic inflammatory response does not necessarily induce superior CTL responses and shows that direct activation of DCs is a more effective approach. Nevertheless, DC-targeted TLRLs unexpectedly induced a similar serum cytokine pattern as high-dose soluble TLRLs, although overall expression levels were lower. This release of cytokines must somehow be initiated by the targeted DCs, because it was not observed after administration of nontargeted low-dose TLRLs, either in soluble form or encapsulated within NPs. The striking similarity in the cytokine pattern induced by both treatments suggests it is unlikely that all cytokines released upon targeted delivery of TLRLs are produced by the targeted DCs themselves, but rather are likely the result of secondary responses induced upon DC activation.
In cancer immunotherapy, one of the major challenges is to break tumor-specific T-cell tolerance. Although exacerbated expression of cytokines can be dangerous, cytokines are crucial to reversing tolerance by DC-based vaccines. Yang et al have shown that a vaccine based on ex vivo Ag-loaded and LPS-matured DCs fails to break CD8+ T-cell tolerance in the presence of regulatory CD4+ T cells. In contrast, ex vivo Ag-loaded immature DCs combined with in vivo injections of LPS or CpG reverses T-cell tolerance.47 In a second study, Yang et al showed that the reversal of tumor-specific CD8+ T-cell tolerance by iDC vaccines in combination with in vivo CpG administration is critically dependent on type I IFN.48 Persistent in vivo TLR activation may not be required when DCs are matured with combinations of TLRLs that act synergistically. Mouse BMDCs activated with poly I:C and R848 release cytokines for prolonged periods of time and overcome the suppressive effects of regulatory T cells on CD4+ and CD8+ T-cell proliferation in vitro.49 Future studies will be needed to determine whether in vivo activation of specific DC subsets by our targeted approach using slow-release particles provides the sustained cytokine release that is required to break T-cell tolerance.
The results of the present study suggest that targeted NPs are well suited to guiding vaccine components to DCs in vivo with high efficacy. Targeted NPs composed of both Ag and adjuvant reach the appropriate cellular compartment for Ag loading and DC activation, resulting in the induction of strong immune responses. Efficient targeting through these NPs also becomes evident from the relatively low amounts of adjuvant that is needed to initiate a productive immune response without any apparent toxicity. Therefore, we believe that NP vaccine carriers are a valuable tool for the further clinical development of targeted vaccination strategies to deliver complete vaccines composed of both Ags and adjuvant to professional APCs.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Charlotte Volgers for her assistance, Karsten Mahnke (University Hospital Heidelberg, Heidelberg, Germany) for supplying the NLDC145 hybridoma, Alexion Pharmaceuticals for supplying the hD1 and 5g1.1 Abs, and Niels Schaft (University Hospital Erlangen, Erlangen, Germany) for supplying the plasmids encoding the gp100-specific TCR.
This work was supported by grants from the Dutch Cancer Society (KUN2008-4189), the European Commission (Immunanomap, MRTN-CT-2006-035946), and The Netherlands Organization for Scientific Research (NWO, Spinoza Prize).
Authorship
Contribution: P.J.T., I.S.Z., L.J.C, M.A.v.H.-K., G.v.d.G., and A.J.A.L. performed the experiments; P.J.T., I.S.Z. L.J.C., and C.G.F. designed and interpreted the experiments; R.G.F analyzed the data; P.J.T., I.S.Z., and C.G.F. wrote the manuscript; and C.G.F. directed the project.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Carl G. Figdor, Department of Tumor Immunology, Nijmegen Centre for Molecular Life Sciences, Radboud University Nijmegen Medical Centre, Post Box 9101, 6500 HB Nijmegen, The Netherlands; e-mail: c.figdor@ncmls.ru.nl.
References
Author notes
P.J.T, I.S.Z., and L.J.C. contributed equally to this work.

![Figure 3. DC-targeted TLRLs enhance CD8+ T-cell stimulation potential of human DCs in vitro. DCs matured and activated by the various TLRL preparations (NP-DC-SIGN-TLRL, soluble TLRL, NP-Isotype-TLRL) and untreated controls (iDC, irrelevant [irr] peptide) were pulsed with the tumor-associated gp100280-88 peptide Ag (NP-DC-SIGN-TLRL, soluble TLRL, NP-Isotype-TLRL, and iDC) or irrelevant peptide and cocultured with gp100280-88-specific naive CD8+ T cells. (A) IFN-γ and IL-2 levels were determined in supernatants after overnight culture of DCs and T cells. Part of the T cells were cultured for an additional 4 days to determine the expression levels of the CTL markers (B) perforin and (C) granzyme B by flow cytometry. Three experiments were performed using cells from independent donors, giving similar results. Data represent mean values of one experiment performed in triplicate ± SEM. The x-axis in panels A and C represents the concentration of the respective soluble or encapsulated TLRLs present in the culture medium. Bar graph (B) only shows data obtained at the highest TLRL concentrations tested, 1250 ng/mL of poly I:C in combination with 400 ng/mL of R848. *P < .05 and **P < .01 for the significant difference from soluble TLRL.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/118/26/10.1182_blood-2011-07-367615/4/m_zh89991182190003.jpeg?Expires=1767773444&Signature=mgIhQBhM8z9z9wCs-fKTIX4EF~6y8bfVauaDa2CIVzbtVcUeuacLgEsxVQ~nZpgU1kFWr6tiG-4SKlBSKAlM3YSpiEeIkgFdJDNHK0TZG4jmhOSz32pWFAsPRjxQo-LiLO0IulbzZqAMMZro-m0ge7dx2pFKYlKHyyO20WcLkvjmK7ZDP5N9rXh70Gz-jjo-Db5LzZigzuL4ljDGzU8AXtUAnOPHK~p-tyDxneq5Wg9IPmZUGyZ~8wYy6l~-H-ZnIodfLQxTSljsnaB~uL99K9m0gDniqpDrgbOSDRZ06xrlmSCjgz~P7kHzxwXrGsfrBtgV532ZyykA1MPeiPgH~A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 3. DC-targeted TLRLs enhance CD8+ T-cell stimulation potential of human DCs in vitro. DCs matured and activated by the various TLRL preparations (NP-DC-SIGN-TLRL, soluble TLRL, NP-Isotype-TLRL) and untreated controls (iDC, irrelevant [irr] peptide) were pulsed with the tumor-associated gp100280-88 peptide Ag (NP-DC-SIGN-TLRL, soluble TLRL, NP-Isotype-TLRL, and iDC) or irrelevant peptide and cocultured with gp100280-88-specific naive CD8+ T cells. (A) IFN-γ and IL-2 levels were determined in supernatants after overnight culture of DCs and T cells. Part of the T cells were cultured for an additional 4 days to determine the expression levels of the CTL markers (B) perforin and (C) granzyme B by flow cytometry. Three experiments were performed using cells from independent donors, giving similar results. Data represent mean values of one experiment performed in triplicate ± SEM. The x-axis in panels A and C represents the concentration of the respective soluble or encapsulated TLRLs present in the culture medium. Bar graph (B) only shows data obtained at the highest TLRL concentrations tested, 1250 ng/mL of poly I:C in combination with 400 ng/mL of R848. *P < .05 and **P < .01 for the significant difference from soluble TLRL.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/118/26/10.1182_blood-2011-07-367615/4/m_zh89991182190003.jpeg?Expires=1767773445&Signature=uVclOFKSuJjvpBX3gaHuZ9uUaCdzz-IRKpBk-Tn9BwXIioytF2ENEQzRkCegdeM5zYdjX0Dw7Fg4heYYslwyOPRnlFJENZw5c55txjNlRnCdsSUbaXFHlaawxaXiUfwqmVW0bYOi671ovU3P7acAvJUf8UAfRSiOXKbHpvFgiUDPOw9RbG00u685UsNePrdlLHJrhVRCEzy5H1ieQnKlEmhHbv96BgghuObydy73vqTbp2z7a0dx28JBD3TsYArC1JGYDXFothyX194GeTLI604Sv1PUIqVg7uN8DZz386QSSetVTdRjS0keui2WR5YgxOd0viK-XFijWqrRz2JQ7A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)