Abstract
During systemic infection and inflammation, immune effector cells are in high demand and are rapidly consumed at sites of need. Although adaptive immune cells have high proliferative potential, innate immune cells are mostly postmitotic and need to be replenished from bone marrow (BM) hematopoietic stem and progenitor cells. We here review how early hematopoiesis has been shaped to deliver efficient responses to increased need. On the basis of most recent findings, we develop an integrated view of how cytokines, chemokines, as well as conserved pathogen structures, are sensed, leading to divisional activation, proliferation, differentiation, and migration of hematopoietic stem and progenitor cells, all aimed at efficient contribution to immune responses and rapid reestablishment of hematopoietic homeostasis. We also outline how chronic inflammatory processes might impinge on hematopoiesis, potentially fostering hematopoietic stem cell diseases, and, how clinical benefit is and could be achieved by learning from nature.
Introduction
The hematolymphoid system is a paradigmatic, somatic stem cell–maintained organ with enormous cellular turnover, that is, the continuous de novo generation and subsequent death of cells. It is estimated that, in a 70-kg adult human, approximately 3 × 105 erythrocytes and 3 × 104 white blood cells are produced per second in steady-state (steady-state hematopoiesis), and it is documented that during specific demand, for example, on blood loss or infectious challenges, cellular production can increase several-fold over baseline levels (demand-adapted hematopoiesis). Obviously, such high cellular throughput requires tight control, as hematopoietic failure (eg, aplasia) or excess (eg, leukemia) within a short time is not compatible with life. It is equally obvious that the control mechanism needs to involve “long-distance” regulatory feedback loops as the blood cell production sites in bone marrow (BM) are spatially disconnected from blood cell effector sites in the periphery.
Blood cell production is hierarchically organized with hematopoletic stem cells (HSCs) at the top, which ensure via both self-renewal and differentiation to multipotent and stepwise blood lineage-committed progenitors the continuous production of all different blood cells throughout the life time of an individual.1 This series of differentiation steps allows both homeostatic maintenance of blood cell levels under basal conditions as wells as a high degree of flexible adaptation to increase blood cell output in response to disturbances of the hematopoietic equilibrium and to specific demand, respectively.
The hematopoietic response to acute systemic bacterial infection, often referred to as emergency myelopoiesis, is a prime example for demand-adapted hematopoiesis. In contrast to less severe local infection not causing systemic symptoms and blood count alterations, emergency myelopoiesis is characterized by systemic signs such as blood leukocytosis, neutrophilia, the emergence of immature neutrophils (clinically called left-shift), and increased production of myelomonocytic cells in BM (Figure 1). Hematopoietic responses in acute viral infection as well as in chronic bacterial or viral infection are more variable, ranging from lymphopenia and neutropenia to lymphocytosis and neutrophilia. Despite differences in kinetics and magnitude of the responses elicited by distinct pathogens, the crucial initial step is shared in any of these settings and involves sensing and recognition of a given pathogen.
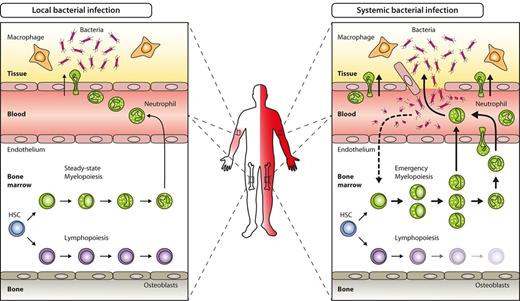
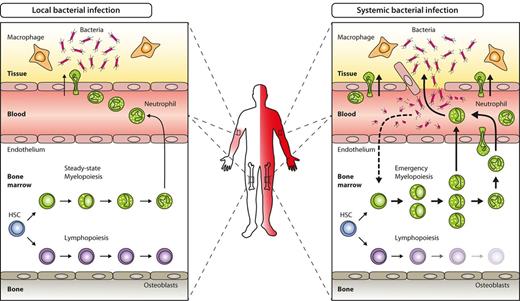
Key hematopoietic differences in local versus systemic bacterial infection. Local bacterial infection leads to activation of resident hematopoietic cells (eg, macrophages) as well as nonhematopoietic cells (eg, parenchymal, vascular, or stromal cells), and to subsequent release of inflammatory cytokines and chemokines. As a net result, myeloid effector cells are attracted to the site of infection, where they exert their antimicrobial functions under the influence of locally produced cytokines. Apart from local signs of infection (calor, dolor, rubor, tumor, and functio laesa), no systemic effects such as peripheral blood leukocytosis or left-shift are induced (left). By contrast, locally noncontrolled infection (eg, sepsis) leads to systemic symptoms such as fever, peripheral blood leukocytosis, and left-shift. Concomitantly, BM myelopoiesis is massively enhanced above steady-state levels (emergency myelopoiesis) to provide myeloid effector cells needed to resolve the infection (right).
Key hematopoietic differences in local versus systemic bacterial infection. Local bacterial infection leads to activation of resident hematopoietic cells (eg, macrophages) as well as nonhematopoietic cells (eg, parenchymal, vascular, or stromal cells), and to subsequent release of inflammatory cytokines and chemokines. As a net result, myeloid effector cells are attracted to the site of infection, where they exert their antimicrobial functions under the influence of locally produced cytokines. Apart from local signs of infection (calor, dolor, rubor, tumor, and functio laesa), no systemic effects such as peripheral blood leukocytosis or left-shift are induced (left). By contrast, locally noncontrolled infection (eg, sepsis) leads to systemic symptoms such as fever, peripheral blood leukocytosis, and left-shift. Concomitantly, BM myelopoiesis is massively enhanced above steady-state levels (emergency myelopoiesis) to provide myeloid effector cells needed to resolve the infection (right).
Specific antigen receptor–independent pathogen recognition in the innate branch of the immune system is accomplished through specialized pattern-recognition receptors (PRRs), which sense pathogen-associated molecular patterns (PAMPs). Toll-like receptors (TLRs) belong to the PRR family and recognize conserved microbial products derived from exogenous pathogens and possibly also some host-derived endogenous ligands.2-5 Ligation of TLRs, via sophisticated downstream signaling cascades, induces cell- and context-dependent proliferation, differentiation, and migration, as well as protein production and secretion, all processes geared to activate and orchestrate efficient immune response against insulting invaders.6
The classic concept of induction of emergency myelopoiesis assumes that activation of PRRs on tissue-resident hematopoietic and nonhematopoietic cells at the site of pathogen entry triggers a cascade of signaling events, leading to release of immune cell attractants (eg, chemokines) and survival and maturation factors (eg, cytokines). Chemokines recruit immune cells such as granulocytes from the blood into the affected tissue, where they exert their effector function.7,8 In parallel, cytokines with pleiotropic functions stimulate immune cells locally, and, on insufficient local control of the infection, increase to reach relevant systemic levels, ultimately enhancing production of myelomonocytic cells from myeloid-committed precursors in the BM to replenish consumed peripheral cells.9,10 Thus, as the kidney senses low oxygen pressure and subsequently increases erythropoietin secretion to stimulate red blood cell (RBC) production on demand,11 environmental contact organs, for instance, the lung with high numbers of local phagocytic cells, might be particularly efficient in producing myeloid cell stimulating and differentiating factors that would ensure appropriate increase of innate immune cell production in BM on need.9
However, recent findings suggest that the hematopoietic response to infection and inflammation has been shaped by evolution in a more sophisticated way involving complementary direct and indirect pathways at inflammatory and hematopoietic sites: cytokines, beyond providing mere stimuli for proliferation, instruct linage choices; hematopoietic and nonhematopoietic cells at primary site of blood production, that is, in BM, are able to sense pathogens and react with appropriate protective responses; and finally, HSCs themselves, that were previously thought to be largely protected from toxic or infectious challenges in their niches,12,13 play a more immediate and active role in the emergency response. In this review, we will try to integrate these novel insights and highlight the short-term protective advantages but also point to potential long-term effects that might support development of hematopoietic disease, especially in an aging population.
Expression of inflammation and infection responsive receptors on hematopoietic cells in BM
In locally noncontrolled infection, a large number of soluble factors are rapidly increased in serum and are able to act at hematopoietic sites in BM. These factors include (1) conserved infectious agent products or “patterns” (eg, bacterial- or fungal-specific membrane products, or species-specific DNA and RNA motifs); (2) cytokines (eg, colony-stimulating factors, interleukins, interferons); and (3) mobility factors (eg, chemokines). To react to any of these, responsive cells need to express the respective receptors.
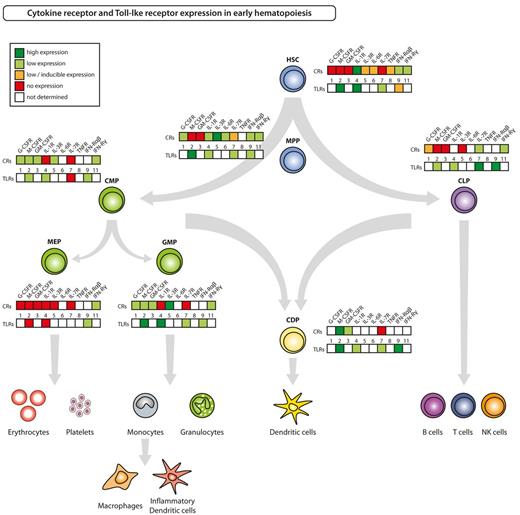
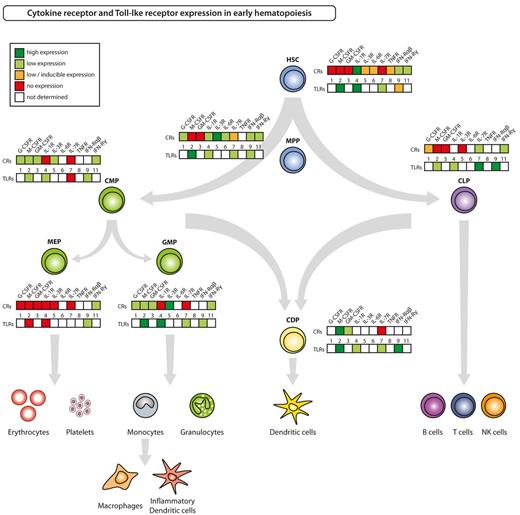
Hematolymphoid differentiation and maturation from HSCs to terminally differentiated cells involves loss of self-renewal capacity, transient acquisition of high proliferative potential, and subsequent restriction to a mature blood cell lineage, that, with the exception of memory T and B cells as well as some tissue macrophages and Langerhans cells, is postmitotic. The sequence of differentiation events goes from HSCs to nonself-renewing multipotent progenitors (MPPs). MPPs, via intermediate populations debated in their specific details elsewhere, give rise to common myeloid progenitors (CMPs), granulocyte-macrophage progenitors (GMPs), megakaryocyte-erythrocyte progenitors (MEPs), common dendritic cell progenitors (CDPs), and common lymphoid progenitors (CLPs), that each have high proliferative potential and are committed to the lineages indicated by their names (Figure 2).1,14-19 With the identification and isolation of HSCs and subsequent downstream hematopoietic progenitors, it has become possible to not only test biologic reactions of these to the aforementioned soluble factors but also to pinpoint respective receptor expression at each developmental stage. Experimental evidence of mouse hematopoietic stem and progenitor cell (HSPC)–expressed growth factor receptors, PRRs, and mobility receptors is summarized and depicted for some selected examples in Figure 2 and in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Cytokine receptor and Toll-like receptor expression in HSPCs. The figure summarizes current knowledge on expression of selected, inflammatory responses relevant cytokine receptors (CRs) and of TLRs on defined hematopoietic progenitor cell populations in steady-state adult mice. Respective references are given in supplemental Table 1.
Cytokine receptor and Toll-like receptor expression in HSPCs. The figure summarizes current knowledge on expression of selected, inflammatory responses relevant cytokine receptors (CRs) and of TLRs on defined hematopoietic progenitor cell populations in steady-state adult mice. Respective references are given in supplemental Table 1.
Some of these receptors have been used to identify and characterize the HSPCs themselves, such as the receptor for stem cell factor (c-Kit), stem cell antigen-1 (Sca-1), CD34, fetal liver kinase-2 (Flk2; also called Flt3), macrophage-colony–stimulating factor (M-CSF) receptor and the IL-7 receptor α (IL-7Rα).1,16,17,20-22 It is thus important to note that these receptor expression patterns might be altered during inflammatory challenges, and steady-state defined phenotypes of HSPCs might thus not apply in these situations. In fact, examples are plenty: In vivo injection of TLR agonists leads to nondetectable surface Flk2 and M-CSF receptor by antibody recognition and thus does not allow to analyze dynamics of the CDP population.23 This might be mediated by excess of the respective ligands, leading to receptor ligation and internalization24 or by other indirect events. Furthermore, altered expression of IL-7Rα, a receptor critical for lymphoid cell commitment, has been observed in experimental malaria infection.25 Lineage marker negative (lin−) c-KithiIL-7Rα+ cells, which do not exist in steady-state hematopoiesis and have similar myeloid and lymphoid potential to MMPs, are induced in response to infection via IFN-γ and contribute to host defense by dominant myeloid cell production.25 Similarly, under pathologic conditions such as infection and cancer, some low-level myeloid colony-forming activity has been unexpectedly detected in Gr-1+CD11b+ cells that, in steady-state, represent both immature as well as fully differentiated granulocytes.26 On the basis of their inhibitory effect on T-cell function, they operationally have been defined as myeloid-derived suppressor cells or activated immature myeloid cells, although their origin, developmental pathways, and existence of putative subsets remains to be determined.26 In addition, it has been demonstrated by many studies that Sca-1, a glycosyl phosphatidylinositol-anchored protein, originally defined as a lymphocyte activation marker because of its up-regulation on T-cell activation27 and now frequently used to define HSPCs,28 is up-regulated on systemic infectious or noninfectious inflammation.29-38 Collectively, the expression of a variety of cytokine and PRRs on HSPCs suggests an active role on modulation of early hematopoiesis during systemic infection and inflammation.
Expression of inflammation and infection responsive receptors on nonhematopoietic cells in BM
The concept that the highly specialized BM microenvironment participates in HSC maintenance as well as guidance of hematopoietic differentiation has been proposed for the last several decades39 and corroborated by experimental observation that long-term survival of HSPCs can be achieved by in vitro coculture on stromal feeder layers.40-42 The first definitive in vivo proof for the existence of a HSC “niche” came from 2 studies in which the authors used genetic and pharmacologic approaches to specifically manipulate osteoblasts function.43,44 Besides osteoblasts, also endothelial cells, endosteal progenitors, CXCL12 abundant reticular cells, and mesenchymal stromal cells have been shown to be constituents of the HSC niche in BM.12,13,45 Although it is now clear that niche cells provide essential fluid and adherent factors for steady-state HSC maintenance,13 the role of nonhematopoietic BM cells during hematopoietic stress conditions such as infection and systemic inflammation is ill-defined. However, recent in vitro and in vivo studies demonstrate that mouse and human mesenchymal stromal cells express functional TLRs and on their ligation increase production of a variety of inflammatory cytokines and chemokines.46-50 Thus, it can be speculated that BM niche cells are responsive toward systemic infectious stimuli and subsequently adapt their hematopoiesis-supporting functions according to respective TLR-agonists.
Evidence for indirect activation of early hematopoiesis by pathogens
Indirect activation of early hematopoiesis by pathogens occurs via the sensing of these by mature hematopoietic cells and nonhematopoietic tissues and a subsequent series of changes in factors that act on early hematopoietic cell expressed receptors, inducing proliferation, differentiation, and migration. The most prominent examples are myeloid differentiation–enhancing cytokines such as granulocyte-CSF (G-CSF), M-CSF, and granulocyte-macrophage-CSF (GM-CSF), as well as early acting cytokines as IL-3, IL-6, and Flt3 ligand (S.B. and M.G.M., unpublished data, February 2012).51-55 It has been demonstrated that in systemic infection those cytokines are released, reaching serum concentrations up to hundred-fold above steady-state levels in both mice and humans.10,55-58 The site of most prominent production, remote from or in BM, the primary hematopoietic site, as well as the relevance of local concentrations of the different soluble and membrane bound factors, still needs to be defined. However, in vitro data on support of myeloid colony-forming activity, and in vivo data on cytokine injections, genetic deletions of cytokines or their respective receptors, clearly demonstrate their key roles in the myeloid response, albeit with some reduncancy.9,59
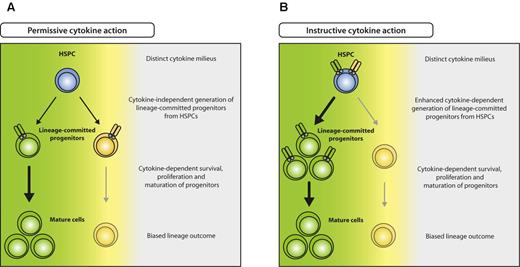
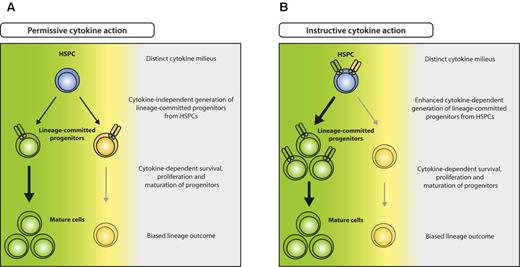
Beyond knowledge on the most prominent proliferation-inducing cytokines in emergency responses, substantial progress has recently been made concerning the long-standing question on how hematopoietic lineage commitment is achieved. Two models on cell fate determination have been proposed: (1) regulation of proliferation or apoptosis of cells that are already committed to a lineage (permissive model),60 and (2) induction of a molecular program in progenitor cells that induces differentiate to a specific lineage (instructive model; Figure 3). In recent studies, it was demonstrated that enforced expression of cytokine receptors or respective transcription factors in either alternatively committed or noncommitted progenitors leads to conversion or instruction to one lineage at cost of others, respectively.61-64 In addition, it was demonstrated by long-term tracking of single-cell differentiation processes that indeed GMPs can be instructed by G-CSF or M-CSF to differentiate into granulocytes or macrophages, respectively.65 It seems thus reasonable to assume that also in vivo, particularly during infection when myeloid-acting cytokines are highly elevated, early noncommitted progenitors are selectively instructed to differentiate into cells of demand, that is, myelomonocytic lineages, resulting in emergency myelopoiesis (Figure 3B). Although less well studied for lymphocyte responses that do not necessarily rely on de novo production from HSPCs, it is interesting to note that lymphopenia causes an elevation of IL-7, possibly also supporting early lymphopoiesis in BM.66
Model for permissive and instructive cytokine effects on HSPCs. (A) In the permissive model, HSPCs generate different lineage-committed progenitors (green and yellow) at intrinsically determined ratios, irrespective of external cues. Committed progenitors express lineage-specific cytokine receptors that transmit survival, proliferation, and maturation signals, resulting in selection of the appropriate progenitors and eventually biased lineage outcome (green), depending on the distinct cytokine milieu (green cytokine > yellow cytokine). (B) In the instructive model, unbiased HSPCs express different cytokine receptors specific for several lineages (green and yellow) and are instructed to differentiate to a lineage according to the cytokine milieu present (green cytokine > yellow cytokine).
Model for permissive and instructive cytokine effects on HSPCs. (A) In the permissive model, HSPCs generate different lineage-committed progenitors (green and yellow) at intrinsically determined ratios, irrespective of external cues. Committed progenitors express lineage-specific cytokine receptors that transmit survival, proliferation, and maturation signals, resulting in selection of the appropriate progenitors and eventually biased lineage outcome (green), depending on the distinct cytokine milieu (green cytokine > yellow cytokine). (B) In the instructive model, unbiased HSPCs express different cytokine receptors specific for several lineages (green and yellow) and are instructed to differentiate to a lineage according to the cytokine milieu present (green cytokine > yellow cytokine).
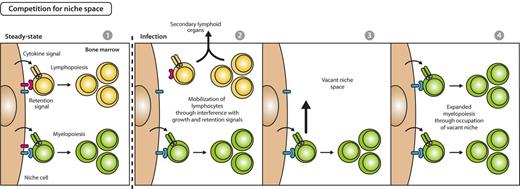
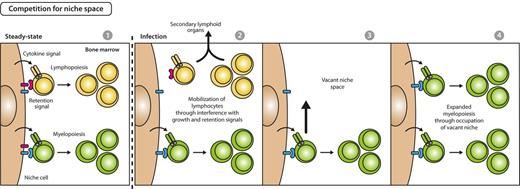
A complementary mechanism to assure enhanced hematopoietic production would be selective provision of BM space for differentiation of cellular lineages in need. In fact, it was demonstrated that during alum-, infection-, or PAMPs-induced emergency myelopoietic responses, secreted inflammatory cytokines (IL-1, IL-3, IL-6, G-CSF, GM-CSF) reduce the expression of growth and retention factors for lymphopoiesis and thus mobilize lymphocytes from BM to the secondary, peripheral lymphoid organs as spleen.36,54,67-69 Vacated BM space then facilitates myelopoiesis that shares a common BM niche with lymphocytes (Figure 4).70,71 This idea is additionally supported by the observation that reduction of B-lymphopoiesis in recombination activating gene-1–deficient mice was compensated by an increase of proliferating granulocytes.70 Further evidences need to be accumulated to gauge the relevance of a differentiation model that acts via liberation of space for development of lineages in need.
Model for competition for space between myelopoiesis and lymphopoiesis during emergency myelopoiesis. In steady state, early myelopoiesis and lymphopoiesis occupy the same niche space in BM, resulting in a stable myeloid to lymphoid ratio on the basis of the intrinsic fitness of the respective lineage to compete for growth and retention signals (first panel). Systemic infectious signals cause a reduced expression of lymphoid-supportive growth and retention signals and lead to lymphocyte mobilization from BM and recruitment to secondary lymphoid organs (second panel), thus creating vacant niche space (third panel). Consequently, BM myelopoietic precursors gain access to enhanced retention and growth stimuli, resulting in expansion of myelopoiesis and increased myeloid cell output (fourth panel).
Model for competition for space between myelopoiesis and lymphopoiesis during emergency myelopoiesis. In steady state, early myelopoiesis and lymphopoiesis occupy the same niche space in BM, resulting in a stable myeloid to lymphoid ratio on the basis of the intrinsic fitness of the respective lineage to compete for growth and retention signals (first panel). Systemic infectious signals cause a reduced expression of lymphoid-supportive growth and retention signals and lead to lymphocyte mobilization from BM and recruitment to secondary lymphoid organs (second panel), thus creating vacant niche space (third panel). Consequently, BM myelopoietic precursors gain access to enhanced retention and growth stimuli, resulting in expansion of myelopoiesis and increased myeloid cell output (fourth panel).
Receptors for lymphoid or myeloid differentiation-supporting cytokines are preferentially expressed at measureable levels on multipotent and lineage-committed hematopoietic progenitors but not HSCs (Figure 2 and supplemental Table 1).14,15,17,72 Therefore, despite their high potential for cell production, HSCs were believed to be unresponsive to lineage differentiation-promoting cytokines during infection and inflammation. This hypothesis could make sense because initiation of differentiation without retention of self-renewal capacity would result in life-threatening HSC loss. Indeed, most HSC are in relative quiescence in steady state, that is, they divide infrequently compared with hematopoietic progenitor cells that lack self-renewal capacity but harbor high proliferation capacity.73-77 However, a lack of response of HSCs to infection and inflammation would also imply that enhanced hematopoiesis during these processes would rely solely on the progenitor compartment, possibly not containing sufficient capacity to support a long-term sustained response. To cope with this demand, an increased self-renewal and progenitor generation response by HSCs might be beneficial for the organism.
IFNs, subdivided into type I (IFN-α/β) and type II IFNs (IFN-γ), are known to be strong immunoregulatory cytokines released from most cells, particularly activated immune cells in response to viral or bacterial infection, and play a pivotal role for regulation of antiviral/bacterial immunity.78 Unexpectedly, both type I and II IFN receptors have been found to be expressed on HSCs (Figure 2), and activation of IFN pathways by in vivo injection of recombinant protein or bacterial products recruits dormant HSCs into proliferation, leading, on chronic excess, even to exhaustion of HSC function.29,30,33 These findings clearly indicate a feedback communication between immune system activation and primary HSC regulation tailored to enhance hematopoiesis on demand. Of note, it seems that even physiologic levels of IFNs are involved in control of HSC turnover and function.29
Evidence for direct activation of early hematopoiesis by pathogens
Besides indirect sensing of pathogens and inflammation via pathogen-induced host factors, in recent studies authors indicated that HSPCs themselves express pathogen recognition receptors (Figure 2 and supplemental Table 1).23,79 Because early hematopoietic cells are not equipped with immediate immune effector functions, it is reasonable to assume that functional consequences of PRR ligation might lie in enhanced provision of hematopoietic cells in need via HSPC survival, proliferation, differentiation, and migration. Indeed, there are 3 major lines of evidence directly supporting this assumption.
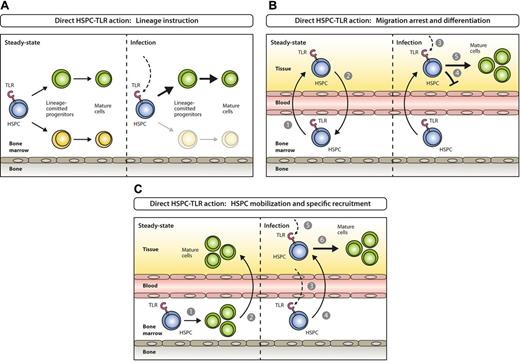
First, in vitro ligation of TLR2 and 4 drives mouse Lin−c-Kit+Sca-1+ (LKS) cells, containing a mixture of HSCs and MPPs, toward proliferation and myeloid cell differentiation, even in the absence of lineage differentiation cytokines required otherwise.79 Particularly, GMPs are driven toward monocyte/macrophage differentiation, and CLPs toward dendritic cells (DCs) at the expense of B-cell differentiation, all via signaling mediated by TLR adaptor molecule, myeloid differentiation primary-response gene 88.79 Moreover, in vivo herpes virus infection revealed that BM lymphoid progenitors, CLPs, are driven toward DC differentiation at the cost of B-cell development via TLR9-dependent pathway.80 Similarly, human CD34+ HSPCs also express TLRs,81-84 and stimulation in vitro bias their lineage commitment to myelopoiesis over lymphopoiesis.82-84 These data indicate that direct pattern recognition via TLR ligation by early hematopoietic cells fosters generation of innate immune cells in need at the expense of other lineages (Figure 5A).
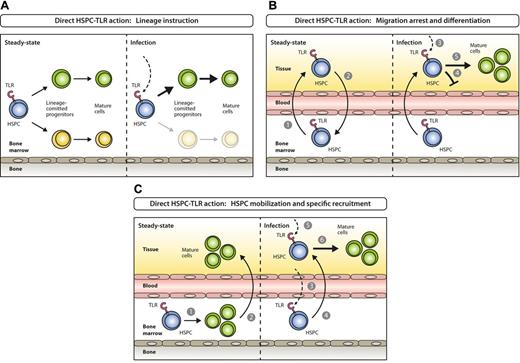
Models for direct activation of BM-resident or circulating HSPCs by PAMPs. (A) Steady-state hematopoiesis ensures homeostatic proliferation and differentiation of different hematopoietic lineages (left). In settings of systemic infection, PAMPs are elevated in circulation and reach the BM, where they directly act on TLR-expressing HSPCs and instruct enhanced generation of a lineage at demand (right). (B) Under steady-state conditions TLR-expressing HSPCs constantly leave the BM (1), enter systemic circulation, perambulate peripheral tissues and some re-home to the BM (2). On the encounter of invading pathogens in peripheral tissues (3), pathogen-mediated triggering of TLRs on HSPCs induces migration arrest (4) and promotes HSPC proliferation and differentiation to ensure local supply of myeloid effector cells (5). (C) In steady state, the majority of blood cell lineages develop in the BM (1) with mostly mature cells leaving (2) to eventually enter their destination tissues where they exert their effector functions. During the course of a systemic immune response when certain lineages are in high demand at specific sites, TLR ligation on lineage-committed progenitors in the BM (3) causes mobilization from the BM and recruitment to these sites (eg, lymph nodes; 4), where they proliferate and differentiate in response to local pathogen (5) to replenish consumed immune effector cells (6).
Models for direct activation of BM-resident or circulating HSPCs by PAMPs. (A) Steady-state hematopoiesis ensures homeostatic proliferation and differentiation of different hematopoietic lineages (left). In settings of systemic infection, PAMPs are elevated in circulation and reach the BM, where they directly act on TLR-expressing HSPCs and instruct enhanced generation of a lineage at demand (right). (B) Under steady-state conditions TLR-expressing HSPCs constantly leave the BM (1), enter systemic circulation, perambulate peripheral tissues and some re-home to the BM (2). On the encounter of invading pathogens in peripheral tissues (3), pathogen-mediated triggering of TLRs on HSPCs induces migration arrest (4) and promotes HSPC proliferation and differentiation to ensure local supply of myeloid effector cells (5). (C) In steady state, the majority of blood cell lineages develop in the BM (1) with mostly mature cells leaving (2) to eventually enter their destination tissues where they exert their effector functions. During the course of a systemic immune response when certain lineages are in high demand at specific sites, TLR ligation on lineage-committed progenitors in the BM (3) causes mobilization from the BM and recruitment to these sites (eg, lymph nodes; 4), where they proliferate and differentiate in response to local pathogen (5) to replenish consumed immune effector cells (6).
Second, shared blood circulation established between surgically joined animals demonstrated that few HSCs physiologically circulate in peripheral blood at any given time and some rehome to BM to participate in blood production.85 Moreover, it was demonstrated that HSPCs leave the vasculature to tissues, enter lymphatic organs, and finally travel via the thoracic duct back to blood and possibly BM again.86 In this process, egress of HSPCs from peripheral tissues to lymph depends on sphigosine-1-phosphate receptor 1 signaling.86 Although not formally proven, the traveling of HSPCs might be a mechanism to keep the equilibrium of HSPCs in different bones, seed the thymus as primary lymphoid organ, or even give rise to hematopoietic offspring involved in fighting infections and tissue repair in peripheral organs. Indeed, first ex vivo and then continued in vivo lipopolysaccharide (LPS) stimulation of HSPCs that were transplanted under the kidney capsule led to local generation of cell clusters containing immunophenotypically defined monocytes, granulocytes, and DCs.86 These data thus indicate that direct pattern recognition via TLR ligation by tissue patrolling HSPCs could lead to differentiation into innate immune cells for local pathogen control (Figure 5B).
Third, we have recently demonstrated that BM CDPs17 express TLR2, 4, and 9 at relatively high levels.23 On in vitro ligation, CDPs down-regulate their BM retention chemokine receptor CXCR4 and up-regulate the lymph node homing receptor CCR7. Subsequent in vivo transfer leads to lymph node homing at the cost of BM homing of progenitors. Moreover, progenitors are specifically recruited to TLR agonist-rich, inflamed lymph nodes, where they give rise to DCs without a change in lineage commitment or proliferation capacity. These data thus indicate that BM-resident HSPCs might directly sense pathogenic insult through TLRs and, by modulation of chemokine receptor expression, subsequently migrate efficiently to inflammatory sites to battle infections and/or rapidly restore lymphoid tissue homeostasis (Figure 5C).
Do BM-resident HSCs also directly respond to TLR ligation on systemically available pathogen components that reach the BM? The demonstration of TLR expression on highly HSC-enriched BM populations (Figure 2)23,79 would suggest that this is the case if BM HSCs are not protected from direct pathogen signals by their niche components.12,13 We have shown by direct in vivo evidence that repetitive, systemic LPS injection activates quiescent HSCs into self-renewing proliferation without leading to lineage read-out bias.76 In addition, it was reported that LPS injection or bacterial infection expands HSC populations by phenotype but impairs their repopulation activity.37,38,87,88 Furthermore, competitive repopulation experiments of wild-type with TLR4- or TLR9-deficient BM cells indicated that TLR-deficient HSCs outcompete wild-type HSCs, arguing for a direct, cell-intrinsic mechanism.89 However, this is area of research is an ongoing one, and it remains to be determined to what extent direct and indirect stimulatory effects are relevant to HSC homeostasis and their response to infection and inflammation (Figure 6).
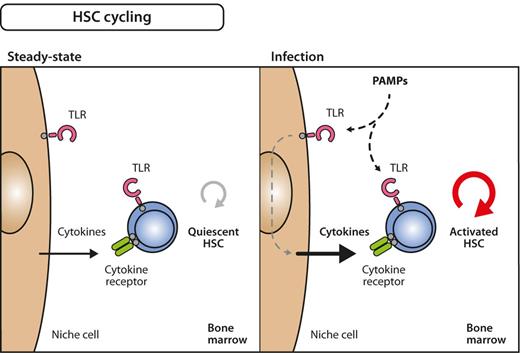
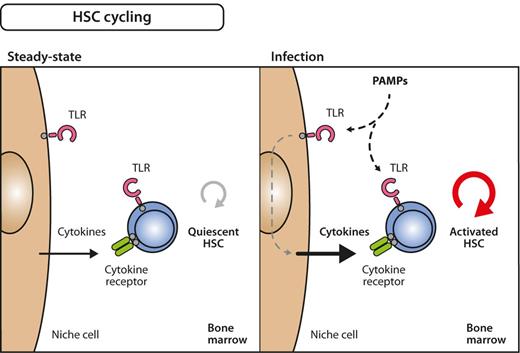
Model for inflammation-induced HSC cycling. Under steady-state conditions, many HSCs are quiescent, that is, only infrequently divide and generate progeny (left). During infection, however, quiescent HSCs become activated into the cell cycle (right). The signals that induce these processes can either be delivered directly on stimulation of TLRs on HSC by PAMPs or via indirect pathways involving growth factors that are secondarily released from PAMP-sensing bystander or niche cells. The relative importance of direct versus indirect influence on HSC division still needs to be determined.
Model for inflammation-induced HSC cycling. Under steady-state conditions, many HSCs are quiescent, that is, only infrequently divide and generate progeny (left). During infection, however, quiescent HSCs become activated into the cell cycle (right). The signals that induce these processes can either be delivered directly on stimulation of TLRs on HSC by PAMPs or via indirect pathways involving growth factors that are secondarily released from PAMP-sensing bystander or niche cells. The relative importance of direct versus indirect influence on HSC division still needs to be determined.
Evolutionary benefits of inflammatory activation of early hematopoiesis
Given the aforementioned discussion, it is obvious that evolution shaped the hematopoietic response during infection and inflammation in a way that early hematopoiesis is involved in fast, versatile, efficient, and sustained defense responses at several layers.
In a first wave of response on local bacterial or fungal infection, mature blood, tissue and lymphoid organ cells, hematopoietic cells, and possibly homeostatically circulating HSPCs become involved via locally produced cytokine, chemokine, and direct TLR-agonist actions on these cells, but no relevant alterations in blood or BM are observed. Although local HSPC differentiation to innate immune effector cells is clearly possible,86 it still needs to be determined how relevant and efficient this time-consuming process in terms of clearing pathogens in a hostile environment really is.
If the infection cannot be controlled locally and thus spread throughout body, a second, systemic wave of response is initiated by systemically released cytokines, chemokines, and infectious patterns that lead to fundamental involvement of early hematopoiesis at all layers: (1) immature myeloid cells as well as immature B cells are released from BM for fighting the infection as well as creating space for more myelopoiesis; (2) stimulated early HSPCs are instructed to expand and differentiate in BM to innate immune effector cells by both cytokines and TLR agonists while at the same time some HSPCs via chemokine-chemokine receptor interactions migrate directly to sites of infection and then differentiate locally; and (3) HSCs are activated to enhance self-renewal and at the same time produce more progenitor cells that subsequently sustain effector cell generation. Finally, all reactions cease once the effector cells cleared the infectious inflammatory drive from the system.
What is then the relative contribution of direct cytokine action versus direct TLR action on HSPCs to quantitative early hematopoietic system responses? This issue has thus far been best evaluated in the context of emergency hematopoiesis on bacterial infection or systemic injection of TLR agonists. Clearly, mice deficient in G-CSF, GM-CSF, IL-6 or deficient in the respective receptors on hematopoietic cells show massive reduction in their response.67,90-93 In sharp contrast, chimeric mice that are deficient for TLR4 expression on only their hematopoietic cells show on LPS stimulation comparable emergency myeloid responses to wild-type animals, whereas respective responses are massively suppressed when TLR4 is expressed only on hematopoietic, but not on nonhematopoietic cells (S.B. and M.G.M., unpublished data, February 2012). Thus, at least in this simple experimental setting of short-term hematopoietic emergency reactions, direct TLR-mediated signals on early hematopoietic cells seem to be of minor importance.
Furthermore, the authors of a recent study also showed that expansion of phenotypically defined HSPCs on pathogen challenge or polymicrobial sepsis, but not on exclusive LPS injection, is independent of TLR adaptor signaling molecules- and type I IFN–mediated pathways.94 In addition, it was suggested by in vivo depletion of neutrophils that creation of BM space recapitulates infection-induced HSPC expansion, and this could be the mechanism for TLR-independent HSPC expansion during infection,94 similar to the reciprocal coupling and competition for space and growth factors between lymphopoiesis and myelopoiesis discussed previously. Collectively, it therefore can be postulated that direct TLR signaling on HSPCs might be more important in fine-tuning the early hematopoietic differentiation and migration response as well as in regulating HSC cycling activity in more complex acute and chronic infectious disease settings. This assumption, as well as the relative contribution of TLR- and non-TLR–mediated pathways, needs to be addressed in ongoing and future studies.
Potential risks of inflammatory activation of early hematopoiesis
Although acute and rapid demand-adapted recruitment of HSCs from quiescence to active cycling and subsequent blood production is likely an economically efficient way to avoid excess generation and storage of otherwise not used differentiation-ready progenitor cells, chronic inflammation or infection might be associated with activation of HSCs that could lead to detrimental effects.
On the basis of extrapolations from HSC turnover studies, it can be assumed that HSCs likely divide approximately 20 and 40-60 times in a relatively unchallenged mouse or human lifetime, respectively.76,77,95 HSC transplantation in both mice and humans demonstrates that the blood-forming capacity of HSCs is not a tightly limited resource. However, HSCs with high proliferative history, for example, during natural aging or experimental serial transplantation, show intrinsic alterations as myeloid biased differentiation capacity,96,97 reduced self-renewal activity,98-101 and an intrinsic drive to rest.76 Furthermore, it was shown by others and us that a fraction of HSCs is in deep quiescence.73,76,77 Although HSC fractions contributing to blood formation and HSC fractions in quiescence likely change dynamically over time,76 the system would ensure at any given time in steady state that a quiescent HSC fraction is largely protected from genome-toxic events by lack of DNA replication and through highly effective nonhomologous end joining, which, however, because of errors might in some cases still allow genetic altered clones to arise.102-105
We speculate that chronic infection and inflammation, inducing high proliferation of most HSCs, might lead to both exhaustion of the HSC pool and an even greater risk to accumulate genetic alterations in HSCs. These genetically altered HSCs, which are supposedly not competitive and die in steady-state environments, might be rescued by stimulants of the inflammatory environment, or “cancer cell niche,”106,107 and finally could lead to early hematologic neoplasia.108 A hypothetical model depicting this process is provided in Figure 7. Furthermore, recently identified myeloid-derived suppressor cells that are expanded and activated in pathologic conditions, have been reported to negatively regulate immune response and promote solid tumor formation.26 Although not evidenced yet, they also might support tumor initiating hematopoietic cells to grow and potentially escape from apoptosis or immune system mediated removal, thereby increasing the accumulation of genetic events and promoting malignant clone formation.
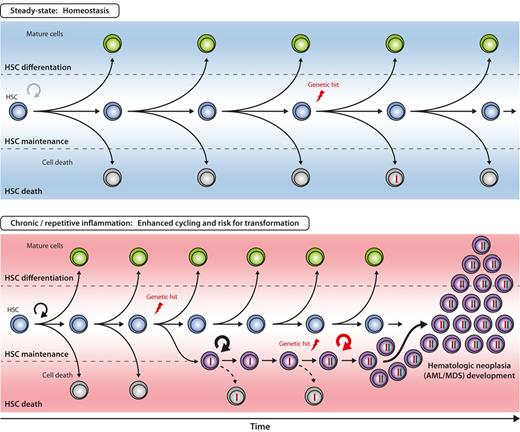
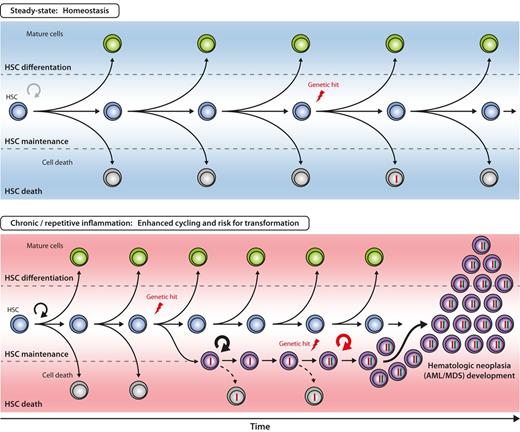
Hypothetical model for inflammation-enhanced malignant transformation in early hematopoiesis. The 3 principle outcomes of HSC divisions are self-renewal, differentiation, and death. The balance between these 3 cell fates is closely regulated to ensure balanced blood cell production over the lifetime of an individual. With increased proliferative history in the HSC compartment, the probability of acquiring genetic alterations (“hits”) in HSCs increases, but efficient counteractive mechanisms (eg, apoptosis, DNA repair) have evolved to remove or correct genetically altered HSCs from the pool (top). It can be hypothesized that under chronic or repetitive inflammation the likelihood of genetic hit acquisition and subsequent accumulation in HSC is increased by two major mechanisms: First, accelerated HSC cycling and self-renewal enhances the statistical probability of acquiring mutations during DNA replication. Second, genetically altered HSC clones are rescued by signals delivered from the inflammatory environment. Persistent and aberrant self-renewal of the respective HSC clone might enhance occurrence of further genetic hits, eventually, resulting in the development of HSC neoplasia, for example, acute myeloid leukemia/myelodysplastic syndrome (AML/MDS, bottom).
Hypothetical model for inflammation-enhanced malignant transformation in early hematopoiesis. The 3 principle outcomes of HSC divisions are self-renewal, differentiation, and death. The balance between these 3 cell fates is closely regulated to ensure balanced blood cell production over the lifetime of an individual. With increased proliferative history in the HSC compartment, the probability of acquiring genetic alterations (“hits”) in HSCs increases, but efficient counteractive mechanisms (eg, apoptosis, DNA repair) have evolved to remove or correct genetically altered HSCs from the pool (top). It can be hypothesized that under chronic or repetitive inflammation the likelihood of genetic hit acquisition and subsequent accumulation in HSC is increased by two major mechanisms: First, accelerated HSC cycling and self-renewal enhances the statistical probability of acquiring mutations during DNA replication. Second, genetically altered HSC clones are rescued by signals delivered from the inflammatory environment. Persistent and aberrant self-renewal of the respective HSC clone might enhance occurrence of further genetic hits, eventually, resulting in the development of HSC neoplasia, for example, acute myeloid leukemia/myelodysplastic syndrome (AML/MDS, bottom).
Indeed, it has been demonstrated that chronic in vivo TLR agonist exposure of mice leads to compromised HSC function that resembles normal HSC aging, ie aspects of HSC exhaustion.88 Although spontaneous hematologic neoplasia is extremely rare in mice, possibly because of their relatively short life-expectancy, recent studies in humans indicated that chronic immune stimulation from infections and autoimmune disease might act as a trigger for acute myeloid leukemia and myelodysplastic syndromes, both bona fide HSC malignancies.109,110 This does not come as a surprise given the long-known association of inflammatory signaling and cancer.111-114 In accordance, recent findings revealed that alterations leading to constitutive active TLR signaling pathways, thus mimicking chronic infection and inflammation, can provide the basis for malignant transformation of HSC115 and that TLR4 and downstream signaling molecules are found to be constitutively up-regulated in HSPCs from myelodysplastic syndromes patients.116,117
Future directions and clinical implications
It has become clear during the last decades that the early hematopoietic system in BM is fundamentally engaged to generate an appropriate hemato-immune response on systemic infection and inflammation. This process involves direct and indirect instruction of hematopoietic cells by cytokines, chemokines, and conserved patterns of infection, leading to tailored hematopoietic generation and directed migration of cells in need. The relative weight of specific actions on target receptors in different infectious and inflammatory situation still needs to be dissected by experimental systems with gain and loss of specific gene function and by clinical observation.
Learning from nature, however, has already led to clinical routine use of cytokines as G-CSF and GM-CSF for enhanced myeloid regeneration,56 thrombopoietin agonists for platelet generation,118 and CXCR4 antagonists for HSPC mobilization.119 More are expected to follow, and it might be envisioned that selective delivery of PRR agonists could also be a therapeutic possibility to enhance or protect hematopoietic cell regeneration in specific situations. Although not dissected in mechanistic detail, a beneficial role of a TLR5 agonist on gut and hematopoietic regeneration after irradiation was recently suggested.120 In addition, naturally evolved early hematopoietic system activating mechanisms might be “hijacked” therapeutically to target hematopoietic malignancies. Quiescent chronic myeloid leukemia–initiating cells and also healthy HSCs might be recruited to cycling by IFN-α treatment.30 After this, only the BCR-ABL1 fusion tyrosine kinase-carrying and -dependent diseased chronic myeloid leukemia cells, but not the healthy HSCs might be eliminated by selective targeting. Although not proving this directly, a recent clinical study suggests that this might indeed be the case.121 Moreover, it can be envisioned that mobilization of leukemia cells and healthy hematopoietic cells from BM, and augmented access to these cells would then help to relatively specifically target diseased cells that otherwise might be protected by their BM niches. First respective studies using CXCR4 antagonists are under way. Finally, it needs to be determined whether some PRRs are expressed or even overexpressed on some early hematopoietic malignancies, and, if so, if they could be targets for future therapies.116,117,122,123 In addition, whereas therapeutic activation of early hematopoiesis is already used in clinical settings, it is thus far not established if the therapeutic interruption of activating signals will be of clinical benefit. This might be envisioned in settings of chronic early hematopoietic stimulation, triggered by infection and inflammation. Although in this review we focus on the consequences of pathogen-host interactions on early hematopoiesis, similar mechanisms and cascades are triggered during various pathologic conditions that do not require an invading pathogen. In this respect, so-called host-derived danger-associated molecular patterns and alarmins released on traumatic, vascular, metabolic, or toxic injury or even during natural aging or tissue repair are recognized as endogenous ligands by PRRs or other receptors and elicit early hematopoietic responses, probably with similar short- and long-term consequences as microbial infections.5,124-126
We are convinced that further research on the versatile demand-adapted response of the hematopoietic system will not only provide new basic insights and therapeutic options for hematologists, oncologists, immunologists, and infectious disease specialists but will likely also instruct on principle mechanisms of homeostasis and regeneration on infection and inflammation in other stem cell maintained organ systems as skin, gut, and brain.
The online version of this article contains a data supplement.
Acknowledgments
This work was supported in part by a Postdoctoral Fellowship of the Japanese Society for the Promotion of Science for Research Abroad to H.T., the Swiss National Science Foundation (310030_131088/1), the Promedica Foundation (Chur, Switzerland), and the Marlis Geiser-Lemken Stiftung (Zurich, Switzerland) to M.G.M.
Note added in proof:
de Bruin et al now demonstrated that IFNγ induces monopoiesis at cost of granulopoiesis from myeloid progenitor cells during viral infections.127
Authorship
Contribution: H.T., S.B., and M.G.M. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Markus G. Manz, Division of Hematology, University Hospital Zürich, CH-8091 Zürich, Switzerland; e-mail: markus.manz@usz.ch.