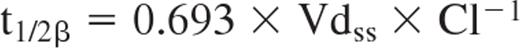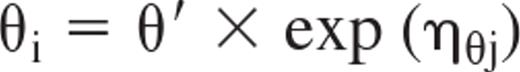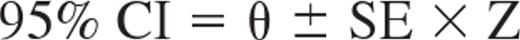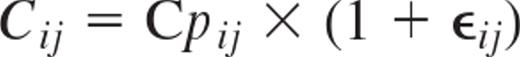Abstract
Pharmacokinetics of 8 doses of rituximab (375 mg/m2) given in combination with 2-week cyclophosphamide, hydroxydaunorubicin, vincristine, and prednisone/prednisolone (CHOP-14) was determined by ELISA in 20 elderly patients with diffuse large B-cell lymphoma (DLBCL) 10 minutes before and after each infusion and 1 week and 1, 2, 3, 6, and 9 months after the last infusion. Population pharmacokinetic modeling was performed with nonlinear mixed-effect modeling software (NONMEM VI). Concentration-time data were fitted into an open 2-compartment model and total clearance, central compartment volume, intercompartment clearance, and volume of distribution at steady-state (Vdss) were investigated. Total clearance was 9.43 mL/h and Vdss was 9.61 l. Rituximab clearance was reduced (8.21 mL/h vs 12.68 mL/h; P = .003) and elimination half-life was prolonged in women compared with men (t1/2β = 30.7 vs 24.7 days; P = .003). Body weight also affected Vdss (0.1 l increase of Vdss per kilogram above median of 75 kg). A sex-dependent effect and the higher weight of males contribute to their faster rituximab clearance, which might explain why elderly males benefit less from the addition of rituximab to CHOP than females. This trial was registered on www.clinicaltrials.gov as numbers NCT00052936, EU-20243 (RICOVER-60 Trial), EU-20534, and NCT00726700 (Pegfilgrastim Trial).
Introduction
Overall survival of patients with diffuse large B-cell lymphoma (DLBCL) has been shown to significantly improve when standard 3-week cyclophosphamide, hydroxydaunorubicin, vincristine, and prednisone/prednisolone (CHOP-21)1-3 or dose-dense 2-week CHOP (CHOP-14)4 are combined with the anti-CD20 mAb rituximab, and the combination of chemotherapy and rituximab is considered the standard treatment for DLBCL. However, despite its widespread use in DLBCL, there are only scarce data on serum levels and pharmacokinetic patterns of rituximab in aggressive B-cell lymphoma. Therefore, the practice of combining rituximab with CHOP (R-CHOP) in 2- or 3-week schedules is based on practical and/or historical reasons and not on scientific data. This practice carries the risk of suboptimal dosing and therefore may not exploit the whole therapeutic potential of rituximab. In all studies of combined chemoimmunotherapy in DLBCL, the 375 mg/m2 standard dose was used and the pharmacokinetic impact of modifying intervals between rituximab administrations was never analyzed. However, rituximab has a complex pharmacology that is thus far only partially understood.5 To better understand the pharmacology of rituximab in aggressive B-cell lymphomas, we investigated serum levels and pharmacokinetic parameters of rituximab when combined with 2-week “dose-dense” CHOP-14 in elderly patients treated in the RICOVER-604 and the Pegfilgrastim6 trials of the German High-Grade Non-Hodgkin Lymphoma Study Group (DSHNHL). Based on the pharmacokinetic data obtained from this study, we performed simulations with various dosing intervals and dose-escalation protocols.
Methods
Study population, patient selection, and study design
Serum rituximab levels were investigated in 20 patients who were treated at 6 different centers participating in the prospective multicenter RICOVER-604 (8 patients) and the R-CHOP-14 Pegfilgrastim6 (12 patients) trials of the DSHNHL. In brief, patients 61-80 years of age with previously untreated and biopsy-confirmed DLBCL were eligible for the study. Patients with a diagnosis or history of indolent lymphoma or other malignancies, HIV infection, or marked impairment of organ or BM function were excluded. The randomized RICOVER-60 trial addressed the question whether 6 or 8 cycles of R-CHOP-14 can improve outcome of these patients compared with 6 or 8 cycles of CHOP-14. In the R-CHOP-14 arms, patients received a total of 8 doses of 375 mg/m2 rituximab, each dose administered 2-24 hours before chemotherapy. The therapy was repeated every 2 weeks with G-CSF support (filgrastim or lenograstim from days 4-13 in the RICOVER-60 trial). In the Pegfilgrastim trial, patients received a second randomization into pegfilgrastim on day 2 versus day 4. Patients who were randomized to receive rituximab were asked to participate in the pharmacokinetic study. The study was conducted in accordance with the Declaration of Helsinki and the study protocol was approved by the ethical review committee of each participating center. All participating patients signed an additional informed consent specific for the pharmacokinetic protocol.
Blood sampling
Ten minutes before and 10 minutes after each rituximab infusion, 10 mL of blood was drawn from each subject to obtain rituximab trough and peak serum levels. Additional samples were taken 1 week and 1, 2, 3, 6, and 9 months after the last rituximab infusion, respectively. Whereas pre-dose samples were drawn from the indwelling venous catheter, postdose samples were drawn from a different catheter or, ideally, from a venous puncture at the contralateral side. Samples were centrifuged at 1000g for 10 minutes at room temperature and stored at −20°C until shipping on dry ice for analysis.
Determination of rituximab concentrations
Rituximab serum levels were determined by Xendo Laboratories. Briefly, rituximab serum levels were measured by ELISA using microtiter high protein-affinity 96-well plates coated with a polyclonal goat anti-rituximab Ab as described previously.7-9 Bound rituximab was detected by goat anti–mouse IgG F(ab′)2 fragments conjugated to peroxidase. Bound peroxidase was then visualized by a chromogenic reaction and rituximab was quantitated using absorbance spectrophotometry with a known standard curve of rituximab. All study samples were analyzed in duplicate at a minimal dilution of 100-fold. The mean value was then used for pharmacokinetic analysis. The validated upper and lower limits of quantitation for 100-fold–diluted samples were 500 and 15.000 ng/mL, respectively. For study samples, the accepted coefficients of variation (relative SD) for the duplicate responses were ≤ 20%. If study samples did not meet this criterion, then they were considered as outliers and were excluded from the analysis.
Pharmacokinetic analysis
Measured data were imported into a standard calculation program (Microsoft Excel 2007; SP2 MSO) and in a first step of analysis, corresponding datasets were processed by the Mw/Pharm Version 3.50 pharmacokinetic program.10 The individual rituximab serum concentrations and pharmacokinetic parameters, respectively, were fitted based on an open 2-compartment model and a Bayesian fitting procedure to estimate pharmacokinetic parameters and typical values as an integral part of the following NONMEM analysis. The following pharmacokinetic parameters were investigated: maximum concentration (Cmax), trough level concentrations (Cmin), elimination half-life (t1/2β), volume of distribution at steady-state (Vdss), and total clearance (Cl).
Population pharmacokinetic analysis and model building
In a second step, the nonlinear mixed-effect software NONMEM VI Version 2.0 (Globomax) was used for compartmental population pharmacokinetic analysis. Compared with a population pharmacokinetic analysis described previously,11 our investigation yielded a structural 2-compartment model as the best-fitting model based on the first-order conditional expectation and the NONMEM subroutines ADVAN3 TRANS3. Based on this subroutine, the corresponding pharmacokinetic parameters Cl, central volume (V1), intercompartmental clearance (Q), and Vdss were calculated. Half-lives corresponding to the exponents for the constants of disposition were calculated as follows:
Model building
A “standard 2-stage” modeling procedure for the evaluation of pharmacokinetics of rituximab was not considered to appropriately estimate pharmacokinetic parameters. For prediction of plasma rituximab concentrations and pharmacokinetic parameters, a population approach was used. In the first step of model building, a 2-compartmental model was developed as a basic structural model (without covariates, but including an interindividual variability on pharmacokinetic parameters). An explorative pharmacokinetic analysis with APO/Mediware was used to get an idea of the “typical values” for the parameter estimates (eg, clearance and distribution volumes).
An exponential error model was chosen for the interindividual variability of the kinetic parameters according to the following equation:
where θi (THETA) represents the estimate for a pharmacokinetic parameter in the ith individual, θ′ is the population mean value of the pharmacokinetic parameter, ηθj (ETA) is the random variable with zero mean, and a variance ω2 (OMEGA, interindividual variability of pharmacokinetic parameters) distinguishes the ith individual pharmacokinetic parameter from the population mean of the basic structural model.
95% confidence intervals (95% CIs) were estimated for the different population model parameters as follows:
where θ is the population parameter estimate and Z = 1.96 is the interval coefficient for a standard normal distribution for 95% CI. A proportional error model was used for the residual variability to describe the deviations between the model predictions and the plasma rituximab concentrations:
where Cij complies with the observed concentration of the ith individual at time j, Cpij represents the model individual predicted concentration in individual i at time j, and ϵij (EPSILON) describes the independent, identically distributed statistical errors with mean zero and variance σ2 (SIGMA).
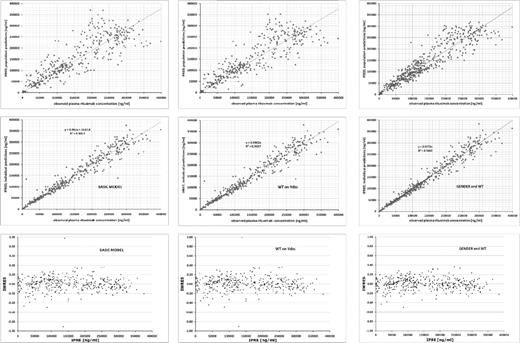
Fitting was performed with the first-order conditional estimates algorithm, not taking interactions between the parameters into account (first-order conditional expectation). A decrease in the objective function of more than 3.84 was considered a significant improvement and a significance level of P < .05 of the structural and covariate model (Figure 1).
Goodness-of-fit plots and improvement within the basic model. Shown is an overview of the goodness-of-fit plots and the improvement of the basic model for rituximab after implementation of covariates (WT and sex). The solid line indicates the line of identity. Top graph shows the predicted (Pred) versus the observed concentrations (DV). The middle graph shows individual predictions (IPRED) versus observed concentrations (DV). The bottom graph shows population model predictions versus weighted residuals of the population model.
Goodness-of-fit plots and improvement within the basic model. Shown is an overview of the goodness-of-fit plots and the improvement of the basic model for rituximab after implementation of covariates (WT and sex). The solid line indicates the line of identity. Top graph shows the predicted (Pred) versus the observed concentrations (DV). The middle graph shows individual predictions (IPRED) versus observed concentrations (DV). The bottom graph shows population model predictions versus weighted residuals of the population model.
Evaluation of the model
Stability of the final pharmacokinetic model parameters was evaluated particularly with regard to a bootstrap analysis generating a total of 500 successfully replicated dataset runs (using the PLTTools software package, a graphical interface for the NONMEM system).
Comparison of the alternative structural model and implementation of covariates to the final model were based on goodness-of-fit plots. A visual predictive check served as an additional instrument for the evaluation of observed and predicted data. Time and observed rituximab concentration profiles were plotted and overlaid with the 95% prediction intervals generated using the structural model.
Simulations
Simulation of the time-concentration profile of rituximab was performed with APO/Mediware to explore the effects of a dose-dense R-CHOP with a 14-day interval of rituximab application on serum levels based on a 2-compartment model that allowed an adequate characterization of rituximab disposition in all patients. The following rituximab parameters were taken into account, corresponding to pharmacokinetic data of NONMEM-analysis: Cl, volume of the central compartment (Vc), Vdss, and elimination half-life (t1/2α and t1/2β).
Statistical analysis
IBM SPSS Version 19.0.0 (2010) software was used for statistical analysis of demographic data.
Results
Pharmacokinetic data were obtained from 20 (11 female and 9 male) patients. There was no correlation between renal and hepatic function and rituximab serum levels within sexes and no differences in renal and hepatic function between the male and female patients included in this pharmacokinetic study (Table 1). None of these patients had a concomitant medication compromising hepatic or renal function.
As shown in Table 1, patients participating in the pharmacokinetic study had less stage III and more stage IV disease and belonged less frequently to the high-intermediate, but more frequently to the high-risk group according to the International Prognostic Index (IPI), but otherwise were representative of the entire RICOVER-60 and R-CHOP-14 Pegfilgrastim study populations, respectively. Treatment results in patients participating in the pharmacokinetics study were similar to the results obtained in the entire RICOVER-60 population: 18 patients achieved a complete remission/complete remission unconfirmed, one a partial remission, and one had progressive disease.
Rituximab serum levels
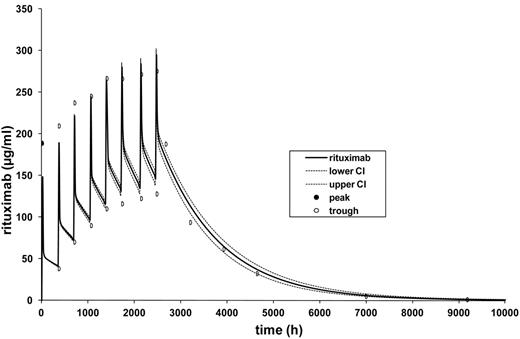
Blood samples were collected between January 2005 and June 2006. During the rituximab treatment period, the complete set of all planned samples was obtained in > 90% of the patients, and after the last infusion of rituximab, pharmacokinetic sampling was obtained in at least 50% of patients at each planned time point. Patients received an absolute median dose of 671 mg (range, 593-881) of rituximab per cycle. Trough rituximab serum levels increased over the entire 8 R-CHOP cycles, indicating a rituximab serum accumulation, and a plateau was not reached until the eighth and last application (Figure 2 and Table 2). After completion of therapy, serum levels decreased continuously, and 9 months after the last cycle, rituximab levels approximated baseline values but remained detectable in 9 of 10 evaluable patients.
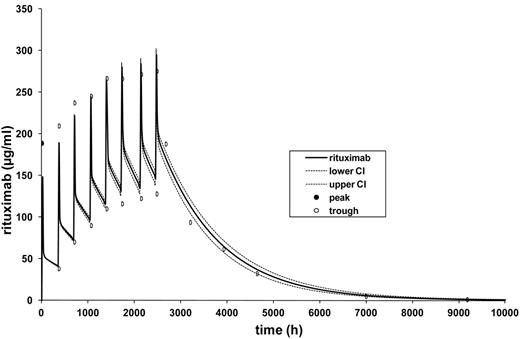
Simulation of rituximab serum levels with APO/Mediware Version 3.50. Depicted are the simulated median concentration profile of rituximab (black line) and the 90% CI (upper and lower 5th percentile over 100; dotted lines). The pharmacokinetics model is based on mean values of pharmacokinetics parameters as follows: Cl = 9.9 mL/h, V1 (l/kgLBMc) = 4.39 l, Vdss = 12.8 l, t1/2α = 8.47h, and t1/2β = 896 hours (37 days) of 20 patients treated with R-CHOP-14 according to a 2-compartment model. LBMc indicates lean body mass corrected.
Simulation of rituximab serum levels with APO/Mediware Version 3.50. Depicted are the simulated median concentration profile of rituximab (black line) and the 90% CI (upper and lower 5th percentile over 100; dotted lines). The pharmacokinetics model is based on mean values of pharmacokinetics parameters as follows: Cl = 9.9 mL/h, V1 (l/kgLBMc) = 4.39 l, Vdss = 12.8 l, t1/2α = 8.47h, and t1/2β = 896 hours (37 days) of 20 patients treated with R-CHOP-14 according to a 2-compartment model. LBMc indicates lean body mass corrected.
Simulation of pharmacokinetic parameters
To explore the effects of a dose-dense R-CHOP with a 14-day interval of rituximab application on serum levels, a rituximab population-based pharmacokinetic simulation was performed using a 2-compartment model that allowed an adequate characterization of rituximab disposition in all patients.
Based on the rituximab trough and peak serum levels of the 8 treatment cycles and on the rituximab serum levels measured at 6 time points after the last infusion, an individual fitting calculation and estimation of the following pharmacokinetic parameters was calculated for each cycle and patient who completed the trial: Cl, t1/2α, t1/2β, Vc, and Vdss (APO/Mediware 3.50). Subsequently, the individual models were combined and a model for the mean pharmacokinetic parameters was adapted (Table 3). In this model, the median t1/2β was 37 days, and the trough levels increased from 38.9 μg/mL after the first administration to 140 μg/mL after the eighth cycle. Steady-state concentrations were not reached because of this relatively long elimination half-life (Figure 2).
NONMEM analysis
The individual serum concentration-time data were fitted to an open 2-compartment model and the following population pharmacokinetic parameters were investigated based on subroutine ADVAN3 TRANS3 as the best fitting: Cl, Vc, Q, and Vdss. The final parameter estimates calculated based on the structural model were: Cl = 9.43 mL/h (95% CI, 8.05-10.81 mL/h) and Vdss = 9.61 l (95% CI, 8.44-10.78 l), with a remaining interindividual variability in Cl not explained by the covariates and characterized by a coefficient of variation of 33.17%. The coefficient of variation for residual variability was 13.49%. For the whole study population, a t1/2β of 27.1 days was calculated.
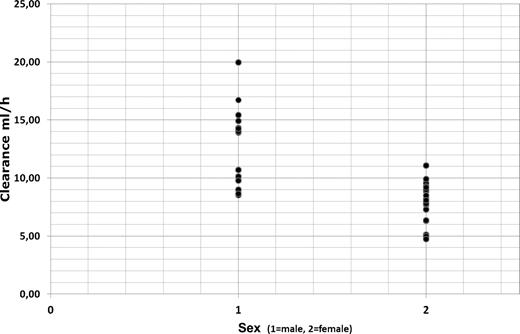
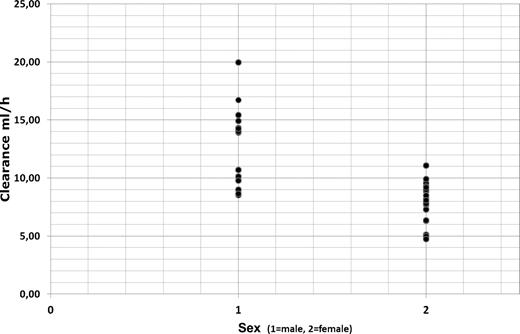
Clearance was markedly influenced by patient sex and weight (Figure 3) and was significantly reduced in female patients (Cl = 8.21 mL/h; 95% CI, 7.16-9.26 mL/h) compared with male patients (Cl = 12.68 mL/h; 95% C, 10.07-15.30 mL/h; P = .003 by t test and P = .003 by Mann-Whitney U test). Calculation of total clearance for females and males was based on the final covariate model: Cl = (θTVCl + θWT × (WT − 75.2)) × exp(ηTVCl).
Rituximab clearance in elderly male (1) and female (2) patients with DLBCL.
Clearance was inversely correlated with elimination half-life of rituximab with a significant shorter t1/2β of 24.7 days in men compared with 30.7 days in women (P = .003 by t test and P = .003 by Mann-Whitney U test). Body weight showed also an impact on volume of distribution (0.1 l increase of Vdss per kilogram of body weight above a median body weight of 75 kg).
Sex and weight independently affected rituximab clearance and serum elimination half-life, respectively. In the example shown in Table 4, the rituximab clearance of a male patient with a body weight 25% above the median body weight of male patients in our study (111.0 kg), is nearly 1.4 fold faster than the rituximab clearance of a patient with a body weight 25% below the median weight (66.6 kg). Conversely, the serum elimination half-life is 18 days and 30 days, respectively, in these 2 sample patients (Table 4).
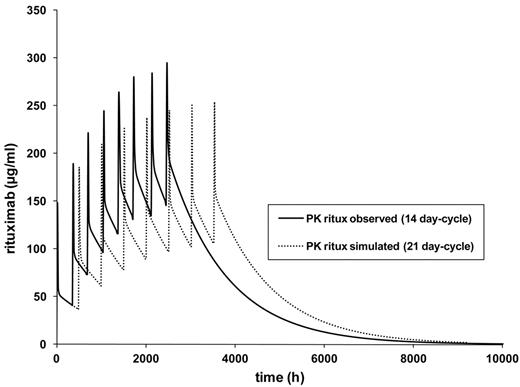
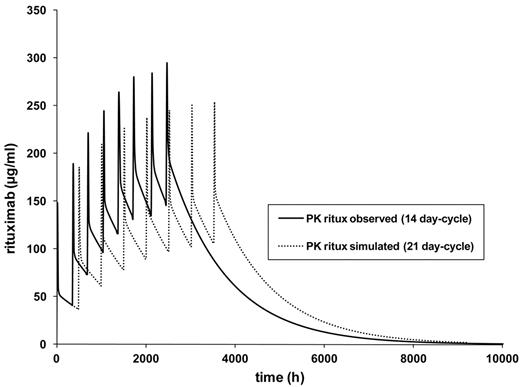
Finally, the model for the standard-dose rituximab dose-dense 14-day cycle was then varied by changing the cycle duration to 21 days (R-CHOP-21). The model showed a markedly slower increase of rituximab trough levels (Figure 4). The highest trough serum levels in this simulation were approximately 105 μg/mL of rituximab for the 3-week schedule compared with 140 μg/mL for the 2-week schedule.
PK simulation R-CHOP-14 versus R-CHOP-21. The pharmacokinetic model is based on median values of pharmacokinetic parameters as follows: Kelm, V1 (l/kgLBMc), k12, and k21 of 20 patients treated with R-CHOP-14 according to a 2-compartment model. The model was then calculated for 21-day intervals. Kelm indicates elimination constant; V1 (I/kgLBMc), central distribution volume; k12, first order rate (constant that describes mean transfer from compartment 1 to compartment 2); and k21, first order rate (mean transfer from compartment 2 to compartment 1)
PK simulation R-CHOP-14 versus R-CHOP-21. The pharmacokinetic model is based on median values of pharmacokinetic parameters as follows: Kelm, V1 (l/kgLBMc), k12, and k21 of 20 patients treated with R-CHOP-14 according to a 2-compartment model. The model was then calculated for 21-day intervals. Kelm indicates elimination constant; V1 (I/kgLBMc), central distribution volume; k12, first order rate (constant that describes mean transfer from compartment 1 to compartment 2); and k21, first order rate (mean transfer from compartment 2 to compartment 1)
Correlation with clinical outcome
The clinical impact of sex of the patients is shown in Table 5. In the RICOVER-60 trial,4 the differences between males and females with respect to progression-free survival (PFS) were considerably bigger after R-CHOP compared with CHOP alone: the rates of PFS of females were 1.0%, 5.1%, and 0.9% better after 2, 3, and 4 years, respectively, than the respective figures in male patients after CHOP-14 only; however, the respective differences in the rates of PFS increased to 5.7%, 7.7%, and 7.6% after 2, 3, and 4 years, respectively, when patients were treated with the same chemotherapy plus rituximab. This demonstrates that female patients, with their slower clearance and longer serum elimination half-life of rituximab, benefit considerably more from the addition of the CD20 Ab.
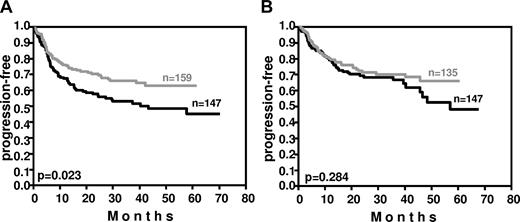
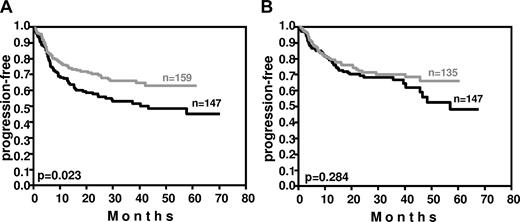
Weight also affected outcome (Figure 5). In the RICOVER-60 trial, patients having a body weight within the lower quartile in the respective sex (≤ 60 kg and ≤ 73 kg for female and male patients, respectively) had a significant improvement in PFS (P = .023) after the addition of rituximab, whereas rituximab improved the PFS of patients with a body weight within the upper quartile of the respective sex (> 77 kg and > 89 kg for female and male patients, respectively) only by a slight margin (P = .284).
Impact of weight on PFS in the RICOVER-60 trial. In patients with a body weight within the lower quartile of the respective sex (≤ 60 kg and ≤ 73 kg for females and males, respectively), the addition of 8 cycles of rituximab to CHOP-14 resulted in a significant (P = .023) improvement of PFS (A). In contrast, in patients with a body weight within the upper quartile of the respective sex (> 77 kg and > 89 kg in females and males, respectively), who have a faster rituximab clearance, this improvement was only minor (P = .284; B). Black curves indicate CHOP-14 without rituximab; gray curves, CHOP-14 with 8 applications of rituximab every 2 weeks.
Impact of weight on PFS in the RICOVER-60 trial. In patients with a body weight within the lower quartile of the respective sex (≤ 60 kg and ≤ 73 kg for females and males, respectively), the addition of 8 cycles of rituximab to CHOP-14 resulted in a significant (P = .023) improvement of PFS (A). In contrast, in patients with a body weight within the upper quartile of the respective sex (> 77 kg and > 89 kg in females and males, respectively), who have a faster rituximab clearance, this improvement was only minor (P = .284; B). Black curves indicate CHOP-14 without rituximab; gray curves, CHOP-14 with 8 applications of rituximab every 2 weeks.
Discussion
Whereas rituximab is supposed to be degraded in the liver and other organs by a process of nonspecific catabolism,5,12-14 little is known about the mechanisms of rituximab metabolism and elimination.15,16 To extend the sparse knowledge of rituximab pharmacokinetics, in the present study, we investigated rituximab disposition and the impact of covariates (ie, sex, age, and weight) on pharmacokinetics in an elderly population of 20 patients with DLBCL in an open prospective study using a population approach. Population-pharmacokinetic information about rituximab is available from only a few studies performed in different patient populations: in patients with rheumatoid arthritis,17 in 8 patients with mostly indolent non-Hodgkin lymphoma,14 and 10 patients with mostly DLBCL.11 To the best of our knowledge, this is the first study on rituximab pharmacokinetics for a dose-dense 2-week R-CHOP-14 schedule in patients with aggressive CD20+ B-cell lymphoma. A comparison of our study with the pharmacokinetic results of previous studies is shown in Table 6.
Based on a 2-compartmental nonlinear, mixed-effects, population pharmacokinetic approach, a Vc of 3.9 l and a Cl of 9.4 mL/h were determined in our study. The pharmacokinetics of rituximab was appropriately described by a 2-compartmental model in our study, confirming previously published studies (Table 6), even though different subroutines for population analysis were used.11,17 Whereas subroutines other than ADVAN TRAN have been suggested as the best-fitting routine for NONMEM, adequate calculation of t1/2β can only be obtained by Vdss calculated with the ADVAN3 TRANS3 subroutine in NONMEM (as demonstrated in this study).
Evaluation of the final model was performed with a bootstrap analysis (500 replicates). The mean population parameters obtained after bootstrap analysis were nearly identical to the parameter estimates generated after a single run of the original dataset because of the high stability of the developed model (Table 3).
In DLBCL, a rapid tumor control is critical to improving outcome because tumors becoming refractory have a poor prognosis,18 particularly when pretreated with rituximab.19 Initial pharmacokinetic studies suggested that high plasma rituximab levels are associated with a favorable outcome in indolent lymphomas.20 In a study by Piro et al, higher efficacy was also associated with higher cumulative doses and more prolonged rituximab exposure in follicular lymphoma.21 In another pharmacokinetic study in patients with relapsed indolent lymphoma and low tumor burden who received 4 weekly doses of rituximab 375 mg/m2 as consolidation after chemotherapy, the median pre-infusion serum concentration of rituximab increased from 80.3 μg/mL (range, 62.8-100.3 μg/mL) after the first dose to 198.1 μg/mL (range, 172.0-267.6 μg/mL) after the fourth.22
In the present study, all patients with DLBCL received 8 dose-dense rituximab cycles and the median serum levels increased slowly and no plateau was reached over the entire 8 applications. Interestingly, we found a rituximab half-life of 27 days in the whole study population, and in 9 of 10 evaluable patients, rituximab serum levels were detectable 9 months after the last infusion. In a study by Berinstein et al, the estimated half-life of rituximab was considerably shorter, but it increased from 3 days after the first cycle to 8.5 days after the fourth cycle.23 Similar to our study, rituximab levels in that study were also detectable as late as 6 months after the last infusion. This difficult-to-explain prolonged half-life and long persistence of measurable rituximab serum levels were speculated to be because of the therapy-induced reduction of tumor mass and saturation of CD20-binding sites, because serum rituximab levels were inversely correlated with both tumor bulk and the number of circulating B cells at baseline. Other studies have found similar results.7,21,23
Our observation that rituximab serum levels are independent of tumor mass are in agreement with a study by Mangel et al in mantle cell lymphoma in which the level of rituximab exposure was similar in patients with minimal disease states and patients with active disease.24
The pharmacokinetics of an mAb depends on a variety of factors, such as the dose and frequency of administration, the metabolic turnover of the Ab, its distribution in the body, and its specific and unspecific clearance. Some of these factors, such as the specific clearance by binding to CD20+ cells, may be influenced by tumor bulk. In contrast, unspecific clearance, such as that caused by binding to Fc receptors, might depend on other factors such as the binding affinity of rituximab to distinct Fc receptor genotypes.25,26 Our results are in agreement with another study in which actual concentrations of the first treatment cycle were used to simulate concentrations of later cycles.11 In that study, the simulated values were compared with the measured values and they did not differ. It was concluded that the pharmacokinetic behavior of rituximab was not altered despite the treatment-induced tumor mass reduction. The determined rituximab half-life in that trial was 21 days.
Whereas age (within the range of 61-80 years, which was an inclusion criterion for the RICOVER-60 and Pegfilgrastim trials) did not influence pharmacokinetic parameters of rituximab in our study, it can only be speculated whether our results can be generalized to other populations with DLBCL (eg, young patients) until data specifically obtained from these subpopulations is available. The most important finding of our study is that clearance is strongly and independently influenced by a patient's sex and, when rituximab dose is adjusted to body surface area, by weight. Clearance was significantly reduced in the female patients included in this study and was 1.5-fold faster (8.21 vs 12.68 mL/h) in male patients. Clearance was inversely correlated with elimination half-life of rituximab, with a significant decrease in men (t1/2β = 24.7 days) compared with women (t1/2β = 30.7 days). These results are similar to the findings of Ng et al using a population approach of rituximab in patients with rheumatoid arthritis.17 These investigators also observed a higher (39%) clearance in men than in women, but also demonstrated an impact of body surface area in their study, which was not observed in our study. This discrepancy, however, can be explained by the fact that patients in our study received a rituximab dose calculated according to body surface area, whereas patients received a fixed total dose of 1000 mg of rituximab in the Ng et al study.17
Clinically relevant sex differences in pharmacology have been described to date in only a few investigations. In one study, the pharmacokinetics of anti-D Ig (144 kD), which is comparable to rituximab with respect to chemical characteristics and molecular weight, was investigated for sex differences in healthy volunteers.27 Women had a higher Cmax of anti-D Ig and a lower volume of distribution than men, most likely also reflecting a decreased clearance of anti-D Ig in females. In another investigation of age and sex effects on pharmacokinetics of lanoteplase, a plasminogen activator, a sex-dependent effect on total clearance was observed, with elderly females having a 32% lower mean clearance than elderly males.28 Whereas the lower cardiac output and hepatic blood flow in women compared with men might contribute to this phenomenon, differences in hepatic enzymatic activities between women and men appear to play the major role in determining pharmacokinetic variability by sex.29
The fact that the significantly faster rituximab clearance in elderly male DLBCL patients resulted in a smaller improvement by the addition of rituximab in males compared with females in RICOVER-604 (Figure 5 and Table 5) suggests that the rituximab dose used for DLBCL patients (375 mg/m2) is suboptimal for elderly male patients. That rituximab pharmacokinetics might also have an impact on outcome when combined with chemotherapy regimens other than CHOP-14 is suggested by a recent observation of patients with relapsed DLBCL, in whom rituximab maintenance treatment had a beneficial effect in younger (18-65 years of age) females, but not in males.30 Because of this and to address the role of lower rituximab serum levels in male patients, a phase 2 study, the SEXIE-R-CHOP-14 study of the DSHNHL, is currently ongoing. This trial is investigating whether male patients receiving a dose of 500 mg/m2 per cycle achieve serum levels similar to those observed in female patients receiving the standard dose of 375 mg/m2, and whether the increased rituximab dose translates into better results in elderly male patients with DLBCL.
We also tested a simulation of a different scheduling of rituximab with 21-day– and 14-day–cycle intervals. If a linear rituximab dose-effect relationship within the different maximum dose ranges indeed existed, a superiority of the dose-dense schedule might be assumed. However, with the dose-dense 14-day schedule, the time of rituximab exposure is shorter. Therefore, if a longer exposure of the aggressive lymphoma cells to rituximab were therapeutically relevant, the 3-week schedule should be more efficacious. That the shortened exposure to rituximab in the R-CHOP-14 regimen (with the last application on day 98 after the start of therapy) compared with R-CHOP-21 (with the last application on day 148) might not exploit the full potential of 8 rituximab applications is suggested by the results of a phase 2 trial, the SMARTE-R-CHOP-14 trial of the DSHNHL in elderly DLBCL patients.31 In this trial, 190 patients received 6 cycles of CHOP-14 together with 8 applications of rituximab over an extended period, with the last rituximab administration given on day 240. This resulted in lower maximum rituximab serum levels than in the RICOVER-60 trial, but in a considerably longer rituximab exposure time. Whereas no differences were observed in elderly patients with good-prognosis DLBCL(IPI score = 1 or 2), the 3-year PFS and overall survival of poor-prognosis patients (IPI = 3-5) were both 13% better in the SMARTE-R CHOP-14 trial than in the respective population of the RICOVER-60 trial, in which 8 cycles of rituximab were given in 2-week intervals. If the short exposure time of rituximab when given in combination with CHOP-14 were indeed detrimental, this would explain why R-CHOP-14 was not superior to R-CHOP-21 in 2 recent randomized studies,32,33 although CHOP-14 was superior to CHOP-21 in elderly patients with DLBCL in another study.34
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all colleagues and patients for participating in the study and the staff of the DSHNHL office for their support.
This work was supported by the Deutsche Krebshilfe funding the RICOVER-60 trial (NCT00052936). Hoffmann-La Roche covered the cost of the measurement of rituximab serum levels.
Authorship
Contribution: N.M., M.W., M.P., and M.R. designed the study; G.H., C.N., N.P., E.L., B.M., and T.R. collected the pharmacokinetics samples and provided the clinical data; C.M. and M.H.J.W. performed the pharmacokinetic modeling; G.H., V.P., T.R., and C.Z. collected the clinical data; S.Z. performed the statistical analysis; and C.M., N.M., M.P., and M.R. wrote the manuscript.
Conflict-of-interest disclosure: M.P. is on the Roche Mabthera Advisory Board and has received research support from Roche. The remaining authors declare no competing financial interests.
Correspondence: Michael Pfreundschuh, Klinik für Innere Medizin I, Saarland University Medical School, D-66424 Homburg (Saar), Germany; e-mail: michael.pfreundschuh@uks.eu.
References
Author notes
C.M. and N.M. contributed equally to this work.