Abstract
Mixed-lineage leukemia (MLL)/AF4-positive acute lymphoblastic leukemia (ALL) is a common type of leukemia in infants, which is associated with a high relapse rate and poor prognosis. IL24 selectively induces apoptosis in cancer cells and exerts immunomodulatory and antiangiogenic effects. We examined the effects of adeno-associated virus type 8 (AAV8) vector-mediated muscle-directed systemic gene therapy in MLL/AF4-positive ALL using IL24. In a series of in vitro studies, we examined the effects of AAV8-IL24–transduced C2C12 cell-conditioned medium. We also examined the effects of AAV8-IL24 in MLL/AF4 transgenic mice. The results revealed the effects of AAV8-IL24 in MLL/AF4-positive ALL both in vitro and in vivo. With regard to the mechanism of therapy using AAV8-IL24 in MLL/AF4-positive ALL, we demonstrated the antiangiogenicity and effects on the ER stress pathway and unreported pathways through inhibition of S100A6 and HOXA9, which is specific to MLL/AF4-positive ALL. Inhibition of S100A6 by IL24 was dependent on TNF-α and induced acetylation of p53 followed by activation of the caspase 8–caspase 3 apoptotic pathway. Inhibition of HOXA9 by IL24, which was independent of TNF-α, induced MEIS1 activation followed by activation of the caspase 8–caspase 3 apoptotic pathway. Thus, gene therapy using AAV8-IL24 is a promising treatment for MLL/AF4-positive ALL.
Introduction
Rearrangements of the mixed-lineage leukemia (MLL) gene located at 11q23 are common chromosomal abnormalities associated with acute leukemia, especially infant leukemia and secondary leukemia after treatment with DNA topoisomerase II inhibitors. In addition, 11q23/MLL abnormalities are now widely recognized as important prognostic factors in acute leukemia. More than 70 chromosomal partners of 11q23 have been identified to date, at least 50 of which have been cloned and characterized at the molecular level.1 The prognosis of leukemia patients with MLL rearrangement varies widely depending on the partner gene, leukemia cell lineage, age of the patient, and treatment administered.2 The most prevalent MLL rearrangement in acute lymphoblastic leukemia (ALL) generates the MLL/AF4 fusion gene because of a t(4;11)(q21;q23) chromosomal translocation. Despite recent improvements in the overall treatment outcome for ALL patients, including allogeneic HSCT (allo-HSCT), MLL/AF4-positive ALL is still associated with a poor prognosis.2
The following are 3 factors that may be related to the poor prognosis of MLL/AF4-positive ALL. First, MLL/AF4-positive ALL cells strongly recruit new blood vessels.3 Second, MLL/AF4-positive ALL escapes from TNF-α–mediated apoptosis by up-regulating the expression of S100A6, which is a 10.5-kDa Ca2+-binding protein belonging to the S100 protein family that has been reported to interact with and alter the conformation of p53.4-11 Up-regulation of S100A6 expression in MLL/AF4-positive ALL inhibits p53 acetylation followed by inhibition of up-regulation of the caspase 8–caspase 3 apoptotic pathway in the presence of TNF-α.4 Third, MLL/AF4-positive ALL shows high levels of HOXA9 expression, which enhances the expansion of hematopoietic stem cells and suppresses MEIS1-mediated apoptosis.12-19
IL24 was identified and cloned using the differentiation induction subtraction hybridization approach after treating HO-1 human melanoma cells with IFN-β and mezerein, which resulted in growth arrest and terminal differentiation.20 Expression of IL24 protein is normally restricted to cells of the immune system and to melanocytes.21,22 Reports of the loss of IL24 expression during the pathologic progression of melanomas, and of the significant correlation between this loss and tumor invasion, suggest that IL24 may function as a tumor suppressor in melanoma.20,21,23,24 Indeed, the results of in vitro studies, in vivo animal studies, and a phase 1 clinical trial all indicate that IL24 can selectively induce apoptosis in cancer cells without affecting normal cells.25-27 In addition, Qian et al recently reported the antitumor activity of a selective conditionally replicating adenovirus in combination with IL24 for B-ALL via induction of apoptosis through endoplasmic reticulum (ER) stress.28 Rahmani et al also showed that IL24 induces apoptosis in human myeloid leukemia cells including MLL cells.29 Intriguingly, IL24 not only induces apoptosis, but also has immunomodulatory and antiangiogenic properties (IL24 bioactivity was 20- to 50-fold more potent than endostatin or angiostatin), as well as showing potent antitumor bystander effects, making it an ideal candidate for anticancer gene therapy.26-31 The antiangiogenic effect of IL24 is favorable for the treatment of MLL/AF4-positive ALL with high angiogenicity described above. Although the inhibitory effects of IL24 on apoptosis by S100A6 or HOXA9 have not been reported, further studies to characterize the immunomodulatory effects of IL24 on S100A6 and HOXA9 are warranted.
Previously, our group reported the effects and feasibility of systemic cancer gene therapy using an AAV vector expressing IL24 in a murine subcutaneous cancer model.32 We recently found that AAV serotype 8 (AAV8) mediates the highest level of long-lasting gene expression in muscle among the 4 known AAV serotypes.33 In addition, our group has also established an MLL/AF4 transgenic (Tg) mouse model with a normal immune system, which is a good model in which to examine the immunomodulatory effects of IL24.34
The present study was performed to evaluate the effects, feasibility, and mechanisms of action of systemic cancer gene therapy using AAV8-IL24 in MLL/AF4-positive ALL, which has a poor prognosis and is the greatest obstacle to overcoming childhood ALL. With regard to the mechanism of systemic cancer gene therapy using AAV8-IL24 in MLL/AF4-positive ALL, we focused on antiangiogenicity and effects on the ER stress pathway, which have already been reported as effects of IL24, as well as unreported pathways such as those mediated through inhibition of S100A6 and HOXA9, which are specific to MLL/AF4-positive ALL.
Methods
Plasmid construction and vector production
The plasmids pAAV-IL24 and pAAV-EGFP were constructed as described previously.32 The AAV vector stocks were generated using an adenovirus-free triple transfection method. Briefly, the cis-AAV vector plasmid (with AAV inverted terminal repeats), the trans-plasmid (with the AAV type 2 Rep gene and AAV type 8 Cap gene), and a helper plasmid (with an essential region from the adenovirus genome, pHelper; Stratagene) were cotransfected into human embryonic kidney 293 cells at a ratio of 1:1:1 by calcium phosphate precipitation. Six hours after transfection, the medium was replaced with fresh culture medium, and the cells were incubated for an additional 50-60 hours at 37°C under a 5% CO2 atmosphere. The cells were then scraped from the culture dishes, pelleted by centrifugation, resuspended in PBS (Sigma-Aldrich), and subjected to 3 cycles of freezing and thawing. The resultant cell debris was centrifuged at 3000g for 20 minutes at 4°C, after which the AAV vectors were purified by ammonium sulfate precipitation and iodixanol continuous gradient centrifugation. The genome titer of the AAV vector was determined by quantitative PCR.
Cell culture
Human embryonic kidney 293 and C2C12 mouse myoblast cells were maintained in DMEM (Sigma-Aldrich) supplemented with 10% FCS (Sigma-Aldrich). The MLL/AF4-positive human ALL cell line RS4;11 was purchased from ATCC. The MLL/AF4-positive ALL cell line SEM was purchased from Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH (DSMZ). SEM cells were maintained in DMEM–high glucose (Sigma-Aldrich) supplemented with 10% FBS (PAN Biowest) at 37°C and 5% CO2. RS4;11 cells were cultivated in RPMI 1640 (Sigma-Aldrich) supplemented with 10% FBS at 37°C and 5% CO2.
ELISA
The concentrations of human IL24 in conditioned medium from C2C12 cells or serum from MLL/AF4 Tg mice that had been treated with AAV8-EGFP or AAV8-IL24 were examined by ELISA in 96-well plates using standard techniques and an Ab pair selected for sensitivity. Briefly, plates were coated with a polyclonal Ab (I) against IL-24 (R&D Systems) overnight at 4°C in standard sodium carbonate coating buffer, after which they were blocked for 2 hours at room temperature in blocking buffer (PBS containing 1% BSA and 1% thimerosal). Samples or recombinant IL-24 (R&D Systems) were diluted in diluent buffer (blocking buffer with 1% Tween 20), added to the plates and incubated for 2 hours at room temperature in diluent containing 2% nonfat dry milk. After extensive washing with 0.1% Tween 20 in PBS, a biotinylated polyclonal Ab (II) against IL-24 (R&D Systems) was added to the plate and incubated for 1 hour at room temperature. After washing, HRP-streptavidin (Amersham Life Science) was added to the plates for 30 minutes at room temperature. The reaction was developed by addition of TMB microwell peroxidase substrate (Moss) and stopped with 1 N H2SO4 after 10 minutes. The OD450 was then recorded using a microtiter plate reader (Dynatech).
Detection of apoptotic cells
Apoptotic cell death induced by conditioned medium from AAV8-IL24–transduced C2C12 cells was assessed by TUNEL assay using the DeadEnd Colorimetric TUNEL system (Promega) according to the manufacturer's instructions. The tumor cells were seeded into 6-well plates (3 × 105 cells/well) and incubated for 24 hours in standard normal culture medium. The culture medium was then replaced with conditioned medium from C2C12 cells that had been treated with AAV8-IL24 or AAV8-EGFP as controls, after which the cells were incubated for an additional 2 days, and subjected to TUNEL assay. To examine the synergistic effects of IL24 and TNF-α, we also used human recombinant TNF-α (Wako). Apoptotic rates (%) were calculated as: number of apoptotic cells/total cell number.
WB analysis
Western blotting (WB) analysis was performed as described previously.35 MLL/AF4-positive ALL cells were seeded into 6-well plates (3 × 105 cells/well) and incubated for 24 hours in standard normal culture medium. The culture medium was then replaced with conditioned medium from C2C12 cells that had been treated with AAV8-IL24 or AAV8-EGFP as controls, after which the cells were incubated for an additional 2 days, and collected for WB analysis. Equal aliquots of lysates from cell lines or homogenized mouse spleen were subjected to 10% SDS-PAGE, transferred onto polyvinylidene difluoride membranes, and immunoblotted with the following primary Abs: anti-S100A6 (calcyclin), anti-MEIS1, and anti-HOXA9 (Santa Cruz Biotechnology), anti-cleaved caspase 8, anti-cleaved caspase 3, and anti-BiP Ab (Cell Signaling Technology), anti-p53 (Santa Cruz Biotechnology), anti-acetyl-p53 (Millipore), and anti–β-actin (Millipore). Can Get Signal (Toyobo) was used to promote the reaction between primary Ab and Ag. Images were captured using a Konica SRX-201 film processor.
Animal experiments using MLL/AF4 Tg mouse model
MLL/AF4 Tg mice, which show pro-B cell (CD45R/B220+CD19+CD43+) lymphoma and leukemia with high-level HOXA9 expression by 12 months of age, at which time lymphoma cells will have infiltrated the liver, lung, and spleen, were established previously.34 This MLL/AF4 Tg mouse model has an essentially normal immune system. We divided 10 male MLL/AF4 Tg mice at the age of 4 months into 2 groups: the IL24 group (n = 5) and the control group (n = 5). The AAV8-IL24 vector (1.0 × 1014 viral genomes/body) or AAV8-EGFP vector (1.0 × 1014 viral genomes/body) suspended in 100 μL of PBS was injected into the left leg muscle of mice belonging to the IL24 group or the control group, respectively. All MLL/AF4 Tg mice were killed 10 months after injection of vector (at the age of 14 months) for histopathology and Western blotting analysis. All animal experiments were performed in accordance with the regulations established by the Ethical Committee of Nippon Medical School and were approved by the Animal Care and Committee of Nippon Medical School.
Flow cytometric analysis
FACS analysis was carried out as described previously,34 after which mononuclear blastic cells that had infiltrated into the liver, lung, BM, peripheral blood, and spleen were collected using 2 Ficoll-Hypaque (Lymphoprep) centrifugation steps. The cells were then incubated with PE-, FITC-, or allophycocyanin-conjugated mAbs specific for CD45R/B220, CD19, and CD43 (BD Pharmingen) and analyzed using a FACScan (BD Biosciences).
Histopathology and immunopathology
For histopathologic analysis, mice were killed and tissues of interest were fixed in 4% paraformaldehyde. H&E staining was performed using standard protocols. For immunopathologic analysis, the slides were fixed for 30 minutes in 4% paraformaldehyde, microwaved for 5 minutes in Vector Antigen Unmasking Solution (pH 6.0; Vector Laboratories) for Ag retrieval, rinsed twice for 10 minutes each time in PBS, and incubated for 15 minutes in 3% hydrogen peroxide in methanol to quench endogenous peroxidase activity. For CD45R/B220 staining, the pretreated slides were incubated for 60 minutes with anti-CD45R/B220 (BD Pharmingen) primary Abs. To visualize anti-CD45R/B220 Ab binding, the slides were incubated for 30 minutes with FITC-conjugated anti–rat IgG (Jackson ImmunoResearch Laboratories). Nuclei were counterstained with Mayer hematoxylin. The photomicrographs shown in the figures were taken with a SPOT Insight digital camera controlled using SPOT Advanced Version 4.0.9 software (Diagnostic Instruments) under a Nikon Eclipse 80i microscope equipped with Nikon Plan 2×/0.08 NA and Plan 40×/0.75 NA objectives.
Statistical analysis
The results of cell growth and gene expression assays were analyzed by Student t test, assuming unequal variances and 2-tailed distributions. Data are shown as the mean values ± SD of at least 3 samples. In all analyses, P < .05 was taken to indicate statistical significance.
Results
IL24 is effective against MLL/AF4-positive ALL cells in vitro
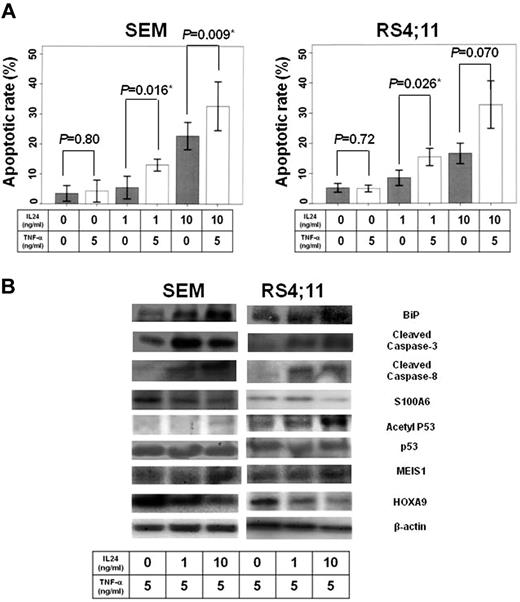
To examine the effects of IL24 on MLL/AF4-positive ALL cells, we first analyzed induction of apoptosis as determined by TUNEL assay after treatment of the MLL/AF4-positive ALL cell lines, SEM and RS4;11, with IL24. Both MLL/AF4-positive ALL cell lines showed significant induction of apoptosis with 10 ng/mL IL24 (SEM, P = .001; RS4;11, P < .001; Figure 1A).
Effects of IL24 on MLL/AF4-positive ALL cell lines. (A) Apoptotic rate of MLL/AF4-positive ALL cell lines by IL24 administration dependent on IL24 concentration. The figure presents a summary of apoptotic rate of MLL/AF4-positive ALL cell lines (SEM and RS4;11). Both MLL/AF4-positive ALL cell lines were significantly induced to undergo apoptosis by 10 ng/mL IL24 (SEM, P = .001; RS4;11, P < .001). (B) WB of lysate of MLL/AF4-positive cell lines under IL24. WB analysis showed that IL24 induced down-regulation of HOXA9 and up-regulation of MEIS1 and Bip followed by up-regulation of cleaved caspase 8 and cleaved caspase 3 although it did not influence p53, acetyl p53, or S100A6 expression.
Effects of IL24 on MLL/AF4-positive ALL cell lines. (A) Apoptotic rate of MLL/AF4-positive ALL cell lines by IL24 administration dependent on IL24 concentration. The figure presents a summary of apoptotic rate of MLL/AF4-positive ALL cell lines (SEM and RS4;11). Both MLL/AF4-positive ALL cell lines were significantly induced to undergo apoptosis by 10 ng/mL IL24 (SEM, P = .001; RS4;11, P < .001). (B) WB of lysate of MLL/AF4-positive cell lines under IL24. WB analysis showed that IL24 induced down-regulation of HOXA9 and up-regulation of MEIS1 and Bip followed by up-regulation of cleaved caspase 8 and cleaved caspase 3 although it did not influence p53, acetyl p53, or S100A6 expression.
IL24 induces up-regulation of the ER stress pathway and down-regulation of HOXA9 in MLL/AF4-positive ALL cell lines
Next, to examine the mechanism of apoptosis induction by IL24 in MLL/AF4-positive ALL, we focused on the effects on the ER stress pathway reported previously to be because of IL24, as well as unreported pathways through inhibition of S100A6 and HOXA9, which are specific to MLL/AF4-positive ALL. The ER stress pathway was examined by examining the expression of BiP, which is a key factor of the pathway.28 The S100A6 pathway was examined by determining the expression of S100A6, p53, and acetyl p53. The HOXA9 pathway was examined by monitoring the expression of HOXA9 and MEIS1. The downstream apoptotic pathway was examined by determining cleaved caspase 8 and cleaved caspase 3 expression. We analyzed the expression of each protein on treatment with IL24 (0 ng/mL, 1 ng/mL, and 10 ng/mL) by WB analysis. WB analysis indicated that IL24 induced down-regulation of HOXA9 and up-regulation of MEIS1 and Bip followed by up-regulation of cleaved caspase 8 and cleaved caspase 3, although it did not influence p53, acetyl p53, or S100A6 expression (Figure 1B).
IL24 induces an increase in TNF-α–induced apoptosis in MLL/AF4-positive ALL cell lines
Next, we analyzed induction of apoptosis by TUNEL assay after treatment of MLL/AF4-positive ALL cell lines, SEM and RS4;11, with a combination of IL24 (1 ng/mL and 10 ng/mL) and TNF-α (5 ng/mL) to examine whether IL24 induces an increase in sensitivity to TNF-α, which is the key factor in immune surveillance, including the GVL effect after allo-HSCT.36 Although we previously reported that MLL/AF4-positive ALL cell lines have poor sensitivity to TNF-α,4 the TUNEL assay showed that IL 24 significantly up-regulates TNF-α–induced apoptosis in MLL/AF4-positive ALL cell lines (SEM, IL24 0 ng/mL vs 1 ng/mL vs 10 ng/mL, P = .80 vs P = .016 vs P = .008; RS4;11, IL24 0 ng vs 1 ng/mL vs 10 ng/mL, P = .72 vs P = .026 vs P = .070; Figure 2A).
Collaborative effects of IL24 and TNF-α on MLL/AF4-positive cell lines. (A) Apoptotic rate of MLL/AF4-positive ALL cell line with IL24 administration dependent on IL24 and TNF-α concentration. TUNEL assay showed that IL24 significantly increased TNF-α–induced apoptosis in MLL/AF4-positive ALL cell lines (SEM, IL24 0 ng/mL vs 1 ng/mL vs 10 ng/mL, P = .80 vs P = .016 vs P = .009; RS4;11, IL24 0 ng/mL vs 1 ng/mL vs 10 ng/mL, P = .72 vs P = .026 vs P = .070). (B) WB of lysate of MLL/AF4-positive cell lines under IL24 and TNF-α Western blotting analysis indicated that S100A6 up-regulation by TNF-α was inhibited and that p53 acetylation was activated in a manner dependent on the IL24 concentration in MLL/AF4-positive ALL cell lines. BiP, HOXA9, and MEIS1 in the presence of both IL24 and TNF-α did not change significantly from those in the presence of IL24 without TNF-α.
Collaborative effects of IL24 and TNF-α on MLL/AF4-positive cell lines. (A) Apoptotic rate of MLL/AF4-positive ALL cell line with IL24 administration dependent on IL24 and TNF-α concentration. TUNEL assay showed that IL24 significantly increased TNF-α–induced apoptosis in MLL/AF4-positive ALL cell lines (SEM, IL24 0 ng/mL vs 1 ng/mL vs 10 ng/mL, P = .80 vs P = .016 vs P = .009; RS4;11, IL24 0 ng/mL vs 1 ng/mL vs 10 ng/mL, P = .72 vs P = .026 vs P = .070). (B) WB of lysate of MLL/AF4-positive cell lines under IL24 and TNF-α Western blotting analysis indicated that S100A6 up-regulation by TNF-α was inhibited and that p53 acetylation was activated in a manner dependent on the IL24 concentration in MLL/AF4-positive ALL cell lines. BiP, HOXA9, and MEIS1 in the presence of both IL24 and TNF-α did not change significantly from those in the presence of IL24 without TNF-α.
IL24 inhibits the up-regulation of S100A6 and also induces acetylation of p53 in the presence of TNF-α
To analyze the mechanism underlying the increase in TNF-α–induced apoptosis in MLL/AF4-positive ALL cell lines by IL24, we analyzed the expression of S100A6, acetyl p53, and p53 in the presence of IL24 (0 ng/mL, 1 ng/mL, and 10 ng/mL) and TNF-α (5 ng/mL) by Western blotting analysis because we have shown previously that the poor TNF-α sensitivity of MLL/AF4-positive ALL is mediated by up-regulation of S100A6 expression followed by inhibition of p53 acetylation.4 Western blotting analysis indicated that up-regulation of S100A6 by TNF-α was inhibited and that P53 acetylation was activated in a manner dependent on the IL24 concentration in MLL/AF4-positive ALL cell lines (Figure 2B). There were no changes in expression of BiP, HOXA9, or MEIS1 in the presence of IL24 and TNF-α compared with those in the presence of IL24 alone (Figure 2B).
Treatment with AAV8-IL24 in MLL/AF4 Tg mouse model: serum ELISA for circulating IL24 levels
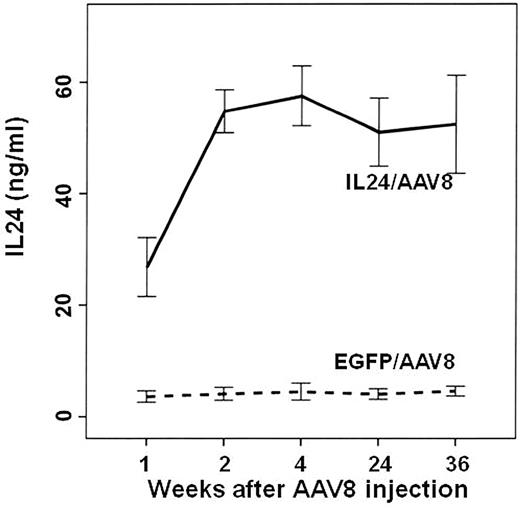
To examine the effects of AAV8-IL24 on MLL/AF4 ALL in vivo, 4-month-old MLL/AF4 Tg mice were IM injected with AAV8-IL24 or AAV8-EGFP as controls. To determine the circulating IL24 levels, we developed a sandwich ELISA as described in “ELISA.” Plasma samples were then obtained from all animals 1, 2, 4, 24, and 36 weeks after IM injection of AAV8-IL24 or AAV8-EGFP and subjected to ELISA. The results indicated that following IM administration of AAV8-IL24, the concentration of IL24 in plasma increased gradually, reaching a plateau (50 ng/mL) 2 weeks after vector injection. Thereafter, plasma IL24 levels remained stable throughout the 36-week observation period (Figure 3).
Time course of changes in plasma IL24 concentration after IM injection of AAV8-IL24 or AAV8-EGFP. In the AAV8-IL24 group, the plasma concentration of IL24 increased gradually, reaching a plateau (60 ng/mL) 2 weeks after vector injection. Thereafter, plasma IL24 levels remained stable during the 36-week observation period.
Time course of changes in plasma IL24 concentration after IM injection of AAV8-IL24 or AAV8-EGFP. In the AAV8-IL24 group, the plasma concentration of IL24 increased gradually, reaching a plateau (60 ng/mL) 2 weeks after vector injection. Thereafter, plasma IL24 levels remained stable during the 36-week observation period.
AAV8-IL24 inhibited infiltration of pro-B-cell lymphoma and leukemia in the MLL/AF4 Tg mouse model
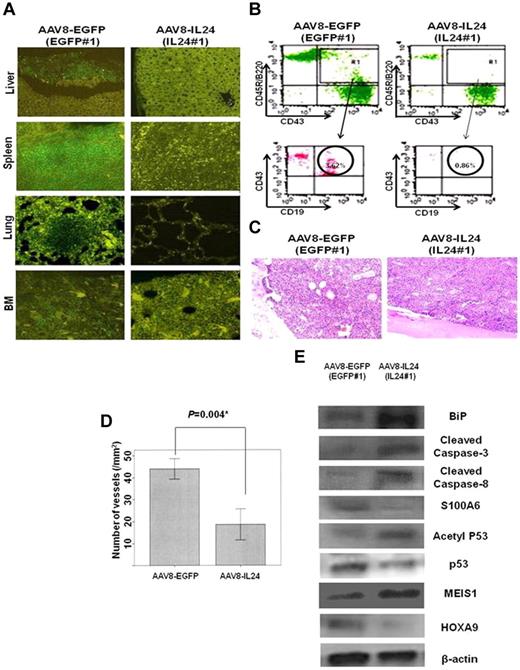
Table 1 shows a comparison of data from killed MLL/AF4 Tg mice between the AAV8-IL24 group (n = 5) and AAV8-EGFP group (n = 5). With regard to the feasibility of therapy, there were no significant toxic side effects of AAV8-IL24 in the mice in the present study. Although pro-B cell lymphoma and leukemia infiltration was observed in all 5 mice in the AAV8-EGFP group, no lymphoma or leukemia was found in 3 of 5 mice in the AAV8-IL24 group. Although leukemia or lymphoma infiltration was observed in 2 mice in the AAV8-IL24 group, the infiltration was less aggressive than that in the AAV8-EGFP group. Figure 4A shows a comparison of the immunohistopathologic analysis results between 1 mouse from the AAV8-IL24 group (IL24#1) and 1 mouse from the AAV8-EGFP group (EGFP#1). Although the liver, lung, spleen, and BM of EGFP#1 showed CD45R/B220+ pro-B cell lymphoma/leukemia infiltration, no lymphoma/leukemia was observed in IL24#1 (Figure 4A). Figure 4B also shows a comparison of FACS analysis results for peripheral blood (PB) between IL24#1 and EGFP#1. In IL24#1, FACS analysis indicated that CD45R/B220+CD19+CD43+ leukemia cells accounted for 0.86% of white blood cells (WBCs) in IL24#1, but 3.62% of WBCs in EGFP#1 (Figure 4B). As controls, CD45R/B220+CD19+CD43+ cells accounted for 0.68% ± 0.46% of WBCs in wild-type C57BL/6N Crj mice (n = 10; data not shown).
Results of therapy with AAV8-IL24 in MLL/AF4 Tg mice. (A) Comparison of the results of immunohistopathologic analysis between one mouse from the AAV8-IL24 group and one mouse from the AAV8-EGFP group. Although CD45R/B220+ pro-B-cell lymphoma/leukemia infiltration (green fluorescence) was observed in the liver, lung, spleen, and BM in EGFP#1 (AAV-EGFP group), no lymphoma/leukemia infiltration was observed in IL24#1 (AAV-IL24 group). (B) Comparison of the results of FACS analysis of PB between 1 mouse from the AAV8-IL24 group and 1 mouse from the AAV8-EGFP group. FACS analysis showed that CD45R/B220+CD19+CD43+ leukemia cells accounted for 3.62% of WBC in IL24#1 (AAV-IL24 group), but only 0.86% of WBCs in EGFP#1 (AAV-EGFP group). (C) H&E-stained BM sections from MLL/AF4 Tg mice (AAV8-EGFP group vs AAV8-IL24 group). (D) Comparison of the number of microvessels in MLL/AF4 Tg mice (AAV8-EGFP group vs AAV8-IL24 group). The number of microvessels in MLL/AF4 Tg mice transduced with AAV8-IL24 was significantly decreased in comparison with MLL/AF4 Tg mice transduced with AAV8-EGFP (18.6 ± 6.1/mm2 vs 44.0 ± 4.0/mm2, P = .004). (E) WB analysis confirmed in vivo that up-regulated S100A6 and HOXA9 were inhibited and p53 acetylation, MEIS1, BiP, cleaved caspase 8, and cleaved caspase 3 were activated in the AAV8-IL24 group.
Results of therapy with AAV8-IL24 in MLL/AF4 Tg mice. (A) Comparison of the results of immunohistopathologic analysis between one mouse from the AAV8-IL24 group and one mouse from the AAV8-EGFP group. Although CD45R/B220+ pro-B-cell lymphoma/leukemia infiltration (green fluorescence) was observed in the liver, lung, spleen, and BM in EGFP#1 (AAV-EGFP group), no lymphoma/leukemia infiltration was observed in IL24#1 (AAV-IL24 group). (B) Comparison of the results of FACS analysis of PB between 1 mouse from the AAV8-IL24 group and 1 mouse from the AAV8-EGFP group. FACS analysis showed that CD45R/B220+CD19+CD43+ leukemia cells accounted for 3.62% of WBC in IL24#1 (AAV-IL24 group), but only 0.86% of WBCs in EGFP#1 (AAV-EGFP group). (C) H&E-stained BM sections from MLL/AF4 Tg mice (AAV8-EGFP group vs AAV8-IL24 group). (D) Comparison of the number of microvessels in MLL/AF4 Tg mice (AAV8-EGFP group vs AAV8-IL24 group). The number of microvessels in MLL/AF4 Tg mice transduced with AAV8-IL24 was significantly decreased in comparison with MLL/AF4 Tg mice transduced with AAV8-EGFP (18.6 ± 6.1/mm2 vs 44.0 ± 4.0/mm2, P = .004). (E) WB analysis confirmed in vivo that up-regulated S100A6 and HOXA9 were inhibited and p53 acetylation, MEIS1, BiP, cleaved caspase 8, and cleaved caspase 3 were activated in the AAV8-IL24 group.
Administration of AAV8-IL24 reduces tumor microvessel density
To examine the antiangiogenic effects of AAV8-IL24, we determined the microvessel density in the BM of mice from the AAV-8-IL24 and AAV8-EGFP groups. Immunologic staining for Factor VIII or CD31 did not yield results because of the strong red color of the BM. However, the microvessels composed of a single layer of endothelial cells were morphologically distinguishable from other cells in H&E-stained sections (Figure 4C). Compared with the MLL/AF4 Tg mice transduced with AAV8-EGFP, the number of microvessels was decreased in MLL/AF4 Tg mice transduced with AAV8-IL24 (44.0 ± 4.0/mm2 vs 18.6 ± 6.1/mm2, P = .004; Figure 4D). The number of microvessels in wild-type C57BL/6N Crj mice was 16.8 ± 8.8/mm2 (n = 5; data not shown).
AAV8-IL24 inhibits S100A6 and HOXA9 up-regulation and up-regulates the ER stress pathway followed by the caspase 8–caspase 3 apoptotic pathway in MLL/AF4 Tg mice
To examine the mechanism underlying the suppression of leukemia or lymphoma by AAV8-IL24 in MLL/AF4 Tg mice, we performed WB analysis of the lysates from the spleens of MLL/AF4 Tg mice and compared the results with those for MLL/AF4 Tg mice treated with AAV8-EGFP. WB analysis indicated the inhibition of S100A6 and HOXA9 up-regulation, and up-regulation of p53 acetylation as well as MEIS1, BiP, cleaved caspase 8, and cleaved caspase 3 levels in the AAV8-IL24 group in comparison with the AAV8-EGFP group (Figure 4E).
Discussion
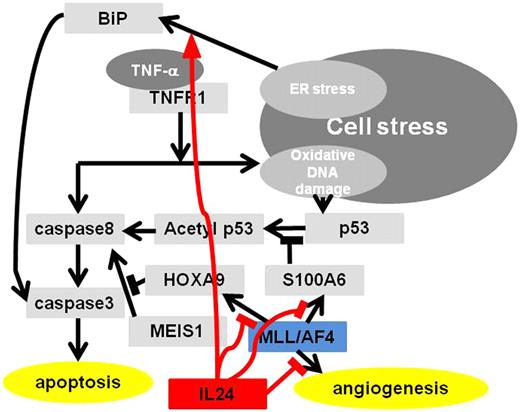
The results of the present study demonstrated the effects of systemic cancer gene therapy using AAV8-IL24 in MLL/AF4-positive ALL in vitro and in vivo. With regard to the mechanism underlying the therapeutic effects using AAV8-IL24 in MLL/AF4-positive ALL, we demonstrated the antiangiogenicity and effects on the ER stress pathway, which have been reported to be because of IL24 and unreported pathways through inhibition of S100A6 and HOXA9, which is specific to MLL/AF4-positive ALL. Inhibition of S100A6 by IL24 is dependent on the presence of TNF-α and it induces acetylation of p53 followed by activation of the caspase 8–caspase 3 apoptotic pathway. Inhibition of HOXA9 by IL24, which is independent of TNF-α, induces MEIS1 activation followed by activation of the caspase 8–caspase 3 apoptotic pathway. The working hypotheses regarding the effects of IL24 on MLL/AF4-positive ALL are shown in Figure 5.
Working hypothesis of IL24 on MLL/AF4-positive ALL. IL24 induces MLL/AF4-positive ALL to caspase 8–caspase 3 apoptotic pathways through down-regulation of HOXA9 and S100A6 followed by p53 acetylation and it also induces apoptosis through ER stress pathway and antiangiogenesis.
Working hypothesis of IL24 on MLL/AF4-positive ALL. IL24 induces MLL/AF4-positive ALL to caspase 8–caspase 3 apoptotic pathways through down-regulation of HOXA9 and S100A6 followed by p53 acetylation and it also induces apoptosis through ER stress pathway and antiangiogenesis.
Among the above effects of AAV8-IL24, we would like to emphasize the effect through inhibition of S100A6 dependent on TNF-α. We produced another immune-deficiency model in which SCID mice received 2 × 107MLL/AF4-positive ALL cells (SEM) transduced with lentiviral vectors expressing luciferase. After engraftment of the SEM cells, we injected the AAV8-IL24 vector (1.0 × 1014 viral genomes/body) or AAV8-EGFP vector (1.0 × 1014 viral genomes/body). Using this MLL/AF4-positive ALL murine model, we could detect tumor growth and metastasis by real-time in vivo imaging analysis (supplemental Figure 1B, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). In this model, although a slight increase in survival was detected in AAV8-IL24-treated mice (median 49M vs 63M, P = .022), the date were not satisfactory because all of the AAV-IL24–treated mice died soon after AAV-EGFP–treated mice (supplemental Figure 1A). Based on the data, we speculated that the satisfactory effectiveness of AAV8-IL24 in the MLL/AF4-Tg mouse model may be mainly dependent on the collaborative mechanism between IL24 and immune surveillance, including TNF-α. The mechanism, inhibition of S100A6, which is dependent on the presence of TNF-α and induces acetylation of p53 followed by activation of the caspase 8–caspase 3 apoptotic pathway may be an important factor in AAV8-IL24 treatment.
As mentioned in the “Introduction,” MLL/AF4-positive ALL is associated with poor prognosis. With regard to the clinical features of MLL/AF4-positive ALL, although the rate of complete remission is high, the relapse rate is also high and the effectiveness of allo-HSCT is limited.2 These features suggest that MLL/AF4-positive ALL may escape immune surveillance, including GVL effects, after allo-HSCT. Three mechanisms have been proposed for the poor prognosis of MLL/AF4-positive ALL: (1) high angiogenicity, (2) apoptosis escape mechanism through activation of S100A6 followed by inhibition of p53 acetylation, and (3) apoptosis escape mechanism through HOXA9 activation.4-19 Our findings indicated that AAV8-IL24 suppressed the above 3 specific mechanisms in MLL/AF4-positive ALL.
With regard to the feasibilities of AAV-IL24 treatment, no obvious side effects were observed in the present study. AAV8-IL24 for administration of IL24 has several strong points with regard to the feasibility of treatment. First, IV cytokine injection at doses barely resulting in any antitumor effects often results in severe toxic side effects.37,38 However, our group has already demonstrated the effectiveness and feasibility of systemic cancer gene therapy using an AAV vector expressing IL24 in a murine subcutaneous cancer model.24 Second, AAV vectors are nonpathogenic and less immunogenic than adenoviral vectors. In addition, the AAV vector has low genotoxicity. Vector genome integration has been reported to be associated with adverse events; integration of γ-retroviral vectors was implicated in the clonal expansion of transduced cells in 3 clinical studies, 2 for X-linked SCID39,40 and the other for chronic granulomatous disease.41,42 However, the low genotoxicity of AAV vectors has been supported by many studies.43-47
With regard to clinical application, administration of AAV8-IL24 after allo-HSCT may be feasible because the effect of AAV8-IL24 can suppress the ability of MLL/AF4-positive ALL to escape from TNF-α as we demonstrated, which is an important factor of GVL effects through up-regulation of S100A6.
In summary, we demonstrated the effects and feasibility as well as the specific mechanisms of action of systemic cancer gene therapy using AAV8-IL24 in MLL/AF4-positive ALL through down-regulation of HOXA9 and S100A6 followed by p53 acetylation and antiangiogenesis in vitro and in vivo. Thus, gene therapy using AAV8-IL24 is a promising strategy for the treatment of MLL/AF4-positive ALL, which has a poor prognosis and is the greatest obstacle to overcoming childhood ALL.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr R. Van Etten of Harvard Medical School for the kind gift of the pMSCVneop230 BCR/ABL plasmid for establishment of our MLL/AF4 Tg mice.
This work was supported in part by grant 19591142 from the Ministry of Health and Welfare of Japan and the Ministry of Education, Science and Culture of Japan.
Authorship
Contribution: H.T. designed research, performed experiments, analyzed data, and wrote the paper; M.T. performed experiments and analyzed data; and H.Y., K.D., K.I., and T.S. analyzed data and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hayato Tamai, MD, Department of Hematology, Nippon Medical School, Sendagi 1-1-5, Bunkyo-Ku, Tokyo 113-8603, Japan; e-mail: s6056@nms.ac.jp.