To the editor:
β-thalassemia is a genetic disorder of hemoglobin production characterized by ineffective erythropoiesis and anemia.1 Iron overload, a major source of morbidity, results from inappropriately low expression of the gene encoding hepcidin (Hamp1).1 Hamp1 controls plasma iron concentration by facilitating the degradation of the iron efflux protein ferroportin.2 In healthy individuals, hepcidin istranscriptionally responsive to iron via bone morphogenetic protein (Bmp)/Smad pathway-mediated phosphorylation of Smad 1,5,8.3 Phosphorylated Smad 1,5,8 is an essential component of the transcriptional complex that induces Hamp1 expression in response to iron.4 We investigated therelationship between iron status, Bmp/Smad signaling, and Hamp1 expression in mouse models of β-thalassemia intermedia (th3/+) and major (th3/th3).5
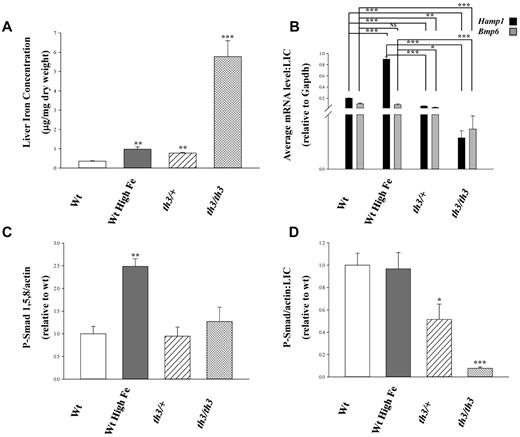
Wild-type (wt) C57BL/6 mice were placed on a high iron diet to match the elevated liver iron concentrations (LICs) observed in th3/+ mice (Figure 1A). The severity of iron overload in th3/th3 was even higher, by 5-fold, relative to iron-loaded wt mice (Figure 1A). Dietary iron loading of wt mice resulted in the expected increase in Hamp1 and Bmp6 expression (measured by quantitative RT-PCR). However, despite the similarly elevated LICs in th3/+ mice, neither Bmp6 nor Hamp1 expression was significantly and proportionally increased. Moreover, the highly iron loaded th3/th3 mice demonstrated a decrease in expression of both Bmp6 and Hamp1. Thus, relative to hepatic iron load, expression of these genes is significantly decreased in both β-thalassemia models (Figure 1B). Furthermore, hepatic phosphorylated Smad 1,5,8 protein concentration is significantly increased only in liver samples from wt mice fed a high-iron diet relative to controls (Figure 1C). When normalized to LIC, our data reveal significantly decreased phosphorylated Smad 1,5,8 in th3/+ and th3/th3 samples compared with wt and wt fed a high-iron diet (Figure 1D). These results present strong evidence of a blunted relationship between LIC and Bmp6 mRNA expression in β-thalassemic mice.
Bmp/Smad signaling is hyporesponsive to iron in β-thalassemic mice. (A) Liver iron concentrations (μg/mg dry weight) from wt mice fed a standard diet (200 ppm iron), wt mice fed a high iron diet (standard chow supplemented with 2.5% carbonyl iron), th3/+, and th3/th3 mice were determined by atomic absorption and are expressed as mean ± SEM. (B) Average fold difference in the transcription levels of the Bmp/Smad pathway member Bmp6 and target gene Hamp1 were normalized to LIC. Data were normalized to mouse Gapdh and are presented as the mean ± SEM (n ≥ 3). All averaged values are the product of duplicate determinations. (C) Chemiluminesence signals from Western blots of liver lysates reacted with antibodies to phosphorylated Smad 1,5,8 and to β-actin were quantified and data expressed as the mean ± SEM of their ratios. (D) Phosphorylated Smad 1,5,8 levels normalized to β-actin are expressed relative to LIC. Data are presented as mean ± SEM. Statistical significance was determined using the Student t test. *P ≤ .05, **P ≤ .01, ***P ≤ .001. Significance with respect to wt or iron-loaded wt values are indicated by the brackets.
Bmp/Smad signaling is hyporesponsive to iron in β-thalassemic mice. (A) Liver iron concentrations (μg/mg dry weight) from wt mice fed a standard diet (200 ppm iron), wt mice fed a high iron diet (standard chow supplemented with 2.5% carbonyl iron), th3/+, and th3/th3 mice were determined by atomic absorption and are expressed as mean ± SEM. (B) Average fold difference in the transcription levels of the Bmp/Smad pathway member Bmp6 and target gene Hamp1 were normalized to LIC. Data were normalized to mouse Gapdh and are presented as the mean ± SEM (n ≥ 3). All averaged values are the product of duplicate determinations. (C) Chemiluminesence signals from Western blots of liver lysates reacted with antibodies to phosphorylated Smad 1,5,8 and to β-actin were quantified and data expressed as the mean ± SEM of their ratios. (D) Phosphorylated Smad 1,5,8 levels normalized to β-actin are expressed relative to LIC. Data are presented as mean ± SEM. Statistical significance was determined using the Student t test. *P ≤ .05, **P ≤ .01, ***P ≤ .001. Significance with respect to wt or iron-loaded wt values are indicated by the brackets.
No prior studies have examined Bmp6 expression or Smad signaling in models of β-thalassemia. Suppressed Hamp1 transcription relative to the degree of iron overload in β-thalassemic mice has been postulated to occur as a consequence of expanded or ineffective erythropoiesis and effects of certain erythroid factors (eg, GDF15 or TWSG1) on hepatocellular hepcidin expression.6-8 Our results suggest that the effects of ineffective erythropoiesis on Hamp1 expression include a suppression of Bmp6 mRNA expression relative to LIC, with consequent decreased Smad signaling. In contrast, studies in mice examining the acute effects of erythropoietin administration on Bmp6 and Hamp1 demonstrated decreased Hamp1 expression but no effect on Bmp6 expression.9 This observation supports the proposal that different erythroid signals regulate hepcidin in normal and ineffective erythropoiesis.8 Whether or not as a consequence of an erythroid factor, our findings demonstrate that in β-thalassemic mice the normal relationship between iron status and liver Bmp6 mRNA expression is blunted. This dysregulation results in low Hamp1 levels relative to iron stores and excess iron absorption relative to tissue demands. Continued exploration of the mechanisms underlying blunted Bmp/Smad signaling in β-thalassemia will enhance understanding of the iron overload that is central to the pathophysiology of this disease.
Authorship
Acknowledgments: The authors thank the members of the Pasta and Red Cells Society of New York for technical support and helpful discussions. This work is supported by the Children's Cancer and Blood Foundation, Cooley's Anemia Foundation (CAF) and the Associazione Veneta Lotta alla Talassemia (AVLT; Veneta Association for the Fight Against Thalassemia, Italy).
This work is supported by grants from the National Institutes of Health (NIDDK-1R01DK090554-01 to S.R.) and a grant from the American Portuguese Biomedical Fund (APBRF, USA)/Inova (to P.R.). S.G. is a fellow of the Cooley's Anemia Foundation. P.R. is a fellow of the Fundação para a Ciência e Tecnologia, Portugal (SFRH/BD/24 813/2005). Y.M.S. was supported by National Institutes of Health Grant CA148828 and by a grant from the University of Michigan Gastrointestinal Peptide Center.
Contribution: N.L.P. performed research, analyzed results and wrote the paper; S.G., P.R., R.W.G, H.L., E.R.A., Y.M.S., R.E.F. and C.C. performed research, and analyzed results; and R.E.F., Y.Z.G. and S.R. designed research, analyzed results and wrote the paper.
Conflict-of-interest disclosure: S.R. is a consultant for Novartis. In addition, he is a co-inventor for the patents U58058061 B2 C12N29111115 and U57541179 B2 C12N 20090602. The consulting work and intellectual property of S.R. did not affect in any way the design, conduct, or reporting of this research. The remaining authors declare no competing financial interests.
Correspondence: Stefano Rivella, PhD, Associate Professor of Genetic Medicine, Weill Cornell Medical College, 515 E 71st St, S702, Box 284, New York, NY 10021; e-mail: str2010@med.cornell.edu; and Robert E. Fleming, MD, Professor of Pediatrics and the Edward A. Doisy Department of Biochemistry and Molecular Biology, Saint Louis University School of Medicine, 1465 South Grand Ave, St Louis, MO 63104; e-mail: flemingr@slu.edu.
References
National Institutes of Health