Abstract
The aim of this phase 2 study was to evaluate the efficacy and safety of trastuzumab, a humanized monoclonal antibody targeted against the human epidermal growth factor receptor 2 (HER2), for adult patients with relapsed/refractory HER2-positive B-ALL. Fifteen patients, with a median age of 62 years, received trastuzumab according to the schedule approved for breast cancer patients (ie, 4 mg/kg intravenous loading dose followed by 2 mg/kg weekly). The overall response rate was 13% with 2 patients achieving partial response and partial remission cytolytic response, respectively. Two other patients were documented with blast clearance. Only 1 reversible grade 3 cardiac toxic event occurred. This phase 2 study showed that trastuzumab monotherapy can allow for some responses in a very high-risk refractory/relapsed HER2-positive adult B-ALL population. Combination of trastuzumab with chemotherapy or other therapeutic monoclonal antibodies should be tested in the future. This trial was registered at www.clinicaltrials.gov/ct as NCT00724360.
Introduction
Besides conventional chemotherapy, immunotherapy has become a very attractive means to treat and improve the prognosis of leukemia. Several surface antigens present on the leukemic cells, such as CD3, CD19, CD20, CD22, CD33, CD52, or HER2, represent target candidates for specific mAbs.1-3 Promising results have already been obtained in the setting of B-acute lymphoblastic leukemia (B-ALL) using rituximab, a chimerical murine/human anti-CD20 mAb,4,5 epratuzumab, a humanized anti-CD22 mAb,6 and to a lesser extend gemtuzumab ozogamicin, a humanized anti-CD33 mAb conjugated with the cytotoxic antitumor antibiotic, calicheamicin,7 or alemtuzumab, a humanized anti-CD52 mAb,8 and more recently blinatumomab, the bispecific mAb targeting both the CD3/CD19 antigens.1,2
Trastuzumab (rhu-mAb-HER2, Herceptin, F. Hoffmann-La Roche) is the humanized equivalent of the murine 4D5 mAb targeting the HER2 cell-surface receptor. The major mechanisms of action of trastuzumab are proliferation blockade, antibody-dependent cell cytotoxicity, and inhibition of DNA repair.9 In combination with chemotherapy, trastuzumab has significantly improved the outcome of women with HER2-positive breast cancer10-14 or patients with HER2-positive advanced gastric cancer.15 We have previously shown that HER2 surface antigen is up-regulated on blasts of approximately one-third of adult B-ALL (but not in T-lineage subset) and is associated with chemoresistance in these patients.16 We report here an original phase 2 study evaluating the safety and efficacy of trastuzumab in refractory/relapsed HER2-positive adult B-ALL patients.
Methods
Before inclusion, HER2 positivity was assessed using multiparameter flow cytometry with the phycoerythrin-conjugated HER2 Neu 24.7 antibody (BD Biosciences) and a CD19+CD45lo gating strategy. The mean fluorescence intensity ratio was obtained by dividing the mean fluorescence intensity of HER2 with that of the relevant isotypic control. HER2 positivity threshold was defined by a mean fluorescence intensity ratio of at least 2. HER2 oncogene amplification was assessed by FISH analysis using the HER2 DNA probe kit (Vysis). Relapsed/refractory B-ALL patients older than 18 years and with HER2-positive expression on at least 20% of the leukemic blast population in peripheral blood (PB) and/or BM were included. Left ventricular ejection fraction had to be more than 50%. Trastuzumab was administered according to the schedule approved for breast cancer patients as a 4-mg/kg intravenous loading dose followed by 2 mg/kg weekly until progression. There was no corticosteroid premedication. Trastuzumab was provided by Roche. The primary endpoint was the response rate (complete response + partial response + partial remission cytolytic response). Complete response was defined as less than 5% blasts in a normocellular or hypercellular BM with no evidence of circulating blasts or extramedullary disease after recovery of peripheral counts (absolute neutrophil count 1 × 109 and platelet count > 100 × 109). Partial response was defined as BM blasts between 5% and 25% with complete disappearance of circulating blasts and adequate peripheral counts. Partial remission cytolytic response was defined as complete disappearance of circulating blasts and achievement of at least 50% reduction from baseline in the BM blast count.6 Toxicity was graded according to the National Cancer Institute Common Toxicity Criteria Version 3.0. Written informed consent was obtained from each patient in accordance with the Declaration of Helsinki. The study was approved by the Nantes ethical committee and performed according to institutional guidelines.
Results and discussion
Of 50 patients screened, 35 (70%) and 15 patients were found negative and positive for the HER2 expression, respectively. All of the 15 HER2-positive patients (8 males and 7 females) have been included in the study between November 2006 and July 2011. The median age was 62 years (range, 24-80 years). They were considered to be a very high-risk population: 2 had refractory disease after 2 induction courses and 2 were in first untreated relapse, whereas 11 patients were beyond first relapse. The median percentage of HER2-positive leukemic blast cells was 94% (range, 0%-100%) in PB and 100% (range, 31%-100%) in BM. Cytogenetics, available for 14 patients, showed normal and complex karyotypes in 7 and 3 patients, respectively, a Philadelphia-chromosome (Ph+) in 3 and a monosomy 7 in 1. Patients' characteristics are given in Table 1.
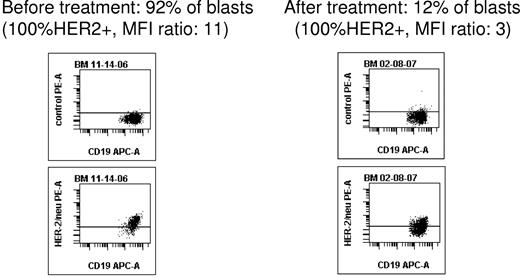
The median number of trastuzumab infusions was 4 (1 month of treatment; range, 1-20). The overall response rate (complete response, partial response, or partial remission cytolytic response) was 13%. No complete response was observed. One patient achieved partial response (decrease of BM blasts from 25% to 11% after 4 infusions, total number of infusions: n = 13). One patient achieved partial remission cytolytic response (decrease of BM blasts from 92% to 12% after 12 infusions with quasi-loss of HER2 expression after 12 infusions, Figure 1; total number of infusions: n = 18). Finally, blast clearance was observed in 2 other patients (96%-57% of BM blasts after 8 infusions, total number of infusions: n = 20; 20% to 0% of PB blasts after 3 infusions, total number of infusions: n = 3). Individual results are given in Table 1. All responders had a high HER2 expression (≥ 99% of the blast population), suggesting perhaps a better efficacy of trastuzumab in these patients, but no correlation between HER2 expression levels and trastuzumab response can be made at this point because of the small cohort studied.
Using multiparameter flow cytometry, a CD19+CD45low blast cell gating strategy allowed to monitor blast population and HER2-positive expression after trastuzumab infusions. In this example of patient 1, HER2 expression became quasi-undetectable in bone marrow after 12 trastuzumab infusions.
Using multiparameter flow cytometry, a CD19+CD45low blast cell gating strategy allowed to monitor blast population and HER2-positive expression after trastuzumab infusions. In this example of patient 1, HER2 expression became quasi-undetectable in bone marrow after 12 trastuzumab infusions.
Not surprisingly, our study shows that trastuzumab, administered as a single agent, is safe and feasible in adult relapsed/refractory HER2-positive B-ALL patients, allowing an overall response rate of 13%. Nevertheless, it should be kept in mind the very high risk of our patient population and the poor results of salvage regimen for relapsed/refractory ALL patients.17-20 In addition, use of trastuzumab as a single agent for breast cancer provides similar results with approximately 15% to 26% of objective overall response.10,11 Finally, the use of therapeutic mAb in monotherapy is generally safe but of limited efficacy, although results could be improved by combining the antibody to chemotherapy, as observed with epratuzumab.6 Such an approach, combining mAb and chemotherapy, has been also documented for breast cancer.12,21 This suggests either an increased sensitivity to chemotherapy of HER2-positive cells through the proliferation blockade induced by the mAb or an adequate decrease of the tumor load by antibody-dependent cell cytotoxicity, as exemplified here by the decrease of HER2 blasts. Thus, 1 way to improve the results might be to incorporate trastuzumab to an ALL-adapted chemotherapy. Besides using it in less advanced patients, another way might be to combine several mAbs together, as supported by recent in vitro3,22 or in vivo studies.22,23 For example, we have reported that all HER2-positive B-ALL patients also express surface CD22 and CD52.3 Second-generation anti-HER2 mAb (pertuzumab) could also be added to trastuzumab, as it is known to have a complementary mechanism of action (receptor dimerization inhibition) and as the combination of the 2 HER2 mAb with taxanes demonstrated recently in a phase 3 study significant clinical benefit compared with trastuzumab alone with the chemotherapy in breast cancer.24
As documented by the FACS analysis, the blast population remains HER2-positive after trastuzumab infusion, suggesting that all targets are not saturated by the therapeutic antibody. This could be explained perhaps by an antigen sink effect or a peripheral loss of trastuzumab by the lymphoblasts. This pleads for testing, within a prephase, higher dose of trastuzumab and performing pharmacokinetic analyses in a future trial before combining it with chemotherapy or other mAb.
The most clinically significant adverse event after trastuzumab perfusion is cardiac dysfunction, reported to occur in 2% to 5% of the patients.10,11 The cardiac effects are generally reversible after discontinuation of trastuzumab, and adequate left ventricular ejection fraction is required before initiating trastuzumab treatment. Here, as expected, trastuzumab infusion was well tolerated, as only one reversible grade 3 cardiac treatment-related adverse event (consisting of a hypertensive crisis and a concomitant unstable angina) occurred in this population over 60 years (median age) despite proper left ventricular ejection fraction at inclusion.
Surprisingly, none of the patients with HER2 overexpression showed HER2 oncogene amplification after FISH analysis. Although there is no clear explanation for that, ALL clearly represents obviously a different tumor than solid breast or gastric cancers with specific molecular biology. Interestingly, a heterogeneity of HER2 expression has also been reported in gastric cancer where more than 20% of cases may carry HER2 amplification in FISH analysis without HER2 expression in immunohistochemistry.25 These conflicting results emphasize the need for standardized definition of HER2 positivity or a HER2 scoring system to grade HER2-positive tumors.
In conclusion, this phase 2 trial shows that HER2-positive B-ALL can be sensitive to monotherapy with trastuzumab. The latter allowed for some responses in a very high-risk refractory/relapsed adult B-ALL population. These results are encouraging for the design of new patient-adapted trials in HER2-positive B-ALL, associating a combination of anti-HER2 mAbs with chemotherapy or other therapeutic mAbs.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Peggy Ageneau from the DRC of Nantes Hospital and all the data managers from the different participating centers for data management and collection.
This work was supported by Roche France.
Authorship
Contribution: P.C. conceived and designed the study, analyzed data, recruited patients, provided clinical care, performed bibliographic search, and wrote the manuscript; N.R. performed flow cytometric analyses for all patients; P.T. performed the FISH analyses for all patients; and A.C., E.R., S.M., S.C., C.C., C.F., M.B., J.-S.B., A.E., J.D., T.G., M.M., M.-C.B., N.I., and H.D. recruited patients and provided clinical care (or performed biologic analyses) and commented on the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Patrice Chevallier, Service d'Hématologie Clinique, Centre Hospitalier Universitaire, 9 quai Moncousu, BP1005, 44093 Nantes cedex 01, France; e-mail: patrice.chevallier@chu-nantes.fr.