Abstract
NK-cell function is regulated by the integration of signals received from activating and inhibitory receptors. Here we show that a novel immune receptor, T-cell Ig and mucin-containing domain-3 (Tim-3), is expressed on resting human NK cells and is up-regulated on activation. The NK92 NK-cell line engineered to overexpress Tim-3 showed a marked increase in IFN-γ production in the presence of soluble rhGal-9 or Raji tumor cells engineered to express Gal-9. The Tim-3+ population of low-dose IL-12/IL-18–activated primary NK cells significantly increased IFN-γ production in response to soluble rhGal-9, Gal-9 presented by cell lines, and primary acute myelogenous leukemia (AML) targets that endogenously express Gal-9. This effect is highly specific as Tim-3 Ab blockade significantly decreased IFN-γ production, and Tim-3 cross-linking induced ERK activation and degradation of IκBα. Exposure to Gal-9–expressing target cells had little effect on CD107a degranulation. Reconstituted NK cells obtained from patients after hematopoietic cell transplantation had diminished expression of Tim-3 compared with paired donors. This observation correlates with the known IFN-γ defect seen early posttransplantation. In conclusion, we show that Tim-3 functions as a human NK-cell coreceptor to enhance IFN-γ production, which has important implications for control of infectious disease and cancer.
Introduction
Human NK cells are lymphocytes that develop from hematopoietic progenitor cells in the BM and secondary lymphoid tissues.1 Peripheral blood (PB) NK cells are phenotypically defined as expressing the surface receptor CD56 (neural cell adhesion molecule [NCAM]) and lacking expression of CD3.2 They mediate their function through the exocytosis of lytic granules that contain perforin and granzymes,3 the expression of death receptor ligands,4 the expression of FcRγIII, which mediates Ab-dependent cell-mediated cytotoxicity,5 and the secretion of cytokines and chemokines.6 As a result, NK cells take part in both the innate and adaptive immune systems and play important roles in the control of viral infections, pregnancy, tumor immunosurveillance, and hematopoietic cell transplantation (HCT).7,8
The ability of NK cells to differentiate normal healthy cells (self) from virally infected or malignantly transformed cells (nonself) is regulated by a sophisticated repertoire of cell-surface receptors that control their activation, proliferation, and effector functions.9-11 Recently, a novel receptor, T-cell Ig and mucin-containing domain-3 (Tim-3), has been described to have various roles in immune regulation and is highly expressed on NK cells in mice and humans.12-17 Tim-3 is a type I membrane glycoprotein that was first identified as a cell marker of terminally differentiated CD4+ Th1 cells.18 Galectin-9 (Gal-9), a 40-kDa S-type β-galactoside binding lectin, is a known ligand for Tim-3 and is highly expressed in tissues of the immune system, such as the BM, lymph nodes, thymus, and spleen.18,19 The functional role of the Tim-3/Gal-9 pathway in T cells was first described to negatively regulate the Th1 response.18 In contrast, stimulation of Tim-3–expressing dendritic cells (DCs) results in the secretion of proinflammatory cytokines.15,20 Therefore, the Tim-3/Gal-9 interaction is considered to mediate both inhibitory and activating signaling pathways that have important roles in infection, autoimmunity, inflammation, peripheral tolerance, and tumor immunity.21-25
The potential of NK cells to control human hematologic malignancies has been increasingly recognized in recent years.8,26,27 Initial studies were based on the KIR-ligand incompatibility model in which transplantation across the HLA class I barriers triggers alloreactive NK-cell responses.8,26,27 More recently, specific donor NK-cell receptor repertoires have been associated with less relapse and improved survival for patients receiving unrelated donor transplants for both HLA-matched and HLA-mismatched settings.28,29 Therefore, to enhance the therapeutic effects of NK cells, a better understanding of the mechanisms that underlie target cell recognition is needed. Although Tim-3 is highly expressed on NK cells compared with other lymphocyte populations,16,17 the functional relevance of this is not completely understood. These experiments were designed to test the hypothesis that Tim-3 acts to mediate NK-cell effector function.
Methods
Cell isolation
Adult PB was obtained from the Memorial Blood Center (Minneapolis, MN). PBMCs were isolated by centrifugation using a Histopaque gradient (Sigma-Aldrich) and NK cells were negatively selected using the magnetic-activated cell sorting (MACS) NK Cell Isolation Kit as per the manufacturer's protocol (Miltenyi Biotec). Additional samples from patients who received double unit umbilical cord blood transplants using cyclosporine and mycophenolate mofetil GVHD prophylaxis were analyzed. All samples were obtained after informed consent in accordance with the Declaration of Helsinki, using guidelines approved by the Committee on the Use of Human Subjects in Research at the University of Minnesota.
Cell lines
NK92 cells were cultured at 37°C with 5% CO2 in NK92 medium (α medium containing 12.5% FBS, 12.5% horse serum [HyClone Laboratories], 0.2mM inositol, 0.1mM β-mercaptoethanol, 0.02mM folic acid [Sigma-Aldrich], 100 U/mL penicillin, 100 U/mL streptomycin [Invitrogen]) containing 500 U/mL recombinant human IL-2 (Chiron). Raji cells were cultured at 37°C with 5% CO2 in RPMI medium supplemented with 10% FBS. K562 cells were cultured at 37°C with 5% CO2 in Iscove medium supplemented with 20% FBS.
Cell transfection
The full-length human Tim-3 cDNA or Gal-9 cDNA, generated by RT-PCR from RNA obtained from primary human NK cells and Jurkat cells, respectively, was cloned into the murine stem cell virus (MSCV) enhanced GFP (eGFP) vector upstream of the internal ribosomal entry sequence using Gateway Cloning Technology (Invitrogen Life Technologies). The Tim-3 MSCV or Gal-9 MSCV vector was transfected into 293T cells along with a pCL packaging vector to generate virus particles. In a 6-well plate, 2 × 106 NK92 or Raji cells were suspended in 3 mL of thawed retrovirus supernatant containing 10 μg/mL protamine sulfate, centrifuged at 700g for 120 minutes at 33°C, rested for 2 hours at 33°C, 5% CO2 and repeated once again the following day. Cells were then cultured for 24-48 hours. eGFP+ cells were then selected using the FACSAria (BD Biosciences) and protein expression was evaluated via Western blot and FACS analysis using monoclonal anti–human Tim-3 Ab and anti–human Gal-9 Ab (R&D Systems).
Flow cytometry
Single-cell suspensions were stained with the following mAbs: PE/Cy7-conjugated CD56 (HCD56; BioLegend), ECD-conjugated CD3 (UCHT1; Beckman Coulter), PE-conjugated Tim-3 (R&D Systems), PerCP/Cy5.5-conjugated anti–human CD107a (LAMP-1, H4A3; BioLegend), and Pacific Blue–conjugated anti–human IFN-γ (4S.B3; BioLegend). Phenotypic acquisition of cells was performed on the LSRII (BD Biosciences) and analyzed with FlowJo software (TreeStar Inc).
Western blot analysis
Western blot analyses were carried out according to standard protocols with Abs raised against human Gal-9 (R&D Systems; 1:200), IκBa (Cell Signaling; 1:1000), p44/42 MAPK (Erk1/2; Cell Signaling; 1:1000), and β-actin (Cell Signaling; 1:1000; β-actin was used as a loading control). Protein expression was detected using the SuperSignal West Pico Chemiluminescent Substrate kit (Thermo Scientific) according to the manufacturer's instructions. For analysis of IκBa degradation and ERK phosphorylation, purified primary PB NK cells were stimulated with plate-bound anti–Tim-3 mAb (clone 2E2; Biolegend; 10 μg/mL) for the indicated time points, harvested, and centrifuged at 1250g for 5 minutes at 4°C and then immediately lysed in NP-40 lysis buffer (Invitrogen) supplemented with protease and phosphatase inhibitors. Lysates were clarified by centrifugation at 13 200g for 10 minutes at 4°C.
Cytokine production and CD107a degranulation assay
NK92 cells, freshly isolated PBMCs, or purified PB NK cells were incubated for 16 hours with IL-12 (1 ng/mL) and IL-18 (10 ng/mL; both from R&D Systems). Cells were washed in 1× PBS, treated with 10 μg/mL anti-Tim-3 mAb or Ig controls (rat IgG2a; R&D Systems) and incubated for 15 minutes at 37°C. For HLA-blocking experiments using primary acute myelogenous leukemia (AML) and chronic myelogenous leukemia (CML) tumor cells, targets were preincubated for 15 minutes with 20 μg/mL anti–human HLA mAb (kindly provided by Lopez Botet, University Pompeu Fabra, Spain) or Ig control (human IgG1; R&D Systems). Anti–human CD107a mAb was added alone or with target cells (Raji eGFP, Raji Gal-9, primary AML, or primary CML tumor cells; E:T ratios 10:1 or 2:1) or recombinant human Gal-9 (rhGal-9; Gal Pharma) and incubated for 1 hour. GolgiStop (1:1500) and GolgiPlug (1:1000; both from BD Biosciences) were added and cells were further incubated for 5 hours. Cells were then harvested and stained with mAb CD56, CD3, and Tim-3 before fixation and permeabilization. Permeabilized cells were then stained for intracellular IFN-γ using anti–human IFN-γ mAb. IFN-γ and CD107a expression was evaluated by FACS analysis. Select chemokines/cytokines were measured in supernatant. Purified PB NK cells were exposed to 20nM rhGal-9 for 4 hours with or without priming for 16 hours with IL-12 (1 ng/mL) and IL-18 (10 ng/mL). Supernatant levels of IFN-γ, GM-CSF, IL-10, IL-8, MIP-1α, MIP-1β, and RANTES were determined by multiplex assay using the Luminex system and human-specific bead sets (R&D Systems; sensitivity 0.3-2.0 pg/mL).
Results
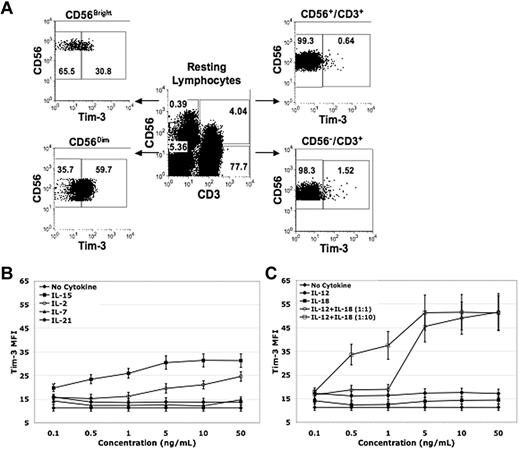
Tim-3 is expressed on human NK cells
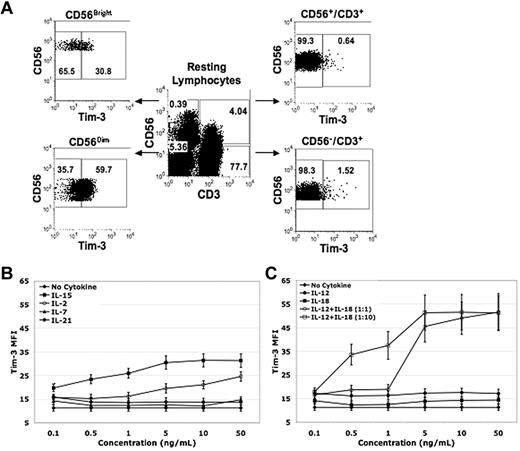
Tim-3 has been described to have diverse innate and adaptive immunomodulatory roles.20-25 Although NK cells express Tim-3,15,16 little is known about its function. We first examined Tim-3 expression on PBMCs from normal healthy volunteers. Among resting lymphocytes, the CD56+/CD3− NK-cell population had the highest percentage of Tim-3–expressing cells (49% ± 2%) compared with CD56+/CD3+ NKT (6% ± 1%, P < .001, n = 10) and CD56−/CD3+ T-cell populations (1% ± 0%, P < .001, n = 10; Figure 1A). The unique subset of IFN-γ–producing CD56Bright NK cells30 exhibited significantly lower resting Tim-3 expression compared with CD56Dim NK cells (53% ± 6% vs 72% ± 5% [P < .001, n = 20]). Tim-3 expression was up-regulated in response to IL-2, IL-15, IL-12, and IL-18 with doses as low as 0.1 ng/mL, and had a minimal response to IL-7 or IL-21 (Figure 1B-C). Together, IL-12 and IL-18 potently increased Tim-3 expression in a 1:1 ratio beginning at 5 ng/mL and in a 1:10 ratio beginning at 0.5:5 ng/mL (Figure 1C). No significant differences were observed in Tim-3 expression between CD56Bright and CD56Dim NK cells with lower concentrations of IL-2 and IL-15 priming. However, with low concentrations of IL-12 and IL-18 priming Tim-3, expression in CD56Bright NK cells was significantly higher compared with CD56Dim NK cells (Tim-3 mean fluorescence intensity [MFI] 31 ± 4 vs 17 ± 2 at IL-12 [0.1 ng/mL] and IL-18 [1 ng/mL; P = .005, n = 8]). Tim-3 expression induced with IL-12 (1 ng/mL) and IL-18 (10 ng/mL) cytokine stimulation decreased 24 hours after cytokine withdraw and was relatively absent after 48 hours. In T cells, it has been shown that the transcription factor T-bet binds to the Tim-3 promoter and is important for Tim-3 transcription.12 Real-time PCR analysis of IL-2, IL-15, IL-12, IL-18, and IL-12/IL-18–stimulated NK cells revealed an increase in Tim-3 mRNA expression (except for IL-18) that also correlated with an increase in T-bet mRNA expression, suggesting a similar mechanism of Tim-3 induction for NK cells as seen in T cells (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Tim-3 is expressed on resting NK cells and is up-regulated on activation. (A-C) PBMCs and purified PB NK cells were stained with anti-CD56, anti-CD3, and anti-Tim-3 mAbs. (A) Tim-3 expression on resting lymphocyte populations was determined by FACS analysis and a representative donor is shown in flow plot A. (B) PBMCs were enriched for CD56+/CD3− NK cells using a negative immunomagnetic bead depletion strategy. NK cells were incubated overnight in basal media or media containing IL-15, IL-2, IL-7, or IL-21 at the indicated concentrations. Tim-3 MFI was determined by FACS analysis (n = 8). (C) PBMCs were enriched for CD56+/CD3− NK cells using a negative immunomagnetic bead depletion strategy. NK cells were incubated overnight in basal media or media containing IL-12 alone, IL-18 alone, IL-12 and IL-18 (concentration ratio of 1:1), and IL-12 and IL-18 (concentration ratio of 1:10) at the indicated concentrations. Tim-3 MFI was determined by FACS analysis (n = 8).
Tim-3 is expressed on resting NK cells and is up-regulated on activation. (A-C) PBMCs and purified PB NK cells were stained with anti-CD56, anti-CD3, and anti-Tim-3 mAbs. (A) Tim-3 expression on resting lymphocyte populations was determined by FACS analysis and a representative donor is shown in flow plot A. (B) PBMCs were enriched for CD56+/CD3− NK cells using a negative immunomagnetic bead depletion strategy. NK cells were incubated overnight in basal media or media containing IL-15, IL-2, IL-7, or IL-21 at the indicated concentrations. Tim-3 MFI was determined by FACS analysis (n = 8). (C) PBMCs were enriched for CD56+/CD3− NK cells using a negative immunomagnetic bead depletion strategy. NK cells were incubated overnight in basal media or media containing IL-12 alone, IL-18 alone, IL-12 and IL-18 (concentration ratio of 1:1), and IL-12 and IL-18 (concentration ratio of 1:10) at the indicated concentrations. Tim-3 MFI was determined by FACS analysis (n = 8).
Tim-3 is an activating coreceptor on NK cells and promotes IFN-γ production
As Tim-3 is highly expressed on resting NK cells and up-regulated with activation, we hypothesized that Tim-3 may be important in mediating NK-cell function. To test this, we first overexpressed Tim-3 in the IL-2–dependent NK-cell line, NK92, which has low endogenous Tim-3 expression (34% ± 4% for NK92 native cell line vs 91% ± 2% for NK92 Tim-3 cell line). The NK92 native, NK92 eGFP control, and NK92 Tim-3 cell lines were then treated with soluble rhGal-9 in the presence of a Tim-3 blocking mAb or isotype control, and intracellular IFN-γ levels were evaluated (Figure 2A). rhGal-9 concentrations of 10nM to 100nM induced significant increases in IFN-γ production in the NK92 Tim-3 cell line compared with the NK92 native and NK92 eGFP control cell lines. Gal-9 has been shown to have a dose-dependent apoptotic effect.22 Although induction of apoptosis was observed at high concentrations (> 50nM) of rhGal-9, there was no apoptosis at lower concentrations used to evaluate function. Blocking the Tim-3/Gal-9 interaction eliminated the induction of IFN-γ levels in the NK92 Tim-3 cell line at all rhGal-9 concentrations tested. These results show that engagement of Tim-3 by rhGal-9 is specific and identifies Tim-3 as an activating coreceptor that induces NK92 cells to produce IFN-γ.
The Tim-3 ligand Gal-9 increases IFN-γ production in the Tim-3–transduced NK92 cell line. (A) NK92 cell lines were stimulated with rhGal-9 for 4 hours in the presence of a blocking Tim-3 mAb or isotype control Ab and intracellular IFN-γ production was determined by FACS analysis (*P < .01, NK92 Tim-3 vs NK92 native [n = 5] and NK92 Tim-3 vs NK92 Tim-3 blocked [anti–Tim-3 mAb, n = 5]). (B) The full-length human Gal-9 gene was cloned into a MSCV vector and the Raji cell line was transduced as described. Gal-9 protein expression (40 kDa) was confirmed by Western blot analysis. The Jurkat cell line has endogenous Gal-9 expression and was used as the positive control. The murine stromal cell line EL08-1D2 was used as the negative control. (C) NK92 cell lines were incubated in the presence of Raji eGFP and Raji Gal-9 target cell lines for 5 hours and intracellular IFN-γ production was determined via FACS analysis (*P < .001, n = 5; error bars represent SEM).
The Tim-3 ligand Gal-9 increases IFN-γ production in the Tim-3–transduced NK92 cell line. (A) NK92 cell lines were stimulated with rhGal-9 for 4 hours in the presence of a blocking Tim-3 mAb or isotype control Ab and intracellular IFN-γ production was determined by FACS analysis (*P < .01, NK92 Tim-3 vs NK92 native [n = 5] and NK92 Tim-3 vs NK92 Tim-3 blocked [anti–Tim-3 mAb, n = 5]). (B) The full-length human Gal-9 gene was cloned into a MSCV vector and the Raji cell line was transduced as described. Gal-9 protein expression (40 kDa) was confirmed by Western blot analysis. The Jurkat cell line has endogenous Gal-9 expression and was used as the positive control. The murine stromal cell line EL08-1D2 was used as the negative control. (C) NK92 cell lines were incubated in the presence of Raji eGFP and Raji Gal-9 target cell lines for 5 hours and intracellular IFN-γ production was determined via FACS analysis (*P < .001, n = 5; error bars represent SEM).
We next evaluated the effect of Tim-3 on NK-cell function when its ligand was presented in a cellular context. The NK-resistant target Burkitt lymphoma–derived Raji cell line, which does not endogenously express Gal-9, was transduced with full-length Gal-9 (Figure 2B). The NK92 cell lines were then coincubated with Raji eGFP control or Raji Gal-9 targets and intracellular IFN-γ levels were measured (Figure 2C). We observed a significant increase in IFN-γ production by the NK92 Tim-3 cell line (compared with the NK92 native and eGFP control cell lines) when exposed to the Raji Gal-9 targets compared with the Raji eGFP control targets (31% ± 1% for Raji eGFP control vs 53% ± 4% for Raji Gal-9 [P < .001]). Moreover, Tim-3 blockade of the NK92 Tim-3 cell line significantly decreased IFN-γ levels, which was greatest in the Raji Gal-9 targets, but also observed in the Raji eGFP control targets. This is consistent with the fact that Tim-3 is capable of engaging other moieties31,32 and suggests that Raji expresses other Tim-3 ligands.
Having established the function of Tim-3 in the NK92 cell line, we were interested in investigating how Tim-3 affects the function of primary NK cells. We first examined the IFN-γ response of primary PB NK cells to rhGal-9 without cytokine stimulation; modest increases in IFN-γ production were noted in resting NK cells with rhGal-9 treatment alone despite Tim-3 expression. These results suggest that Tim-3 acts as an activating coreceptor and that cytokine priming is a requirement for enhanced Tim-3 function. Therefore, NK cells were isolated from normal healthy volunteers and activated overnight with 1 ng/mL IL-12 and 10 ng/mL IL-18 based on Figure 1C and the finding that these concentrations were a weak IFN-γ stimulus. We also chose these cytokines to explore further as murine IL-12 knockout studies have shown that IL-12 has a vital role in the physiologic antitumor and antiviral immune response, which is mediated in part through NK-cell IFN-γ production.33,34 A unique Tim-3 expression pattern was found on post–IL-12 and IL-18–activated NK cells, which divided cells into 4 distinct populations: Tim-3 was homogeneously up-regulated on all CD56Bright NK cells while CD56Dim NK cells were stratified into 3 defined populations based on Tim-3hi, Tim-3lo, and Tim-3neg expression levels. Analysis of these populations revealed that the Tim-3–expressing NK cells were the predominant IFN-γ–producing cells, with the Tim-3hi NK cells producing the highest IFN-γ levels (30% ± 6% for Tim-3hi, 17% ± 5% for Tim-3lo, and 11% ± 4% for Tim-3neg [n = 15]; Figure 3A). We next sorted CD56Bright, CD56Dim/Tim-3−, and the higher fraction of the CD56Dim/Tim-3+ resting NK-cell populations and stimulated these cells overnight with 1 ng/mL IL-12 and 10 ng/mL IL-18. After activation, the majority of the CD56Bright NK cells exhibited a Tim-3hi phenotype (72% ± %5), with 32% ± 3% of the CD56Dim/Tim-3− population and 74% ± 4% of the CD56Dim/Tim-3+ population acquiring a Tim-3hi phenotype (Figure 3B). IFN-γ production within these activated NK-cell populations in response to stimulation with 10nM rhGal-9 was next evaluated. There was a significant increase in IFN-γ production within the Tim-3hi–expressing populations compared with the Tim-3neg populations when exposed to rhGal-9. This increase in IFN-γ production within the Tim-3–expressing NK-cell populations was abrogated by the addition of β-lactose, a β-galactoside that binds and blocks Gal-9 activity. In agreement with the intracellular data, Luminex analysis revealed an increase in IFN-γ production of resting bulk CD56+ NK cells in response to a 4-hour exposure to 20nM rhGal-9 (baseline: 0 ± 0 pg/mL vs rhGal-9 stimulated: 30 ± 10 pg/mL; P < .05; n = 6). To confirm the Tim-3 specificity of this response to rhGal-9, we subsequently sorted the Tim-3+ and Tim-3− fractions of IL-12– and IL-18–activated NK cells and measured IFN-γ production in response to rhGal-9 in the presence of a Tim-3–blocking mAb (Figure 3C). There was a significant decrease in IFN-γ production within the Tim-3+ NK cells with the application of the blocking mAb, confirming that the increase in IFN-γ levels in response to rhGal-9 was induced via interaction with Tim-3.
Primary Tim-3+ NK cells specifically produce IFN-γ in response to rhGal-9. (A) Purified PB NK cells were incubated overnight in media containing IL-12 (1 ng/mL) and IL-18 (10 ng/mL) and intracellular IFN-γ production was determined by FACS analysis. One representative donor is shown in flow plots A. (B) Resting NK-cell populations CD56Bright, CD56DIM/Tim-3−, and CD56DIM/Tim-3+ were sorted and incubated overnight in media containing IL-12 (1 ng/mL) and IL-18 (10 ng/mL). Each sorted, activated population was exposed to 10nM rhGal-9 with and without β-lactose (30mM) blocking and the percentage of control intracellular IFN-γ production (calculated as [% cells IFN-γ+ at 10nM/% cells IFN-γ+ at 0nM] × 100) was determined within the Tim-3neg and Tim-3hi fractions via FACS analysis (*P < .005, n = 5). (C) IL-12 (1 ng/mL) and IL-18 (10 ng/mL) activated NK cells were sorted into Tim-3+ and Tim-3− cell populations and exposed to rhGal-9 (0nM, 10nM, and 20nM) with and without blocking using anti-Tim-3 mAb. The percentage of control intracellular IFN-γ production (calculated as [% cells IFN-γ+ at 10nM or 20nM/% cells IFN-γ+ at 0nM] × 100) determined via FACS analysis (*P < .05, n = 5; error bars represent SEM).
Primary Tim-3+ NK cells specifically produce IFN-γ in response to rhGal-9. (A) Purified PB NK cells were incubated overnight in media containing IL-12 (1 ng/mL) and IL-18 (10 ng/mL) and intracellular IFN-γ production was determined by FACS analysis. One representative donor is shown in flow plots A. (B) Resting NK-cell populations CD56Bright, CD56DIM/Tim-3−, and CD56DIM/Tim-3+ were sorted and incubated overnight in media containing IL-12 (1 ng/mL) and IL-18 (10 ng/mL). Each sorted, activated population was exposed to 10nM rhGal-9 with and without β-lactose (30mM) blocking and the percentage of control intracellular IFN-γ production (calculated as [% cells IFN-γ+ at 10nM/% cells IFN-γ+ at 0nM] × 100) was determined within the Tim-3neg and Tim-3hi fractions via FACS analysis (*P < .005, n = 5). (C) IL-12 (1 ng/mL) and IL-18 (10 ng/mL) activated NK cells were sorted into Tim-3+ and Tim-3− cell populations and exposed to rhGal-9 (0nM, 10nM, and 20nM) with and without blocking using anti-Tim-3 mAb. The percentage of control intracellular IFN-γ production (calculated as [% cells IFN-γ+ at 10nM or 20nM/% cells IFN-γ+ at 0nM] × 100) determined via FACS analysis (*P < .05, n = 5; error bars represent SEM).
The production of other cytokines and chemokines induced by 4-hour exposure to 20nM rhGal-9 was also examined in purified bulk CD56+ NK cells primed with IL-12 (1 ng/mL) and IL-18 (10 ng/mL). An increase in MIP-1α (0nM: 670 ± 120 pg/mL vs 20nM: 2331 ± 203 pg/mL; P < .001, n = 4), MIP-1β (535 ± 92 pg/mL vs 3262 ± 1209 pg/mL; P = .04, n = 4), and RANTES (603 ± 188 pg/mL vs 1530 ± 301 pg/mL; P = .04, n = 4) was observed. No differences were observed for GM-CSF, IL-10, and IL-8. Although the addition of β-lactose potently blocked the effect of 20nM rhGal-9 (MIP-1α: 727 ± 102 pg/mL, P < .001; MIP-1β: 514 ± 86 pg/mL, P = .06; RANTES: 574 ± 220 pg/mL, P = .04), addition of the Tim-3–blocking mAb had a minimal effect. As the activation thresholds and kinetics of NK-cell chemokine and cytokine responses are different,11 our data suggest Tim-3 may be less important for the early chemokine response and have a more prominent role in the IFN-γ response.
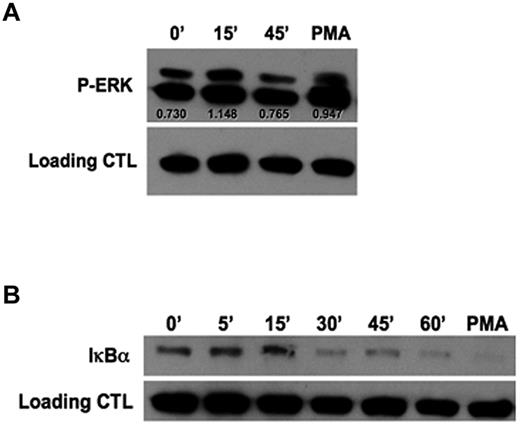
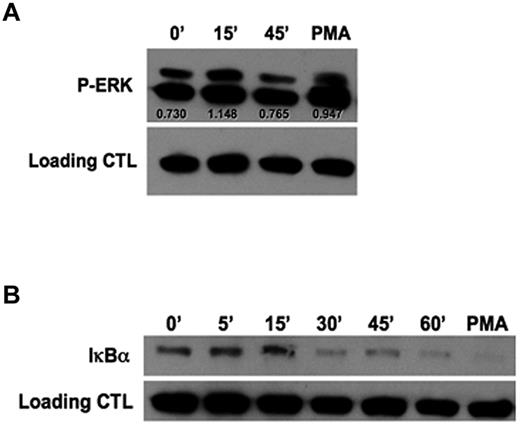
Initial experiments evaluating the mechanism by which rhGal-9 induces NK-cell IFN-γ production revealed the involvement of MEK1/2 and NFκB signaling components (supplemental Figure 2). To further understand the direct mechanism by which Tim-3 enhances IFN-γ, purified NK cells were stimulated with an agonistic anti–Tim-3 mAb and activation of ERK (Figure 4A) and degradation of the NFκB inhibitor IκBα (Figure 4B) were examined. After 15 minutes of Tim-3 engagement, an increase in ERK phosphorylation was observed. Furthermore, 30 minutes of Tim-3 engagement led to degradation of IκBα, indicating Tim-3 is involved in NFκB signaling. Together, these results further establish Tim-3 as an activating coreceptor, which requires IL-12 and IL-18 priming to enhance IFN-γ cytokine production.
Tim-3 engagement induces IκBα degradation and ERK activation. (A-B) Purified PB NK cells were cross-linked with plate-bound anti–Tim-3 mAb for the indicated times. Cells were stimulated with phorbol 12-myristate 13 acetate (PMA) for 15 minutes as a positive control. (A) Western blot analysis of ERK phosphorylation. Scanning densitometry was used to determine the relative expression levels of protein after normalizing to β-actin as a loading control. (B) Western blot analysis of total IκBα degradation. Data in panels A and B are representative of 4 independent experiments.
Tim-3 engagement induces IκBα degradation and ERK activation. (A-B) Purified PB NK cells were cross-linked with plate-bound anti–Tim-3 mAb for the indicated times. Cells were stimulated with phorbol 12-myristate 13 acetate (PMA) for 15 minutes as a positive control. (A) Western blot analysis of ERK phosphorylation. Scanning densitometry was used to determine the relative expression levels of protein after normalizing to β-actin as a loading control. (B) Western blot analysis of total IκBα degradation. Data in panels A and B are representative of 4 independent experiments.
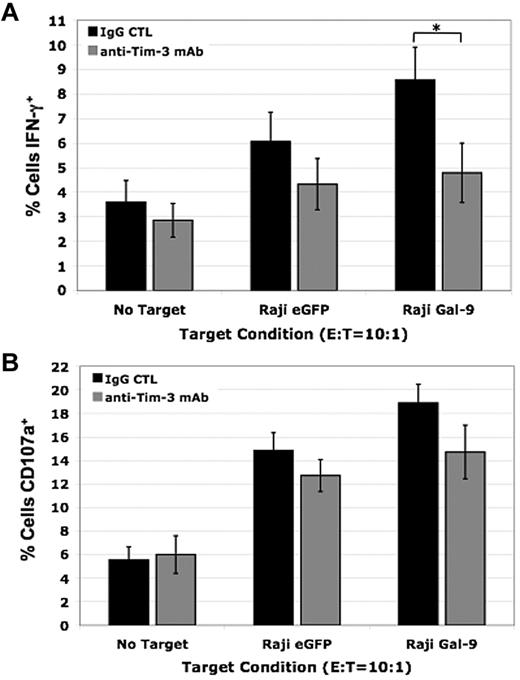
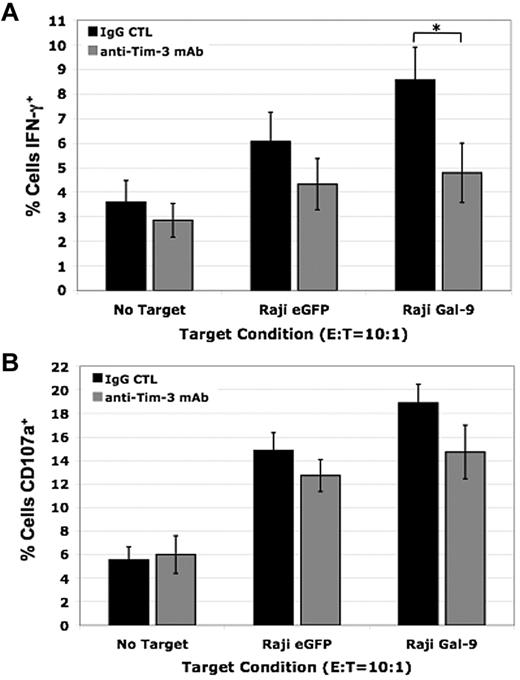
To examine the effect of Tim-3 in primary human NK cells when Gal-9 is presented in a cellular context, we coincubated IL-12 (1 ng/mL) and IL-18 (10 ng/mL) activated PBMCs with Raji eGFP control or Raji Gal-9 target cells. We observed an increase in NK-cell IFN-γ production when exposed to Raji Gal-9 target cells compared with the Raji eGFP control target cells and Tim-3 blockade significantly reduced this increase against the Raji Gal-9 targets (9% ± 1% vs 5% ± 1% [P < .05]; Figure 5A). We also evaluated CD107a expression, a marker of degranulation that correlates with NK cell–mediated lysis of target cells. Although there was a slight increase in CD107a expression after coculture with the Raji Gal-9 target cells compared with the Raji eGFP control target cells and blocking Tim-3 resulted in a small decrease, these differences were not significant (Figure 5B). Collectively, these data demonstrate Tim-3 functions to activate NK cells significantly enhancing their ability to produce IFN-γ and has a lesser effect on degranulation when primed with IL-12 and IL-18.
Blocking Tim-3 decreases IFN-γ production in primary human NK cells and has a minimal effect on degranulation. (A-B) IL-12 (1 ng/mL) and IL-18 (10 ng/mL) activated PBMCs were coincubated with Raji eGFP and Raji Gal-9 target cell lines in the presence of a blocking Tim-3 mAb or isotype control for 5 hours at an E:T ratio of 10:1. (A) Intracellular IFN-γ production (*P < .05, n = 6) and (B) CD107a expression (n = 6) was determined by FACS analysis (error bars represent SEM).
Blocking Tim-3 decreases IFN-γ production in primary human NK cells and has a minimal effect on degranulation. (A-B) IL-12 (1 ng/mL) and IL-18 (10 ng/mL) activated PBMCs were coincubated with Raji eGFP and Raji Gal-9 target cell lines in the presence of a blocking Tim-3 mAb or isotype control for 5 hours at an E:T ratio of 10:1. (A) Intracellular IFN-γ production (*P < .05, n = 6) and (B) CD107a expression (n = 6) was determined by FACS analysis (error bars represent SEM).
Diminished Tim-3 expression on NK cells correlates with impaired IFN-γ production in vivo
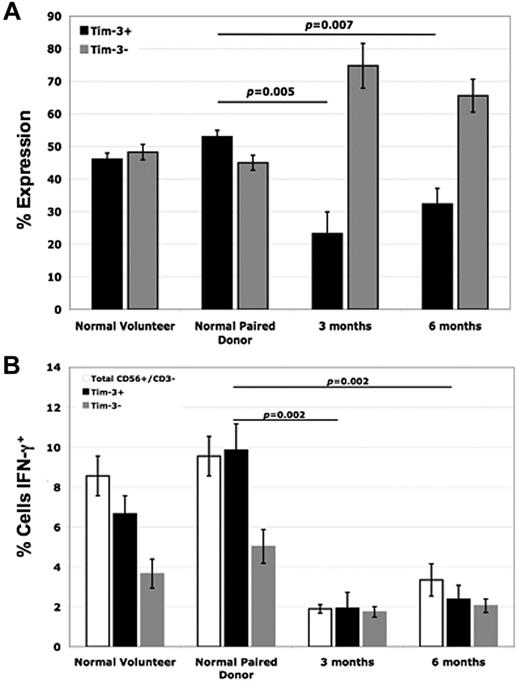
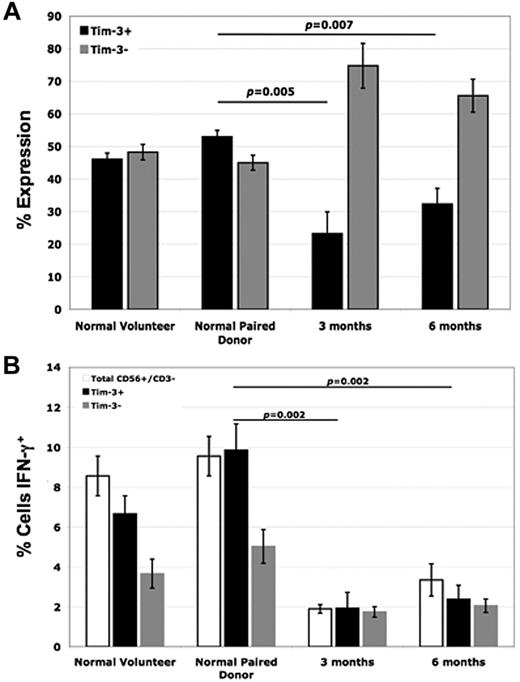
Studying patients undergoing allogeneic HCT for the treatment of leukemia provides a unique in vivo opportunity to investigate donor NK-cell function in the context of human disease.35 Therefore, Tim-3 expression on NK cells from normal healthy volunteers and HCT donor/recipient pair patient samples was next evaluated. In posttransplant recipients at both the 3-month and 6-month time points, Tim-3 NK-cell expression was significantly lower than that of the paired donor (53% ± 2% for paired donor vs 23% ± 7% in the recipient at 3 months [P = .005], and 32% ± 5% in the recipient at 6 months [P = .007]; Figure 6A). When these cells were coincubated with the highly NK-sensitive target cell line K562 and intracellular IFN-γ levels were measured, we observed a significant impairment in the ability of recipient NK cells after transplantation to produce IFN-γ compared with their paired donors (Figure 6B). As our data above demonstrate, Tim-3 has a pivotal role in enhancing NK-cell IFN-γ production. When we evaluated IFN-γ levels within the Tim-3+ populations of the donor/recipient pairs, we also found significant decreases in IFN-γ levels of the posttransplant recipient NK cells compared with the paired donors (10% ± 1% for the donor vs 2% ± 1% in the recipient at 3 months [P < .002]), suggesting that the posttransplantation defect may go beyond just reduced expression of Tim-3 alone.
NK cells from posttransplant recipients have reduced Tim-3 expression compared with normal paired donors, which correlates with decreased IFN-γ production. (A) PBMCs from normal healthy volunteers, normal pair donors, and posttransplant recipient patient samples (from time points of 3 and 6 months) were stained with anti-CD56, anti-CD3, and anti–Tim-3 mAb. Tim-3 expression was evaluated by FACS analysis within the gated CD56+/CD3− NK-cell population (n = 4). (B) PBMCs from normal healthy volunteers, normal pair donors, and posttransplantat recipient patient samples (from time points of 3 and 6 months) were coincubated with the K562 target cell line at an E T ratio of 2:1 for 5 hours and intracellular IFN-γ levels were measured within the total CD56+/CD3−, Tim-3+, and Tim-3− NK-cell populations via FACS analysis (n = 4; error bars represent SEM).
NK cells from posttransplant recipients have reduced Tim-3 expression compared with normal paired donors, which correlates with decreased IFN-γ production. (A) PBMCs from normal healthy volunteers, normal pair donors, and posttransplant recipient patient samples (from time points of 3 and 6 months) were stained with anti-CD56, anti-CD3, and anti–Tim-3 mAb. Tim-3 expression was evaluated by FACS analysis within the gated CD56+/CD3− NK-cell population (n = 4). (B) PBMCs from normal healthy volunteers, normal pair donors, and posttransplantat recipient patient samples (from time points of 3 and 6 months) were coincubated with the K562 target cell line at an E T ratio of 2:1 for 5 hours and intracellular IFN-γ levels were measured within the total CD56+/CD3−, Tim-3+, and Tim-3− NK-cell populations via FACS analysis (n = 4; error bars represent SEM).
Tim-3 blockade decreases NK-cell IFN-γ production against primary AML tumor targets
To evaluate the functional contribution of Tim-3 and its impact in NK-cell targeting of leukemia cells, we examined the Gal-9 expression profile of human primary AML and CML samples via Western blot and IHC. Analysis revealed primary CML samples to be Gal-9 negative while primary AML samples had high levels of Gal-9 expression (Figure 7A; IHC, data not shown). PBMCs were then isolated from normal healthy volunteers and coincubated with primary AML tumor cells from patients and IFN-γ levels were evaluated (Figure 7B). Tim-3 blockade resulted in a significant reduction of NK-cell IFN-γ levels after target cell exposure. To better evaluate the contribution of Tim-3 to NK-cell activation, we applied a pan-HLA blocking Ab to the primary AML target cells to eliminate overriding inhibitory signals and then measured IFN-γ production with and without Tim-3 blockade. As expected, IFN-γ levels increased with the application of the HLA-blocking Ab alone. When both the anti-HLA and anti–Tim-3–blocking Abs were applied, we observed a significant decrease in NK-cell IFN-γ production, thus confirming the functional contribution of Tim-3 in NK-cell targeting of primary AML cells. This effect was specific as CML patient samples, which lack Gal-9 expression, did not reveal significant functional differences with Tim-3 blockade (Figure 7B).
Tim-3 blockade decreases IFN-γ production of activated human NK cells against primary AML tumor cells, but has no effect on degranulation. (A) Western blots were performed on cell lysates of AML (n = 2) and CML (n = 2) tumor cells to evaluate Gal-9 (40 kDa) expression. The Jurkat cell line has endogenous Gal-9 expression and was used as the positive control. The murine stromal cell line EL08-1D2 was used as the negative control. (B-C) PBMCs were incubated overnight in media containing IL-12 (1 ng/mL) and IL-18 (10 ng/mL) and a 5-hour CD107a/IFN-γ assay was performed as described using primary human AML and CML tumor cell targets. The percentage of control intracellular IFN-γ (B) or CD107a (C) production of NK cells when exposed to targets with and without Tim-3 and HLA blocking alone and in combination (calculated for each blocking condition as [% cells IFN-γ+ or CD107a+ in the presence of target/% cells IFN-γ+ or CD107a+ without targets] × 100) was determined by FACS analysis (*P < .005, n = 6; error bars represent the SEM).
Tim-3 blockade decreases IFN-γ production of activated human NK cells against primary AML tumor cells, but has no effect on degranulation. (A) Western blots were performed on cell lysates of AML (n = 2) and CML (n = 2) tumor cells to evaluate Gal-9 (40 kDa) expression. The Jurkat cell line has endogenous Gal-9 expression and was used as the positive control. The murine stromal cell line EL08-1D2 was used as the negative control. (B-C) PBMCs were incubated overnight in media containing IL-12 (1 ng/mL) and IL-18 (10 ng/mL) and a 5-hour CD107a/IFN-γ assay was performed as described using primary human AML and CML tumor cell targets. The percentage of control intracellular IFN-γ (B) or CD107a (C) production of NK cells when exposed to targets with and without Tim-3 and HLA blocking alone and in combination (calculated for each blocking condition as [% cells IFN-γ+ or CD107a+ in the presence of target/% cells IFN-γ+ or CD107a+ without targets] × 100) was determined by FACS analysis (*P < .005, n = 6; error bars represent the SEM).
As data in Figure 5B showed a minimal role of Tim-3 in NK-cell degranulation against the Raji target cell lines when primed with IL-12 and IL-18, we next evaluated whether this was also true for primary leukemia cells. PBMCs isolated from normal healthy volunteers were coincubated with primary AML and CML tumor cells from patients in the presence of a blocking Tim-3 mAb or isotype control, and CD107a expression levels were measured (Figure 7C). In agreement with the Raji cell line data, there were no differences in CD107a degranulation with Tim-3 blockade for either of the primary AML or CML targets.
Discussion
In this study, we have examined the expression and function of a novel immune receptor Tim-3 in primary human NK cells. Among resting lymphocyte populations, NK cells have the highest percentage of cells expressing Tim-3. In response to cytokine stimulation, Tim-3 expression increased and engagement of Tim-3 with the Gal-9 ligand induced significant increases in IFN-γ production that were abrogated by Tim-3 blockade. Tim-3 directly signals as engagement of Tim-3 led to ERK activation and degradation of the NFκB inhibitor IκBα. In contrast to its effect on cytokine production when primed with IL-12 and IL-18, Tim-3 blockade minimally affected NK-cell degranulation. Reconstituted posttransplantation NK cells from HCT recipients have reduced levels of Tim-3 expression that correlated with impaired IFN-γ production and IFN-γ production by NK cells targeting Gal-9–positive primary AML tumors was significantly reduced with Tim-3 blockade. Our data collectively show that Tim-3 functions as an activating coreceptor in human NK cells to enhance IFN-γ production when engaged by its ligand Gal-9 supporting a model whereby Tim-3 is up-regulated by inflammatory cytokines and NK-cell cytokine production is enhanced by Tim-3/Gal-9 interactions.
The functional role of the Tim-3/Gal-9 pathway was first described as a mechanism to negatively regulate the Th1 response, inhibiting IFN-γ production and inducing cell death.18,20 Subsequently, this interaction has been described to have important roles in infection, autoimmunity, inflammation, and tumor immunity serving to down-regulate T-cell responses.20,21,23-25 In contrast, stimulation of Tim-3–expressing DCs or macrophages results in the secretion of proinflammatory cytokines, thus exposing a dual function for Tim-3 in immunoregulation.15 A recent study examining the role of Tim-3 in NK cells in the context of hepatitis B virus (HBV) infection showed increased cytotoxicity of NK cells isolated from chronic hepatitis B patients against HepG2.215 targets with the blockade of Tim-3, while IFN-γ levels were not affected.36 Our results demonstrate an immunopotentiating role for Tim-3, enhancing IL-12– and IL-18–primed NK-cell IFN-γ production with little effect on NK-cell degranulation in the context of tumor immunity. This raises the question of how triggering one receptor can lead to 2 distinct functional outcomes. One possibility is that Tim-3 function is influenced by the surrounding microenvironment. This concept is supported by findings describing Tim-3 function in CD8+ cytotoxic T cells. In a mouse model of fibrosarcoma, increased antitumor activity, as indicated by enhancement of IFN-γ, perforin, and granzyme B function, was reported in CD8+Tim-3+ T cells in the presence of Gal-9–expressing DCs.37 In contrast, shRNA knockdown of Tim-3 in a mouse model of HBV infection resulted in significant increases in IFN-γ production from hepatic CD8+ T cells.38 All of this supports the notion of a functional threshold that governs the activating or inhibitory potential of Tim-3, causing the receptor to act as rheostat that fine-tunes the cell response.
Although the molecular basis for Tim-3 signaling is still evolving, it is proposed that Tim-3 may facilitate both positive and negative signaling cascades through differential tyrosine phosphorylation of the cytoplasmic tail. The intracellular tail of Tim-3 contains 6 conserved tyrosine residues and a Src homology 2 (SH2) binding motif that, upon Tim-3 cross-linking, displayed differential tyrosine phosphorylation patterns in DCs and T cells.15 These results indicate that Tim-3 is able to initiate distinct signaling events that lead to different functional outcomes in a cell-specific manner. Furthermore, a recent study examining the role of the cytoplasmic tail tyrosine residues of Tim-3 in T cells shows that the dominant activity of the 3 most C-terminal tyrosine residues is inhibitory with 2 of the more N-terminal tyrosine residues displaying an activating function.39 Differential function through a single receptor in the same cell, however, is not without precedent in NK-cell biology. One such receptor is 2B4 (CD244), which has been shown to initiate both positive and negative signaling events that are mediated, in part, by differential tyrosine phosphorylation of the cytoplasmic tail and the availability of 2 distinct adaptor proteins, SAP and EAT-2.40-42 Therefore, it is plausible that differential tyrosine phosphorylation within the cytoplasmic tail of Tim-3 may serve to mediate the recruitment of alternative adaptor proteins that ultimately lead to NK-cell activation or inhibition.
Although Gal-9 is a known ligand of Tim-3 and interacts with the N-linked carbohydrates attached to the IgV region via its 2 carbohydrate recognizing domains (CRDs), Tim-3 has been described to contain another conserved binding domain that has Gal-9 independent binding activities.18,31,32 Our data showing an increase in NK92 Tim-3 IFN-γ production in the presence of Gal-9–negative Raji eGFP control targets suggest there are additional ligands for Tim-3 that have a functional role. Therefore, regulation of positive or negative signaling pathways through Tim-3 may be ligand-dependent and rely on a balance between the interplay of the multiple Tim-3–binding activities. This is the case for the related protein Tim-1, where 2 different Abs with distinct binding affinities for the IgV domain have been shown to induce opposing functions.43 Consequently, the Tim-3–interacting ligand may influence the effect of Tim-3 on cell function, ultimately contributing to the dual roles of this receptor exhibited within the same cell.
Stimulation with both soluble rhGal-9 and membrane-bound Gal-9 demonstrated a preferential effect of the Tim-3/Gal-9 pathway on NK-cell IFN-γ production compared with degranulation when primed with IL-12 and IL-18. Emerging evidence has revealed a clear dichotomy in signals that regulate NK-cell cytokine production versus cytotoxicity on stimulation of a single receptor.30,44,45 These divergent pathways involve the use of different kinases and adaptor proteins to facilitate each function. We found that rhGal-9 was not a strong enough Tim-3 stimulus for resting NK cells to elicit either cytokine production or degranulation. However, when we primed with IL-12 and IL-18 Tim-3 enhanced this stimulus and increased IFN-γ production. Taken together, this suggests that Tim-3 is dependent on the recruitment of key adaptor molecules through prior activation before signaling can occur. Furthermore, the function of Tim-3 as a coreceptor may primarily act to fine-tune a cellular response from cytokine exposure and/or signals delivered from other NK-cell receptors.
As NK cells do not require presensitization for antitumor activity and do not induce GVHD in an allogeneic transplantation setting, they are considered to be an attractive option for immunotherapy.8,26,27,46 To enhance the therapeutic effects of donor NK cells, it is critical to identify functional receptors essential for the interaction of malignant tumor cells and effector NK cells. We have demonstrated that engagement of a novel NK-cell receptor Tim-3 positively regulates IFN-γ–producing NK cells, which have a central role in tumor immunosurvelliance. The antitumor mechanisms of IFN-γ include both direct and indirect targeting, functioning to inhibit cellular proliferation,47 promote apoptosis through the up-regulation of caspases, FAS and TRAIL,48 inhibit angiogenesis,49 and prime the transition of the immune response from innate to adaptive immunity. As a result, enhancement of IFN-γ production by Tim-3 has important implications for NK-cell effector function against cancer and infected cells.
Donor-derived NK cells are recognized to mediate beneficial GVL reactions in HCT and ultimately provide protection against relapse for patients with AML.26,46,50 We have shown that Tim-3+ NK-cell IFN-γ function is enhanced against Gal-9–positive AML primary tumors compared with Gal-9–negative CML primary tumors. These results are consistent with the finding that NK-cell effects are strongest in patients with AML compared with other myeloid leukemias. Our results demonstrating reduced Tim-3 expression in posttransplantation reconstituted NK cells from HCT recipients, which correlated with an impairment to produce IFN-γ, indicate that Tim-3 expression on NK cells is developmentally regulated and further supports our hypothesis that Tim-3 function in NK cells is important in the context of HCT. Enhancing Tim-3 expression early posttransplantation may, therefore, have an essential role in augmenting the NK-cell immune response against residual tumor cells. Collectively, our data show that Gal-9–positive tumors are more susceptible to NK-cell recognition and targeting via interaction with Tim-3. Furthermore, the Tim-3/Gal-9 pathway may have a critical role in clearing residual disease. Understanding this mechanism can lead to strategies to overcome early posttransplantation limitations, providing protection against relapse and infection, and ultimately improving survival outcomes.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge sample procurement and cell-processing services from the Translational Therapy Core supported from the National Cancer Institute (P30-CA77598) and Minnesota Masonic Charities.
This work was supported in part by National Institutes of Health grant P01-CA111412-01 (J.S.M., S.A.C., M.R.V.), R01 HL55417 (J.S.M.) and R01 CA72669 (B.R.B.).
National Institutes of Health
Authorship
Contribution: M.K.G. designed the research plan, performed experiments, analyzed and interpreted data, and wrote the manuscript; T.R.L. assisted with cloning experiments and data analysis; V.M. performed experiments, analyzed data, and contributed to manuscript preparation; M.F., S.A.C., M.R.V., and F.C. performed data analysis and contributed to manuscript preparation; M.S.O. assisted with cloning and cell culture, and performed experiments; C.J.H. performed IHC experiments and contributed to manuscript preparation; A.P.-M. performed chemokine/cytokine assays and contributed to manuscript preparation; T.N. and M.H. provided rhGal-9 reagent and contributed to manuscript preparation; B.R.B. designed the research plan, performed data analysis and interpretation, and contributed to manuscript preparation; and J.S.M. designed the research plan, performed data analysis and interpretation, contributed to manuscript preparation, and was responsible for all aspects of the work.
Conflict-of-interest disclosure: T.N. and M.H. are board members of Gal-Pharma, suppliers of Gal-9. The remaining authors declare no competing financial interests.
Correspondence: Jeffrey S. Miller, MD, Professor of Medicine, University of Minnesota Masonic Cancer Center, MMC 806, Division of Hematology, Oncology, and Transplantation, Harvard St at East River Rd, Minneapolis, MN 55455; e-mail: mille011@umn.edu.

![Figure 2. The Tim-3 ligand Gal-9 increases IFN-γ production in the Tim-3–transduced NK92 cell line. (A) NK92 cell lines were stimulated with rhGal-9 for 4 hours in the presence of a blocking Tim-3 mAb or isotype control Ab and intracellular IFN-γ production was determined by FACS analysis (*P < .01, NK92 Tim-3 vs NK92 native [n = 5] and NK92 Tim-3 vs NK92 Tim-3 blocked [anti–Tim-3 mAb, n = 5]). (B) The full-length human Gal-9 gene was cloned into a MSCV vector and the Raji cell line was transduced as described. Gal-9 protein expression (40 kDa) was confirmed by Western blot analysis. The Jurkat cell line has endogenous Gal-9 expression and was used as the positive control. The murine stromal cell line EL08-1D2 was used as the negative control. (C) NK92 cell lines were incubated in the presence of Raji eGFP and Raji Gal-9 target cell lines for 5 hours and intracellular IFN-γ production was determined via FACS analysis (*P < .001, n = 5; error bars represent SEM).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/119/13/10.1182_blood-2011-06-360321/4/m_zh89991288370002.jpeg?Expires=1765987068&Signature=OFiC851Y-aIiO-19ugOIbvun8fPKwVI-ZqHlmpKILc~18w5ZeJ-G7kE6TZ-oghh7NuFT1P8Ml79iC96y6PO2LuTY-Av4~nnjIRBaegWZZrpko7RKu1VYJ4~lw045ENS9bDpay-7RtJKO4fhMvGLr3a20g23L15CUZ-9GUZho7tmwGkLfKO608wnSPHuUUcrkv2USIuM9H~jo1xj7WwfBRJS6QqbRcuL6thVPMEbA3nfQbvA-zVfA1dBjebiT4QOPlcAYrgeR2RBSe~zuhdRJSr8nwijcc7yw5bgG~ljN2aJnmiir~yfSsnjf-fYRYNI3C4JaZgAvQxh7YlDKIJ28Iw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. Primary Tim-3+ NK cells specifically produce IFN-γ in response to rhGal-9. (A) Purified PB NK cells were incubated overnight in media containing IL-12 (1 ng/mL) and IL-18 (10 ng/mL) and intracellular IFN-γ production was determined by FACS analysis. One representative donor is shown in flow plots A. (B) Resting NK-cell populations CD56Bright, CD56DIM/Tim-3−, and CD56DIM/Tim-3+ were sorted and incubated overnight in media containing IL-12 (1 ng/mL) and IL-18 (10 ng/mL). Each sorted, activated population was exposed to 10nM rhGal-9 with and without β-lactose (30mM) blocking and the percentage of control intracellular IFN-γ production (calculated as [% cells IFN-γ+ at 10nM/% cells IFN-γ+ at 0nM] × 100) was determined within the Tim-3neg and Tim-3hi fractions via FACS analysis (*P < .005, n = 5). (C) IL-12 (1 ng/mL) and IL-18 (10 ng/mL) activated NK cells were sorted into Tim-3+ and Tim-3− cell populations and exposed to rhGal-9 (0nM, 10nM, and 20nM) with and without blocking using anti-Tim-3 mAb. The percentage of control intracellular IFN-γ production (calculated as [% cells IFN-γ+ at 10nM or 20nM/% cells IFN-γ+ at 0nM] × 100) determined via FACS analysis (*P < .05, n = 5; error bars represent SEM).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/119/13/10.1182_blood-2011-06-360321/4/m_zh89991288370003.jpeg?Expires=1765987068&Signature=T0AQ65gXpfhX8umJPNveL1IySlOZSso2ZxK5RdQ~2vrnwXQF~~Ja-tzBZP13kqmfl-Z506Ib1QcS5rWCQ~P6Z1LkAy1GQOcUOWwBD2xdZB~aTCDxmemBLDHELsf9Ub0fhmRcPHamgm7t55Dg0890T3zH22qjlN2ZS4sU-5gfhaJ3oRGZzVJxb6bwIV1aXbpjRCubH1RZyfErKHTH-OHwoZh2YJTTeytWoUDBNKby5MFRDm-qNhfwgMU9dBdjsDpDvK2R7oTKqkggnM1x8lcV7uU8TsmGJV89uRnOm~XqdUsORzozWcBMCu4I7v5MrsPp8gfdcd2U9g4HecZVqeCtHg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 7. Tim-3 blockade decreases IFN-γ production of activated human NK cells against primary AML tumor cells, but has no effect on degranulation. (A) Western blots were performed on cell lysates of AML (n = 2) and CML (n = 2) tumor cells to evaluate Gal-9 (40 kDa) expression. The Jurkat cell line has endogenous Gal-9 expression and was used as the positive control. The murine stromal cell line EL08-1D2 was used as the negative control. (B-C) PBMCs were incubated overnight in media containing IL-12 (1 ng/mL) and IL-18 (10 ng/mL) and a 5-hour CD107a/IFN-γ assay was performed as described using primary human AML and CML tumor cell targets. The percentage of control intracellular IFN-γ (B) or CD107a (C) production of NK cells when exposed to targets with and without Tim-3 and HLA blocking alone and in combination (calculated for each blocking condition as [% cells IFN-γ+ or CD107a+ in the presence of target/% cells IFN-γ+ or CD107a+ without targets] × 100) was determined by FACS analysis (*P < .005, n = 6; error bars represent the SEM).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/119/13/10.1182_blood-2011-06-360321/4/m_zh89991288370007.jpeg?Expires=1765987068&Signature=HsyUUO4s-0JZUSk~idGf2VjCxAUXlmHEGt6KGYfXlq~ZmSpYZ0drhj1wo-yPVQZgnsqMuMx9lKdcCuzDvbPsZ4QSSbG7JUrXnbqhAt8gXPFrXM7lrZG~pND3BGdDDobhOhQLZ9amj0fEn5q~mlZxIAT~riW~b3qccyBgcZEfmaqykn29EPTYEO1tdqROef585AOW2CAIlG-0-mKNZeZF5DxXDkxoWVg605GQxv6MtImE54UHLzM1s9h9L7NEN2YbEDSNS~xUghtSj7w-0Efiy2aSNBr6BVcetHSaavPgLOFonAVvMNCasl3~UxHYeV0TiAvtQVh6s-kq-p5i8zXLYg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 2. The Tim-3 ligand Gal-9 increases IFN-γ production in the Tim-3–transduced NK92 cell line. (A) NK92 cell lines were stimulated with rhGal-9 for 4 hours in the presence of a blocking Tim-3 mAb or isotype control Ab and intracellular IFN-γ production was determined by FACS analysis (*P < .01, NK92 Tim-3 vs NK92 native [n = 5] and NK92 Tim-3 vs NK92 Tim-3 blocked [anti–Tim-3 mAb, n = 5]). (B) The full-length human Gal-9 gene was cloned into a MSCV vector and the Raji cell line was transduced as described. Gal-9 protein expression (40 kDa) was confirmed by Western blot analysis. The Jurkat cell line has endogenous Gal-9 expression and was used as the positive control. The murine stromal cell line EL08-1D2 was used as the negative control. (C) NK92 cell lines were incubated in the presence of Raji eGFP and Raji Gal-9 target cell lines for 5 hours and intracellular IFN-γ production was determined via FACS analysis (*P < .001, n = 5; error bars represent SEM).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/119/13/10.1182_blood-2011-06-360321/4/m_zh89991288370002.jpeg?Expires=1766078656&Signature=axwwlTN52cp4ZcpUgccDdmeFwVX2m8OH3ArgkoDiEfZ-8itI~GiYlfC~UXkavEIzFYWeadhuth2D0vd~svzdB-v7-q9X4735DvqLKmOVCWOLj9BkYseV5MV3QwqHT5JR98cWglSZ8hIuhh9Vxm~Oh1dbY9Ob-xX-pJVA3hwR3spNTJkZQkBCJ6A0I0GyV853PdCGZOlP73rhLXXteVdlDGlfb7-yiIg~Zg-HMrR0KkJeraIWc7ft0BmK91Kf~1m8qbI7swJ6-DwdscXs00OoWzPtMdFxK4a53HOIBv6Ove~fqa6ETNxMk4~KTbNufcgSM-fUzmtQmDM9g02wb84j9g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. Primary Tim-3+ NK cells specifically produce IFN-γ in response to rhGal-9. (A) Purified PB NK cells were incubated overnight in media containing IL-12 (1 ng/mL) and IL-18 (10 ng/mL) and intracellular IFN-γ production was determined by FACS analysis. One representative donor is shown in flow plots A. (B) Resting NK-cell populations CD56Bright, CD56DIM/Tim-3−, and CD56DIM/Tim-3+ were sorted and incubated overnight in media containing IL-12 (1 ng/mL) and IL-18 (10 ng/mL). Each sorted, activated population was exposed to 10nM rhGal-9 with and without β-lactose (30mM) blocking and the percentage of control intracellular IFN-γ production (calculated as [% cells IFN-γ+ at 10nM/% cells IFN-γ+ at 0nM] × 100) was determined within the Tim-3neg and Tim-3hi fractions via FACS analysis (*P < .005, n = 5). (C) IL-12 (1 ng/mL) and IL-18 (10 ng/mL) activated NK cells were sorted into Tim-3+ and Tim-3− cell populations and exposed to rhGal-9 (0nM, 10nM, and 20nM) with and without blocking using anti-Tim-3 mAb. The percentage of control intracellular IFN-γ production (calculated as [% cells IFN-γ+ at 10nM or 20nM/% cells IFN-γ+ at 0nM] × 100) determined via FACS analysis (*P < .05, n = 5; error bars represent SEM).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/119/13/10.1182_blood-2011-06-360321/4/m_zh89991288370003.jpeg?Expires=1766078656&Signature=LwT0IWHGT3RNbrISbfzMmgRmmYaXkyBc6Gkv3pqWltlscZi3DjZccP9KpYX5~GbUqRAJQ0TShB5WjZZL6OblrjFpvs-M9abuNwfKh8oTZTHfvdQZknK8WAdUw1jKCCQigEcVnjqJst9eE5zCTkfYHQ8KQPJRCPMLzV-GtMc3-yrTecToK4-tbgXrPgjeZxCE4A6oELnJOBAnm37lurXlWEAsIhKfRKn7ZpHhs2iRu0Wla~UHeo6svzwg5vkIQIwACGcGtMe2yY4NyyzznOzH23iqjdKKT1WuPpTP034~9oJ-gxU1G7aq20SJ3mozxwq7OZJ3hzs66-2CkTlV1EVqOQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 7. Tim-3 blockade decreases IFN-γ production of activated human NK cells against primary AML tumor cells, but has no effect on degranulation. (A) Western blots were performed on cell lysates of AML (n = 2) and CML (n = 2) tumor cells to evaluate Gal-9 (40 kDa) expression. The Jurkat cell line has endogenous Gal-9 expression and was used as the positive control. The murine stromal cell line EL08-1D2 was used as the negative control. (B-C) PBMCs were incubated overnight in media containing IL-12 (1 ng/mL) and IL-18 (10 ng/mL) and a 5-hour CD107a/IFN-γ assay was performed as described using primary human AML and CML tumor cell targets. The percentage of control intracellular IFN-γ (B) or CD107a (C) production of NK cells when exposed to targets with and without Tim-3 and HLA blocking alone and in combination (calculated for each blocking condition as [% cells IFN-γ+ or CD107a+ in the presence of target/% cells IFN-γ+ or CD107a+ without targets] × 100) was determined by FACS analysis (*P < .005, n = 6; error bars represent the SEM).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/119/13/10.1182_blood-2011-06-360321/4/m_zh89991288370007.jpeg?Expires=1766078656&Signature=E5RdIVO0eQyFp4JNRdpkjBhYRdACRgVoSsCimBqHp4RhWbo2QiesN71~pwNGHzjPUNvTyQcZLMjWOeiR2fMZyYZJOGF7TwsH2MEWNVMuuOCLSao1-hpdK2R5~BsnPhLGe5FBf3R0JXTiU9C4U1qH-vffmCyJOlne5on8RkEZPxng4pWnnk4yDPmlkjG6fRjgcXxVmpv7lplhFQ5z7gbL6TrVgirkzrEW-eCOXiT-jeqc1NEo5PHGjaJeJ87m~9TZxSnLrNTltQkED8zfAFAHuX84fpd0HwmPCsDpCbEDBPGhH8CWbzvMt4GTST8I~vBYldPTDUWgo5ZlzOXUD~YXgw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)