Abstract
Ultimate success of hematopoietic stem cell transplantation (HSCT) depends not only on donor HSCs themselves but also on the host environment. Total body irradiation is a component in various host conditioning regimens for HSCT. It is known that ionizing radiation exerts “bystander effects” on nontargeted cells and that HSCs transplanted into irradiated recipients undergo proliferative exhaustion. However, whether irradiated recipients pose a proliferation-independent bystander effect on transplanted HSCs is unclear. In this study, we found that irradiated mouse recipients significantly impaired the long-term repopulating ability of transplanted mouse HSCs shortly (∼ 17 hours) after exposure to irradiated hosts and before the cells began to divide. There was an increase of acute cell death associated with accelerated proliferation of the bystander hematopoietic cells. This effect was marked by dramatic down-regulation of c-Kit, apparently because of elevated reactive oxygen species. Administration of an antioxidant chemical, N-acetylcysteine, or ectopically overexpressing a reactive oxygen species scavenging enzyme, catalase, improved the function of transplanted HSCs in irradiated hosts. Together, this study provides evidence for an acute negative, yet proliferation-independent, bystander effect of irradiated recipients on transplanted HSCs, thereby having implications for HSCT in both experimental and clinical scenarios in which total body irradiation is involved.
Introduction
Hematopoietic stem cell transplantation (HSCT) may provide cures for many congenital diseases, immunodeficiencies, and hematopoietic or nonhematopoietic malignancies.1 However, the broader use of HSCT in patients is hindered in part by the limited number of HSCs contained in each harvest. This issue may prove especially problematic in cases when HSCs are collected from human cord blood, where the number of HSCs per harvest is not sufficient for an adult patient.2 Successful HSC engraftment depends not only on the dosage and quality of transplanted HSCs but also the conditioning of recipients. Given the current unsatisfactory status of ex vivo HSC expansion, manipulating the bone marrow (BM) microenvironment or the stem cell niche has been thought to be an attractive strategy3–6 to improve the outcome of HSCT.
On transplantation, HSCs go through a series of processes, including homing, lodgment, proliferation, and differentiation, all of which are important determinants for short- and long-term engraftment. One of the major factors that may impact these processes is the conditioning regimen administered to the HSCT recipient before transplantation to reduce the chances of graft rejection and in the case of cancer treatment, to reduce the burden of disease and to make niches available for donor stem cells. Of particular interest is the effects of total body irradiation (TBI) on these processes as this remains a component in many HSCT conditioning regimens and has been associated with a number of effects on the BM microenvironment, including oxidative stress, genotoxicity, and other physical disruptions.7,8 In addition, previous studies have demonstrated that HSCs and their descendants may develop DNA damage and other effects after exposure to irradiated marrow (ie, “bystander or untargeted effects”).9,10 Some of these effects may be mediated not only by TNF-α, nitric oxide, and superoxide, and other factors produced by irradiated macrophages in the BM microenvironment, but probably also other operative mechanisms as well.9 Together, these observations suggest that irradiation of a transplant recipient as part of the conditioning regimen could result in unintended and potentially deleterious effects on transplanted HSCs through these “bystander” mechanisms. Currently, however, a number of aspects of this process and its effects on HSCs remain unclear, including whether there are any effects on HSC homing and early engraftment, if there are any long-term effects on the transplanted HSCs, and the nature of the cellular and molecular mechanisms that may underlie these effects.
To answer these important questions, we have examined the functionality, phenotype, localization, and inflammatory stress response of transplanted HSCs from lethally irradiated (IR) mice compared with transplanted HSCs from nonirradiated (NR) mice and further explored the mediatory factors that are involved. Our results demonstrated that IR recipients have an immediate negative bystander effect on long-term hematopoietic engraftment of transplanted HSCs. Such an acute effect occurs before the input cells start to proliferate (17 hours after transplantation). It is marked by c-Kit down-regulation that is caused by increased generation of reactive oxygen species (ROS). Our current study provides insights into mechanisms underlying the irradiation induced “bystander” effects and suggests both possible clinical sequelae of these effects as well as potential interventions that deserve further studies.
Methods
Mice
C57BL/6 (CD45.2+) and congenic strain B6.SJL (CD45.1+) mice were purchased from either The Jackson Laboratory or Taconic Farms. F1 mice (CD45.1+/CD45.2+) were generated by crossing C57BL/6 and B6.SJL breeders. All mice were maintained in a certified animal facility at the University of Pittsburgh Cancer Institute per procedures approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh.
Exposure to irradiated BM
All recipients of bone marrow transplantation (BMT) were treated with 10 Gy of total body irradiation at the dose rate of 0.84 Gy/min (cesium 137; model MKL-68 IRRAD, JL Shepherd & Associates) on the day before transplantation. For in vitro coculture, BM stromal cells or the AFT024 stromal cell line were irradiated with 12 Gy at the rate of 13.28 Gy/min (cesium 137; AECL Gamma Cell 1000D, Nordion International). The in vivo experimental procedure is illustrated in Figure 1A.
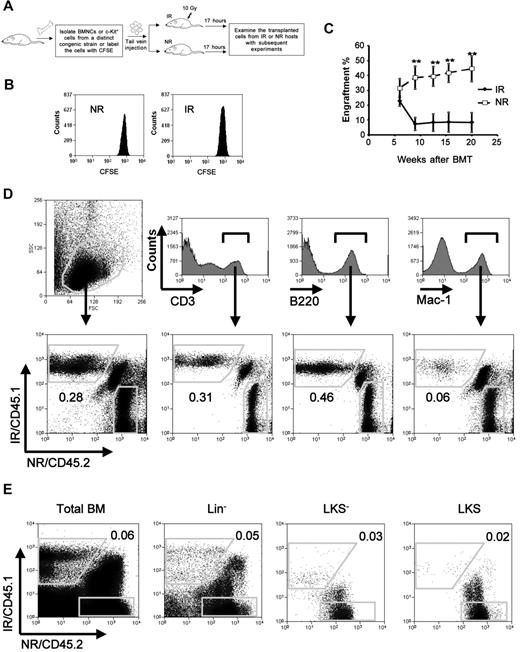
Impaired long-term engraftment of bystander hematopoietic cells. (A) An overall experimental design for the bystander exposure in vivo. Recipient mice of IR received 10 Gy irradiation 1 day before transplantation. (B) A cell proliferation assay. Lin−c-Kit+CFSE+ cells from 20 pooled mice were sorted and injected into IR or NR recipients. Seventeen hours after transplantation, the transplanted cells were analyzed for their divisions according to the intensity of CFSE by flow cytometry. Data presented were obtained from 1 of the 2 experiments with identical results. (C) Engraftment levels at different time points in blood. Homed Lin−c-Kit+ donor cells from IR and NR recipients were sorted 17 hours after transplantation and transplanted into lethally irradiated congenic recipients (1-2 × 104 cells/mouse) in the cBMT model. Engraftment levels of donor cells at different time points after transplantation were represented by the frequencies of donor cells in blood. **P < .01. n = 6. This is a representative summary from 3 experiments with consistent results. (D-E) Representative ratios in blood (D) and BM (E) 20 weeks after the transplantation. The ratios of CD45.1+ (IR) to CD45.2+ (NR) in blood at whole or different lineages (D), and in BM at whole or different hematopoietic subsets (E) are shown. The figures show the representative data from 1 of 3 mice.
Impaired long-term engraftment of bystander hematopoietic cells. (A) An overall experimental design for the bystander exposure in vivo. Recipient mice of IR received 10 Gy irradiation 1 day before transplantation. (B) A cell proliferation assay. Lin−c-Kit+CFSE+ cells from 20 pooled mice were sorted and injected into IR or NR recipients. Seventeen hours after transplantation, the transplanted cells were analyzed for their divisions according to the intensity of CFSE by flow cytometry. Data presented were obtained from 1 of the 2 experiments with identical results. (C) Engraftment levels at different time points in blood. Homed Lin−c-Kit+ donor cells from IR and NR recipients were sorted 17 hours after transplantation and transplanted into lethally irradiated congenic recipients (1-2 × 104 cells/mouse) in the cBMT model. Engraftment levels of donor cells at different time points after transplantation were represented by the frequencies of donor cells in blood. **P < .01. n = 6. This is a representative summary from 3 experiments with consistent results. (D-E) Representative ratios in blood (D) and BM (E) 20 weeks after the transplantation. The ratios of CD45.1+ (IR) to CD45.2+ (NR) in blood at whole or different lineages (D), and in BM at whole or different hematopoietic subsets (E) are shown. The figures show the representative data from 1 of 3 mice.
Isolation of HSC enriched cell population.
BM cells were first enriched with CD117 micromagnetic beads (Miltenyi Biotec) per the manufacturer's protocol. Enriched cells were then stained with the antibodies against mouse c-kit− (2B8) and lineage (Lin) markers: CD3 (CT-CD3), CD4 (CT-CD4), CD8 (CT-CD8a), B220 (RA3-6B2), Gr-1 (RB6-8C5), Mac-1 (M1/70.15), and TER-119 (BD Biosciences). Dead cells were discriminated by propidium iodide uptake. Live Lin−c-Kit+ cells were sorted using a MoFlo high-speed cell sorter (Beckman Coulter/Dako).
cBMT
Two test cell populations or 1 test cell population and 1 competitor cell population with distinct congenic markers were mixed at a 1:1 ratio (1-2 × 104 cells) and cotransplanted into lethally irradiated mice. Blood from transplanted mice was collected every 3 weeks and stained with anti-CD45.1–PE and anti-CD45.2–FITC antibodies. The frequencies of CD45.1 and CD45.2+ cells were measured by a Beckman Coulter XL cytometer. The level of transplanted cell engraftment from IR or NR recipients was calculated as the frequency of CD45.1 or CD45.2 single-positive cells in total CD45+ cells. To measure the effect of N-acetylcysteine (NAC), recipients were administered 3 doses of NAC (Sigma-Aldrich) at 1 g/kg 1 hour before irradiation, overnight after irradiation, and 1 hour before transplantation.
Real-time RT-PCR
Total RNA from 5000 cells sorted directly into lysis buffer was extracted with the RNA Nanoprep Kit (Strategene) according to the manufacturer's protocol. cDNA was generated using oligo-dT(12-18) and M-MLV reverse transcriptase (Ambion) according to the manufacturer's protocol. Real-time PCR reactions consisting of DyNAmo SYBR Green Master Mix (Finnzymes), 0.3μM of specific forward and reverse primers, and diluted cDNA were carried out with the Chromo 4 Detector System (MJ Research). The gene specific primers used in our study are listed in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Apoptosis analysis.
Following the manufacturer's protocol, annexin V (BD Biosciences) and 4′,6-diamidino-2-phenylindole, dihydrochloride (DAPI) were used for the assay.
In vivo tracking and proliferation assay.
BM cells were labeled with 5- (and 6-) carboxyl fluorescein diacetate succinimidyl ester (CFSE; Invitrogen) before transplantation, and the number of cell divisions was measured after transplantation based on the fluorescent intensity of CFSE in different hematopoietic cell subpopulations as described previously.11
Homing assay
Total BM or Lin−c-kit+ cells were injected into IR (overnight, 10 Gy) or NR congenic recipients. The recipients were scarified 17 hours after transplantation, and BM cells were stained with anti–Sca-1–PE, anti–c-Kit–APC, and lineage markers conjugated with PE-Cy7, anti-CD45.1–PE–Cy5.5, and anti-CD45.2–FITC for quantifying the homing efficiency of the donor cells and harvesting the donor cells for subsequent transplant experiments.
Histologic examination of homed HSC localization in the endosteal region
c-Kit–enriched bone marrow nucleated cells (BMNCs) were labeled with lineage markers and CFSE as described previously.11 CFSE+Lin−c-Kit+ cells were sorted and transplanted into IR or NR recipients. Seventeen hours after transplantation, mouse femurs were collected after perfusion with 4% paraformaldehyde (Fisher Scientific). Femurs were fixed, decalcified, dehydrated, paraffin-embedded, and sectioned as described previously.6 CFSE+ transplanted cells within the endosteal (< 12 cells of the endosteum) or central region were counted separately under a fluorescent microscope (Nikon Eclipse TE 300). The percentage of lodgment of the hematopoietic cells was calculated as the proportion of transplanted cells located within the endosteal region.
Detection of ROS in the hematopoietic cells.
Cells were loaded with 5μM of the ROS probe, 6-carboxy-2′,7′-dichlorodihydrofluorescein diacetate (H2DCF), di(acetoxymethyl ester) (Invitrogen) at 37°C for 30 minutes and then stained with anti-CD45–PE antibody at room temperature for 10 minutes. The stained cells were analyzed on a CyAN cytometer (Beckman Coulter).
Cytokine antibody array.
BM cells were harvested and lysed with cell lysis buffer (BayBiotech). Cell lysate was then sent to BayBiotech for the membrane-based cytokine antibody array. NR mouse BM cells were used as control. Western blot was used for the confirmation of PF4 expression. The activity of MMP9 was measured by ELISA kit (GE Healthcare).
Overexpression of catalase in BM hematopoietic cells
Retrovirus particles were produced by cotransfection of HEK 293T cells with retroviral vector overexpressing catalase or vector with green fluorescence protein as indicator through plasmids: vesicular stomatitis virus glycoprotein and pKat. Supernatants were then collected and used to infect lineage-depleted mouse BM cells, which were prestimulated with 50 ng/mL of recombinant mouse stem cell factor, 10 ng/mL of thrombopoietin, and 10 ng/mL of Flt3-ligand in the RetroNectin (Takara Bio) coated 24-well plates. Green fluorescence protein-positive–transduced cells were sorted in a MoFlo sorter. The transduced cells were used for evaluating the effect of catalase on HSCs in the competitive bone marrow transplantation (cBMT) model.
Statistical analysis
Mean values were compared using the Student 2-tailed t test for independent means or paired means. A P value < .05 is considered as a significant difference between groups.
Results
Long-term reconstitution of the bystander hematopoietic cells in recipients
Transplanted HSCs in irradiated hosts encounter proliferative stress that is thought to cause ultimate HSC exhaustion after serial transplantation.12,13 To distinguish the bystander effect of irradiated hosts on transplanted HSCs (specifically the Lin−c-Kit+ enriched population) from the proliferative response of the cells, we sought to focus on the time window in which the transplanted hematopoietic cells had not begun to divide after their arrival in BM. According to previous studies by others and us,14,15 we chose 17 hours after transplantation as the time point at which the cells from IR or NR recipients were harvested for subsequent studies (Figure 1A). We applied the cytoplasmic dye CFSE to examine the cell divisions of the transplanted Lin−c-Kit+ cells.11 At this time point, virtually all of the transplanted Lin−c-Kit+ cells had not divided (Figure 1B), which was highly consistent with the studies by others.15 The functions of bystander hematopoietic cells from IR as well as NR recipients were directly compared with the cBMT model.11 We isolated Lin−c-Kit+ cells and injected them into IR (10 Gy) or NR congenic recipients. Seventeen hours after transplantation, homed cells from IR and NR recipients were individually sorted. Both isolated cell types were mixed at a 1:1 ratio (1-2 × 104 cells) and transplanted into lethally irradiated recipients (F1 of C57BL/6 and SJL.B6 breeders, CD45.1+/CD45.2+). A total of 1 × 105 of total BMNCs from the CD45.1+/CD45.2+ mice were cotransplanted as supporting cells in the recipient animals. The engraftment levels of donor cells were measured at different time points after transplantation (Figure 1C). We found that the engraftment of the cells from IR recipients decreased significantly 9 weeks after transplantation. At week 20, the average engraftment levels of the cells exposed to IR recipients and NR recipients were 8.35 ± 16.03 and 44.42 ± 21.00, respectively (P < .01, n = 6). Specific ratios between the 2 test cell populations in different lineages and an HSC–enriched cell population were examined by costaining of multilineage or HSC markers in conjunction with multicolor flow cytometric analysis. The ratio of CD45.1+ (from IR recipients) to CD45.2+ (from NR recipients) cells in the blood decreased to 0.22 ± 0.11 in total leukocytes, 0.34 ± 0.22 in CD3+ T cells, 0.27 ± 0.19 in B220+ B cells, and 0.05 ± 0.01 in Mac-1+ myeloid cells (Figure 1D; n = 3). It suggests that the negative effect on myeloid differentiation is more severe than that on lymphoid differentiation. In BM, the ratio of CD45.1+ to CD45.2+ cells was observed to be much lower than those in blood, particularly in the HSC-enriched Lin−c-Kit+Sca-1+ (LKS) and hematopoietic progenitor cell (HPC)–enriched Lin−c-Kit+Sca-1− (LKS−) populations (0.02 ± 0.01 and 0.01 ± 0.01, respectively, n = 3; Figure 1E), thereby suggesting a more specific negative effect of IR BM on donor HSCs/HPCs. Given the possibility that the hematopoietic cells from CD45.1+ and CD45.2+ mice may not be absolutely equal in their ability to engraft,16 2 independent cBMT experiments in which both CD45.1+ and CD45.2+ congenic strains were inversely used or each of the congenic cell types was cotransplanted with the same unmanipulated competitor cells in separate groups, were also performed (data not shown). The disadvantage of the Lin−c-Kit+ cells from IR recipients compared with the cells from NR recipients was further confirmed by these different approaches.
Homing of transplanted hematopoietic cells in the endosteal region of recipient BM
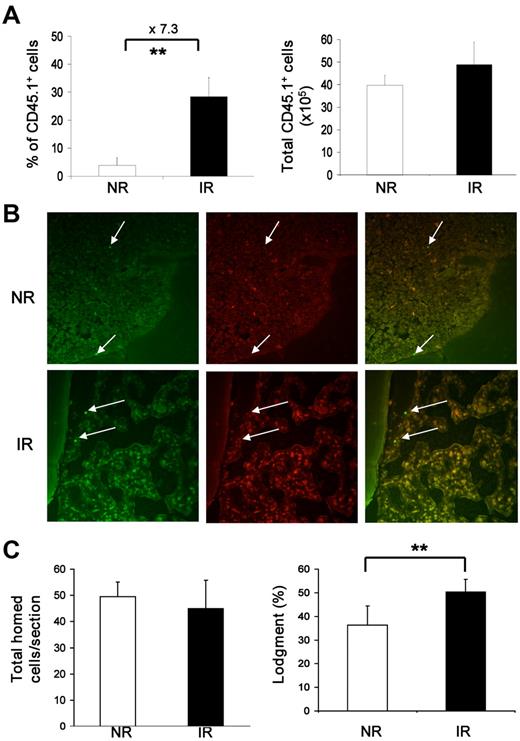
Homing of transplanted HSCs is the earliest event critical for the long-term engraftment and repopulation of transplanted HSCs. It involves multiple steps, including rolling and adhesion on vascular endothelium, migration across endothelial cells, and finally anchoring into the endosteal HSC niche.3,5,6,17 A decreased homing ability of transplanted HSCs in BM was reported based on a lower recovery of specific immunophenotypes18 but not measured by the number of cells localizing to the endosteal HSC niche in IR recipients. To further verify the effect of irradiated BM microenvironment on the arrival of transplanted hematopoietic cells, we first measured the total homed cells (CD45.1+) in BM 17 hours after injection of BMNCs into IR and NR recipients. Although the percentage representation of the transplanted cells in total BMNCs was significantly higher in IR recipients because of the massive death of endogenous BM cells in these hosts after TBI, the absolute number of homed cells in the BM was not significantly different between IR and NR recipients (Figure 2A).
Normal homing and localization of transplanted hematopoietic cells in recipient BM. (A) Frequencies and total numbers of homed BMNCs in recipient BM. BM cells pooled from 3 mice (CD45.1+) were injected into IR or NR recipients (CD45.2+). Seventeen hours after transplantation, BM cells were harvested and stained with anti-CD45.1 and CD45.2. The frequency of donor cells was then measured by flow cytometry. Total absolute number of donor cells was also calculated. **P < .01. n = 4. (B) Localization of the homed cells. CFSE-labeled Lin−c-kit+ cells were injected into IR or NR recipients that were scarified 17 hours after transplantation. The homed cells were identified on the sections of femurs based on CFSE staining with fluorescence microscopy. Images show the cells under a 490/20-nm filter (green, left), 555/28-nm filter (red, center column) and their overlay (right). Arrows indicate homed cells. The top panel was from a NR recipient, and the bottom panel was from an IR recipient. The photograph was taken under 40×/0.60 by a digital camera (RT Slider-Spot, model 2.3.1.1.) with Spot Version 4.0.9 software (Diagnostic Instruments). Adobe Photoshop CS Version 8.0 (Adobe System) and Microsoft Office PowerPoint 2003 software were used for image sizing and displaying. (C) Enumeration of the homed cells in proximity to the endosteal niche. Total homed cells from NR and IR recipients per section were counted, and the lodgment was quantified by the proportion of homed cells located within the endosteal region. **P < .01. n = 20 sections.
Normal homing and localization of transplanted hematopoietic cells in recipient BM. (A) Frequencies and total numbers of homed BMNCs in recipient BM. BM cells pooled from 3 mice (CD45.1+) were injected into IR or NR recipients (CD45.2+). Seventeen hours after transplantation, BM cells were harvested and stained with anti-CD45.1 and CD45.2. The frequency of donor cells was then measured by flow cytometry. Total absolute number of donor cells was also calculated. **P < .01. n = 4. (B) Localization of the homed cells. CFSE-labeled Lin−c-kit+ cells were injected into IR or NR recipients that were scarified 17 hours after transplantation. The homed cells were identified on the sections of femurs based on CFSE staining with fluorescence microscopy. Images show the cells under a 490/20-nm filter (green, left), 555/28-nm filter (red, center column) and their overlay (right). Arrows indicate homed cells. The top panel was from a NR recipient, and the bottom panel was from an IR recipient. The photograph was taken under 40×/0.60 by a digital camera (RT Slider-Spot, model 2.3.1.1.) with Spot Version 4.0.9 software (Diagnostic Instruments). Adobe Photoshop CS Version 8.0 (Adobe System) and Microsoft Office PowerPoint 2003 software were used for image sizing and displaying. (C) Enumeration of the homed cells in proximity to the endosteal niche. Total homed cells from NR and IR recipients per section were counted, and the lodgment was quantified by the proportion of homed cells located within the endosteal region. **P < .01. n = 20 sections.
Because of the down-regulation of c-Kit (see Figure 4) and known alterations of other cell surface markers, such as CD3419 in IR recipients, it was not possible to measure HSCs in the recipient with a stringent immunophenotype for HSCs, such as CD34−LKS.20 Instead, we quantified the fraction of transplanted Lin−c-Kit+ cells that had localized in the endosteal region, which has been demonstrated to contain the osteoblastic niche for HSCs in vivo3,5,21,22 and in particular be associated with the long-term repopulating HSCs.3,5 A total of 2 × 106 Lin−c-kit+ cells labeled with CFSE were transplanted into IR or NR recipients. Seventeen hours later, mice were perfused and the femurs were collected for histologic examination according to an established method.6 The lodgment of the injected cells was quantified by the proportion of transplanted cells (CFSE+) located within the endosteal region (within 12 cells of the endosteum; Figure 2B). Overall, there was “empty space” in irradiated BM, whereas nonirradiated BM was filled with cells, indicating that irradiation with 10 Gy eliminated the majority of endogenous hematopoietic cells within 17 hours. The percentage of transplanted cells in IR recipient marrow (50.29 ± 8.04) was statistically higher than that in the NR recipient marrow (36.34 ± 5.45; P < .01). The total transplanted cells per section, however, were not different between IR (44.95 ± 5.62) and NR recipients (49.45 ± 10.74; P > .05; Figure 2C). Our data suggest that irradiated BM does not have a significant negative effect on the homing of transplanted HSCs, as defined by the localization of Lin−-c-Kit+ cells to the endosteal region, a major HSC niche in BM. Taken together, our data present an argument that the impaired long-term engraftment (reconstitution) of bystander HSCs was not the result of a decreased homing efficiency of transplanted HSCs in IR recipients. However, a possible defective interaction between transplanted HSCs and the stem cell niche at the molecular level cannot be ruled out by these data.
Measurements for apoptosis, proliferation, and senescence of the bystander hematopoietic cells
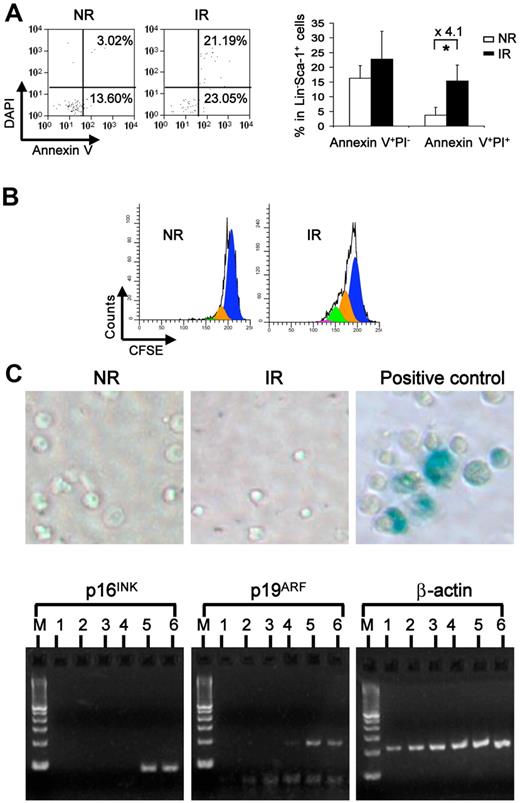
The impaired long-term engraftment of the bystander hematopoietic cells may be with a consequence of increased cell death, senescence, or altered cell proliferation of the homed HSCs. To test these possibilities, we first examined cell apoptosis by staining cells with annexin V. There was an increased dead cell fraction (annexin V+DAPI+) but not early apoptotic fraction (annexin V+DAPI−) in the transplanted cells from IR recipients (Figure 3A). To examine the proliferative potential of injected cells, we injected the CFSE-labeled cells into IR or NR recipients and harvested the cells 3 days later for cytometric analysis. We observed a greater dilution of the CFSE dye in IR recipients, which indicated more divisions of the transplanted cells (Figure 3B). This data are consistent with an original observation that pre-irradiated hosts increase proliferation of donor BM cells.23 In addition, we also examined the activity of the enzyme β-galactosidase (SA-β-gal), which was shown to be associated with the senescent state of cells,24,25 as well as p16INK4A and p19ARF, both of which have been shown to be specific molecular biomarkers for senescent cells.26 Neither SA-β-gal staining nor the expression of p16 and p19 was detected in either cells isolated from IR or NR recipients (Figure 3C). These data demonstrate that the bystander effect may cause a decrease of hematopoietic cell survival associated with late increased proliferation but does not directly cause measurable senescence of the transplanted hematopoietic cells in irradiated hosts at the cell population level.
Increased apoptosis and proliferation without overt senescence of bystander hematopoietic cells. (A) Apoptosis of the resided hematopoietic cells. Apoptosis was measured in homed Lin−Sca-1+ cells with multicolor flow cytometry combining surface markers of CD45.1, CD45.2, lineage, Sca-1 with annexin V, and DAPI. Plots were gated on homed donor Lin−Sca-1+ cells (left). Percentage of early apoptosis (annexin V+DAPI−) and late apoptosis was compared between NR and IR recipients (right). *P < .05. n = 3 or 4. (B) Cell proliferative potential. Transplanted BMNCs were labeled with CFSE before injection. Three days later, the division of donor Lin− cells in NR or IR recipients was analyzed according to the intensity of CFSE in flow cytometry. The representative plots were generated from ModFit Version 3.1 software. (C) Senescence of the bystander cells from the irradiated hosts. Homed Lin−Sca-1+ cells from NR and IR were sorted and stained with X-gal for detecting the β-gal activity. Blue color in the cells indicated positive activity of β-gal. Lin−Sca-1+ cells cultured in the medium containing 50 ng/mL of stem cell factor, 50 ng/mL of Flt3, and 10 ng/mL of thrombopoietin at 37°C, 5% CO2 for 10 days were used as positive control. Real-time RT-PCR was performed for examining the mRNA expression of p16INK4A and p19ARF in homed cells 17 hours after transplantation. M indicates DNA ladder. Lanes 1 and 2 represent homed Lin−Sca-1+ cells from IR recipients; lanes 3 and 4, homed Lin−Sca-1+ cells from NR recipients; and lanes 5 and 6, positive controls.
Increased apoptosis and proliferation without overt senescence of bystander hematopoietic cells. (A) Apoptosis of the resided hematopoietic cells. Apoptosis was measured in homed Lin−Sca-1+ cells with multicolor flow cytometry combining surface markers of CD45.1, CD45.2, lineage, Sca-1 with annexin V, and DAPI. Plots were gated on homed donor Lin−Sca-1+ cells (left). Percentage of early apoptosis (annexin V+DAPI−) and late apoptosis was compared between NR and IR recipients (right). *P < .05. n = 3 or 4. (B) Cell proliferative potential. Transplanted BMNCs were labeled with CFSE before injection. Three days later, the division of donor Lin− cells in NR or IR recipients was analyzed according to the intensity of CFSE in flow cytometry. The representative plots were generated from ModFit Version 3.1 software. (C) Senescence of the bystander cells from the irradiated hosts. Homed Lin−Sca-1+ cells from NR and IR were sorted and stained with X-gal for detecting the β-gal activity. Blue color in the cells indicated positive activity of β-gal. Lin−Sca-1+ cells cultured in the medium containing 50 ng/mL of stem cell factor, 50 ng/mL of Flt3, and 10 ng/mL of thrombopoietin at 37°C, 5% CO2 for 10 days were used as positive control. Real-time RT-PCR was performed for examining the mRNA expression of p16INK4A and p19ARF in homed cells 17 hours after transplantation. M indicates DNA ladder. Lanes 1 and 2 represent homed Lin−Sca-1+ cells from IR recipients; lanes 3 and 4, homed Lin−Sca-1+ cells from NR recipients; and lanes 5 and 6, positive controls.
A molecular profile of the bystander hematopoietic cells marked by dramatic c-Kit down-regulation
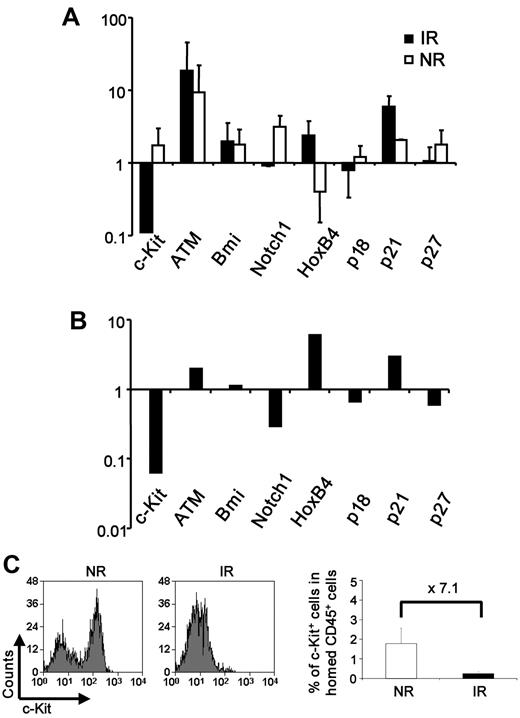
We further attempted to explore the intrinsic molecular players by measuring the mRNA expression of a number of genes known to be involved in HSC regulation, including c-Kit, HoxB4, Bmi-1, Notch-1, p16, p19ARF, p18, p21, and p27.11,14,27–31 Lin−Sca-1+ cells were isolated from IR or NR recipients 17 hours after transplantation and immediately lysed for real-time RT-PCR. Although both groups showed alterations of gene expression as opposed to the unmanipulated Lin−Sca-1+ cells, irradiated BM had a more significant effect on the gene expression (Figure 4A). In direct comparison with nonirradiated BM, irradiated BM down-regulated the expression of c-Kit, Notch1, p18, and p27 and up-regulated HoxB4, p21, and ATM in the bystander HSCs (Figure 4B). The significant down-regulation of c-Kit was also observed at the protein level by flow cytometric analysis (Figure 4C). Given the well-known role of c-Kit signaling in hematopoietic cell survival, dramatic down-regulation of c-Kit in hematopoietic cells on transplantation may be at least partly responsible for the impaired function of bystander HSCs.
Altered expression of stem cell regulators in the bystander hematopoietic cells. (A-B) Relative expression of genes in the cells from IR or NR recipients. Homed Lin−Sca-1+ cells were sorted from IR or NR recipients, and mRNA expression of selected genes was examined with real-time RT-PCR. (A) Relative expression of the genes in cells from IR or NR recipients compared with unmanipulated cells. (B) Direct comparison of the gene expression between the cells isolated from IR and NR recipients; n = 3. The primer sequences for those genes are listed in supplemental Table 1. (C) Down-regulation of c-Kit in the IR host. c-Kit expression was measured by flow cytometry 17 hours after BMT. The plots were gated on the donor Lin− population. Bar graphs represents expression of the c-Kit+ fraction in total CD45+ cells homing to BM. **P < .01. n = 2 to 4.
Altered expression of stem cell regulators in the bystander hematopoietic cells. (A-B) Relative expression of genes in the cells from IR or NR recipients. Homed Lin−Sca-1+ cells were sorted from IR or NR recipients, and mRNA expression of selected genes was examined with real-time RT-PCR. (A) Relative expression of the genes in cells from IR or NR recipients compared with unmanipulated cells. (B) Direct comparison of the gene expression between the cells isolated from IR and NR recipients; n = 3. The primer sequences for those genes are listed in supplemental Table 1. (C) Down-regulation of c-Kit in the IR host. c-Kit expression was measured by flow cytometry 17 hours after BMT. The plots were gated on the donor Lin− population. Bar graphs represents expression of the c-Kit+ fraction in total CD45+ cells homing to BM. **P < .01. n = 2 to 4.
Increased levels of inflammatory proteins in irradiated BM
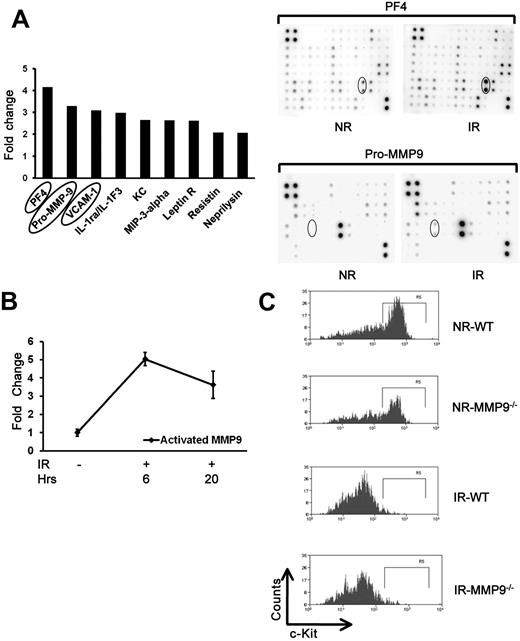
Radiation damage causes a cytokine “storm” in the host.32 To define the inflammatory cytokines that may contribute to the bystander effects, especially the interaction between HSCs and their niches, we used a mouse cytokine antibody array to measure the levels of 144 cytokines in the irradiated BM. Several cytokines and other proteins significantly elevated in the cell lysate of irradiated mice (Figure 5A), such as PF4 (platelet factor-4), Pro-MMP9 (matrix metalloproteinase-9), and VCAM-1. PF4 has been reported to provoke strong formation of oxygen radicals.33 VCAM-1 is a known molecule on the endothelial niche of HSCs.15 Pro-MMP9 in its active forms releases the soluble c-Kit ligand, which can down-regulate c-Kit expression on HSCs.34 To further demonstrate whether the level of activated MMP9 is also elevated in irradiated BM cells, we analyzed both pro-MMP9 and activated MMP9 levels using an ELISA method. As expected, we found a 3.6- to 5.0-fold increase of activated MMP9 in irradiated BM cells than that in nonirradiated BM cells at both 6 and 20 hours after irradiation (Figure 5B). To define whether MMP9 is required for the down-regulation of c-Kit in the bystander hematopoietic cells, we used flow cytometry to examine c-Kit down-regulation of the transplanted cells in MMP9−/− versus MMP9+/+ recipients. Unexpectedly, there was no difference of c-Kit down-regulation between MMP9+/+ and MMP9−/− recipients (Figure 5C), suggesting that MMP9 does not play an essential role in down-regulating c-Kit in the bystander cells.
Increased level of inflammatory cytokines in irradiated BM cells. (A) Cytokine antibody array analysis of irradiated and nonirradiated BM cells. Cell lysates of BM cells were collected from 10 Gy IR mice (n = 3) and NR mice 17 hours after treatment. Cell lysates were sent for membrane-based mouse cytokine antibody array. The cytokines increased more than 2-fold in irradiated cells compared with nonirradiated controls. Lower panels: Representative membrane image. (B) Increased level of activated MMP9 in irradiated BM cells. Cell lysates were also used for activated MMP9 measurement using ELISA. The fold change in irradiated BM compared with nonirradiated BM lysates is shown based on 3 experiments. (C) Down-regulation of c-Kit expression. C-kit-enriched BM cells were injected into lethally IR or NR MMP9+/+ or MMP9−/− mice, respectively. Mice were killed at 17 hours after transplantation, and the expression of c-Kit in the donor cells was measured by flow cytometry. Panels represent the representative flow charts gated in the donor Lin− cells.
Increased level of inflammatory cytokines in irradiated BM cells. (A) Cytokine antibody array analysis of irradiated and nonirradiated BM cells. Cell lysates of BM cells were collected from 10 Gy IR mice (n = 3) and NR mice 17 hours after treatment. Cell lysates were sent for membrane-based mouse cytokine antibody array. The cytokines increased more than 2-fold in irradiated cells compared with nonirradiated controls. Lower panels: Representative membrane image. (B) Increased level of activated MMP9 in irradiated BM cells. Cell lysates were also used for activated MMP9 measurement using ELISA. The fold change in irradiated BM compared with nonirradiated BM lysates is shown based on 3 experiments. (C) Down-regulation of c-Kit expression. C-kit-enriched BM cells were injected into lethally IR or NR MMP9+/+ or MMP9−/− mice, respectively. Mice were killed at 17 hours after transplantation, and the expression of c-Kit in the donor cells was measured by flow cytometry. Panels represent the representative flow charts gated in the donor Lin− cells.
The causative role of ROS in the bystander effects
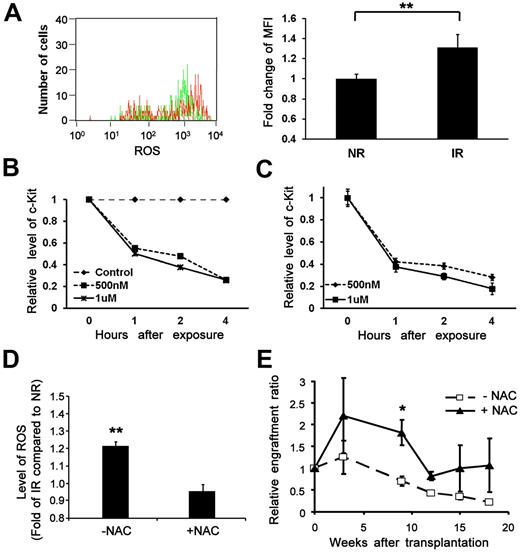
ROS has been considered as one of the key mediators for bystander effects in other cell types.35,36 It has been reported that irradiated stromal cells can induce free radicals in nonirradiated cells in a coculture system37 as well as in directly irradiated cells.38 A previous study demonstrated an increased level of ROS in serially transplanted HSCs over long-term engraftment.39 We first quantified the level of ROS by H2DCF-di (acetoxymethyl ester), a ROS (hydrogen oxide, H2O2) probe, in bystander HSC-enriched Lin−Sca-1+ cells by flow cytometry. There was a statistically significant increase of intracellular ROS in the bystander cells from IR recipients compared with NR recipients (Figure 6A). Furthermore, to explore the effect of ROS on c-Kit down-regulation, c-kit–enriched hematopoietic cells were treated with different doses of hydrogen peroxide (H2O2). As shown in Figure 6B, exposure to H2O2 (500nM to 1μM) was able to reduce c-Kit protein expression as early as 4 hours after exposure. Consistent with the flow cytometric analysis (Figure 6A), we have also detected down-regulation of c-Kit at the mRNA level after exposure to H2O2 (Figure 6C), thereby indicating that elevated ROS in the IR BM microenvironment is at least partially responsible for the dramatic down-regulation of c-Kit in the bystander cells via transcriptional regulation.
Increased generation of ROS in the bystander cells and the effects of NAC. (A) An elevated level of ROS in bystander cells. Lin−Sca-1+ BM cells transplanted into IR or NR mice were collected 17 hours later and subjected to ROS measurement. Top panel: Representative flow chart. Bottom panel: Fold change of mean fluorescence intensity (MFI).**P < .01. n = 3. (B-C) Down-regulation of c-Kit expression at the protein and transcript levels by exposure to hydrogen peroxide. (B) c-Kit–enriched BM cells were exposed to different doses of hydrogen peroxide. Cells collected at different time points after exposure were subjected to c-Kit analysis by flow cytometry or RT-PCR. The relative expression of c-Kit at the protein level (B) and the transcript level (C) compared with nonexposed controls was plotted. (D) An elevated level of ROS reversed by administration of NAC in vitro. Lin−c-kit+ cells plated onto an IR (12 Gy) or NR stromal cell line (AFT024) layer treated with or without NAC were collected 40 hours later. The intracellular level of ROS was compared among groups. **P < .01. n = 3. (E) Improved reconstitution of bystander hematopoietic cells by administration of NAC in vivo. Homed Lin−c-kit+ cells in NR or IR recipients treated with or without NAC were sorted 17 hours after transplantation and retransplanted into lethally irradiated congenic recipients (3000 cells/mouse). Engraftment ratios of donor cells from IR recipients to that from NR recipients were calculated; n = 3. *P < .05. n = 4.
Increased generation of ROS in the bystander cells and the effects of NAC. (A) An elevated level of ROS in bystander cells. Lin−Sca-1+ BM cells transplanted into IR or NR mice were collected 17 hours later and subjected to ROS measurement. Top panel: Representative flow chart. Bottom panel: Fold change of mean fluorescence intensity (MFI).**P < .01. n = 3. (B-C) Down-regulation of c-Kit expression at the protein and transcript levels by exposure to hydrogen peroxide. (B) c-Kit–enriched BM cells were exposed to different doses of hydrogen peroxide. Cells collected at different time points after exposure were subjected to c-Kit analysis by flow cytometry or RT-PCR. The relative expression of c-Kit at the protein level (B) and the transcript level (C) compared with nonexposed controls was plotted. (D) An elevated level of ROS reversed by administration of NAC in vitro. Lin−c-kit+ cells plated onto an IR (12 Gy) or NR stromal cell line (AFT024) layer treated with or without NAC were collected 40 hours later. The intracellular level of ROS was compared among groups. **P < .01. n = 3. (E) Improved reconstitution of bystander hematopoietic cells by administration of NAC in vivo. Homed Lin−c-kit+ cells in NR or IR recipients treated with or without NAC were sorted 17 hours after transplantation and retransplanted into lethally irradiated congenic recipients (3000 cells/mouse). Engraftment ratios of donor cells from IR recipients to that from NR recipients were calculated; n = 3. *P < .05. n = 4.
To functionally demonstrate the role of ROS in mediating the negative effect, we examined how ROS reduction might minimize the bystander effect. NAC is a well-known antidote to acetaminophen toxicity that restores intracellular glutathione, a predominant antioxidant in the cytoplasm of cells.39 We cocultured sorted Lin−c-Kit+ cells on irradiated or nonirradiated BM stromal cells40 at 37°C, 5% CO2. Cells were then loaded with H2DCF-di and the anti-CD45 antibody to distinguish the input cells from stromal cells by flow cytometry. ROS generation in the input Lin−c-Kit+ cells was significantly higher on the irradiated stroma than that on nonirradiated stroma after coculture (Figure 6D). In contrast, no significant difference of ROS generation between the input Lin−c-Kit+ cells on the irradiated and nonirradiated stromal layers was seen when we treated the stromal cells with NAC. Further, we evaluated whether NAC can restore the compromised long-term repopulation of transplanted HSCs from IR recipients. We treated recipient mice with NAC at multiple time points before transplantation in the cBMT assay. Our data showed that the impaired long-term engraftment of the transplanted HSCs from IR hosts could be partially restored (Figure 6E), further supporting a role of the ROS pathway in the bystander effect of irradiated microenvironment on transplanted HSCs in vivo.
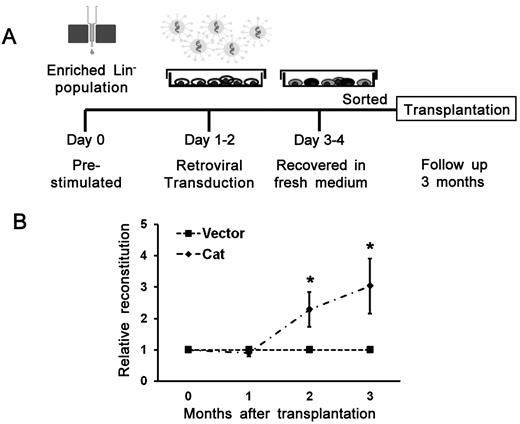
To provide direct evidence for a protective effect on the transplanted HSCs in the irradiated BM when intrinsic levels of ROS are reduced, an independent approach involving a genetic manipulaton of a major ROS-metabolizing enzyme, catalase (degrading H2O2 to H2O), was also used. We transduced hematopoietic cells with a recombinant retrovirus that ectopically expresses catalase (Figure 7A). As shown in Figure 7B, 3 months after transplantation, the relative repopulation ability was statistically higher (3.4-fold) in the catalase group than that of the control group. Taken together, these data demonstrate that ROS plays a critical role in the bystander effect, and its deleterious effect can be partially reversed by reduction of ROS in microenvironment or transplanted HSCs.
Improved engraftment of bystander hematopoietic cells by ectopically expressing catalase. (A) A schematic illustration of retrovirus generation, transduction into hematopoietic cells, and transplantation. (B) Improved engraftment by ectopically expressing catalase in cBMT assay. Catalase (Cat) overexpressed HSCs showed higher engraftment potential than that of control vector transduced cells. Data are mean ± SEM of relative ratios of catalase to control in the peripheral blood of 3 independent transplantation experiments. *P < .05. n = 19 in the vector group; n = 13 in the Cat group (3 experiments in total).
Improved engraftment of bystander hematopoietic cells by ectopically expressing catalase. (A) A schematic illustration of retrovirus generation, transduction into hematopoietic cells, and transplantation. (B) Improved engraftment by ectopically expressing catalase in cBMT assay. Catalase (Cat) overexpressed HSCs showed higher engraftment potential than that of control vector transduced cells. Data are mean ± SEM of relative ratios of catalase to control in the peripheral blood of 3 independent transplantation experiments. *P < .05. n = 19 in the vector group; n = 13 in the Cat group (3 experiments in total).
Discussion
Our current study demonstrates that the irradiated BM microenvironment has a negative impact on transplanted HSCs, and this impact may occur within 17 hours after transplantation, a time-frame during which HSCs have not begun to divide. We observed that, when c-transplanted into a new irradiated host, Lin−c-Kit+ enriched HSCs harvested 17 hours after infusion into IR recipients were significantly compromised in their ability to sustain long-term engraftment compared with the cells harvested after infusion into NR recipients (Figure 1). Although using an irradiated secondary recipient may also have a TBI effect on the host environment, the harvested cells from the 2 different groups of primary recipients (IR and NR) would encounter the same compounding effects. As such, the decreased engraftment ability of HSCs harvested after exposure to IR recipients should reflect the effect of the primary transplant host environment. This negative impact was not the result of an overt homing defect of transplanted HSCs (Figure 2); rather, it involved an increase of acute cell death associated with accelerated proliferation (Figure 3). In addition, we observed an ROS-induced rapid down-regulation of c-Kit at the transcriptional level (Figures 4 and 6), which is apparently independent from the potential effect of MMP9 activation (Figure 5C). In addition, although DNA damage in bystander HSCs, as assessed by γ-H2AX staining, was not increased shortly (< 17 hours) after transplantation, there was an increase of phosphorylated γ-H2AX in T and B cells 20 weeks after transplantation (supplemental Table 2). This suggests that the bystander effect may induce DNA damage, but in a delayed fashion in vivo, as suggested in previous studies.41 Taken together, these observations indicate that when TBI is included in the transplant conditioning regimen, the irradiated BM microenvironment causes a number of both immediate and delayed effects, which may be deleterious to transplanted HSCs and long-term hematopoiesis.
Although many molecules may be involved in mediating these negative bystander effects, our data (Figures 6 and 7) and previously published data35,36 indicate that ROS are important factors in these processes. Ito et al previously demonstrated that Atm−/− mice, where HSCs were exposed to elevated ROS levels, had premature BM failure resulting from a defect in HSC regeneration.42 This raises the possibility that exposure of the transplanted bystander HSCs to ROS after TBI administration, as we observed in our current study, could result in premature BM failure after transplantation using TBI-containing conditioning regimens. This could be of important clinical relevance, particularly in the pediatric population where transplanted HSCs need to survive and generate hematopoiesis for a number of decades. A second potential implication of our observations is that the bystander effects could also cause DNA damage in the transplanted hematopoietic cells, which could contribute donor-derived myelodysplasia and leukemia. This has been previously reported and may be significantly under-reported as in most cases, testing to determine whether leukemia occurring after allogeneic transplant is donor or recipient in origin is not performed.43 In addition, most persons undergoing allogeneic transplant have done so only in the last 30 years, and there are surprisingly little data available on long-term donor engraftment or very late effects in these patients, so studying these potential issue further in both the clinical setting and in preclinical models appears warranted. In future studies, it would also be important to compare chemotherapy conditioning regimens to TBI-containing regimens to determine whether any bystander effects are specific to TBI or may be more generic.
An additional clinical concern raised in our studies is related to ROS-mediated down modulation of c-Kit. It is widely recognized that c-Kit signaling is critical for many physiologic functions of hematopoietic cells, including self-renewal and proliferation, and may be involved in HSC homing and engraftment as well.44,45 Although we observed no significant alteration of overall homing of hematopoietic cells in BM and localization of the cells to the endosteal niche area (Figure 2), the complete enumeration of multiple types of dynamic HSC niches in BM is not feasible given the current technologies and controversies.46,47 Consequently, it remains possible that c-Kit down regulation could interfere with an effective interaction between homed HSCs and their niches, thereby in part compromising the ability of the newly arrived donor HSCs, whose optimal functions are dependent on their cross-talk with the niche.48 In addition, effects of down-regulated c-Kit on HSC proliferation and self-renewal could also have long-term consequences.
Although this negative bystander effect may not be prominent in allogeneic transplants performed with BM or peripheral blood stem cell sources where HSC numbers are plentiful, it could be an important consideration in cord blood transplants where the number of engrafting HSCs is relatively low, particularly in adults.49 If the bystander effect on HSCs is confirmed to cause late effects or impair engraftment with limiting number of HSCs, then our studies also suggest that treatment with NAC or other antioxidants help mitigate this effect. Alternatively, inhibitors of p38 MAPK may also be useful to abrogate bystander effects as p38 MAPK is a downstream mediator of ROS in HSCs which mediates some of their deleterious effects on hematopoiesis.39 Another possible route could be ectopic genetic expression of catalase as this abrogated many of the bystander effects in our studies as well (Figure 7) and has previously been reported to improve HSC self-renewal in long-term hematopoietic cell cultures.50
In conclusion, mechanisms for the negative impact of irradiated hosts on transplanted HSCs appear to be multifactorial. However, the connections between these molecular events remain to be further defined. Especially, the manner in which remote factors versus local microenvironmental cues contribute to the bystander effect and how it is potentially related to genomic stability of HSCs or their progeny are important issues for future investigations. Nevertheless, our current study demonstrates that negative bystander effects occur on transplanted HSCs in a very short time frame after transplantation when TBI is used as part of the conditioning regimen. In addition, our results offer some key molecular targets for potential pharmacologic interventions that may improve the short- and long-term efficacy of HSCT. In addition, when HSCs are evaluated in irradiated hosts, the bystander effects on transplanted HSCs should be taken into consideration for experimental design and data interpretation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Saonan Cao for technical assistance in some experiments and Drs Joel Greenberger and Weimin Miao for their helpful comments on this work.
This work was supported by the National Program on Key Basic Research Project (2011CB964801), National Institutes of Health (NIH grants AI080424 and HL070561), and National Science Foundation of China (NSFC; research grant 81090410). T.C. was a recipient of the Scholar Award from the Leukemia & Lymphoma Society (1027-08) and the Outstanding Young Scholar Award from the NSFC (30825017).
National Institutes of Health
Authorship
Contribution: H.S. designed the experiment, performed flow cytometry, analyzed the data, and prepared the manuscript; H.Y. performed bone marrow sampling, immunostaining, long-term culture, and transplantation, prepared the manuscript, and summarized the data; P.H.L. cared for animals, performed bone marrow sampling immune staining, summarized the data, and edited the manuscript; H.C. provided assistance in ROS and cytokine assays; R.X. performed retroviral transduction; Y.Y. performed immunostaining and transplantation; P.Z. performed bone marrow transplantation; C.A.S. provided input on clinical relevance and edited the manuscript; and T.C. performed overall research design, analyzed the data, and prepared the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Tao Cheng, Hillman Cancer Center Research Pavilion, 5117 Center Ave, Office Suite 2.42e, Pittsburgh, PA 15213-1863; e-mail: chengt@upmc.edu or chengtao@ihcams.ac.cn.
References
Author notes
H.S. and H.Y. contributed equally to this study.