In this issue of Blood, Jian and coworkers report on a gain-of function variant of ADAMTS13 that is resistant to the autoantibodies responsible for acquired thrombotic thrombocytopenic purpura (TTP).1
Auto-antibodies directed toward ADAMTS13 prohibit cleavage of von Willebrand factor (VWF) resulting in systemic platelet aggregation in the microcirculation. Our knowledge on the etiology of the misrouted immune response at the onset of acquired idiopathic TTP is still very limited. A genetic predisposition is suggested by the observation of severe autoantibody-mediated ADAMTS13 deficiency leading to acute TTP episodes in identical twin sisters2 and the over-representation of the HLA-DRB1*11 allele in TTP patients.3 A number of different bacterial and viral infections preceding a first acute episode or relapse have been reported (reviewed by Pos et al4 ), although specific triggers have not been identified yet, molecular mimicry as documented in other autoimmune disorders cannot be excluded. Moreover, a recent study suggests that ADAMTS13 is efficiently internalized by antigen-presenting cells, thereby potentially contributing to initiation of CD4+ T-cell responses to ADAMTS13 in previously healthy individuals.5
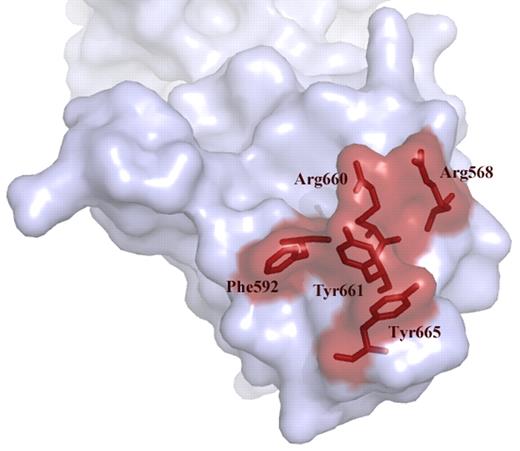
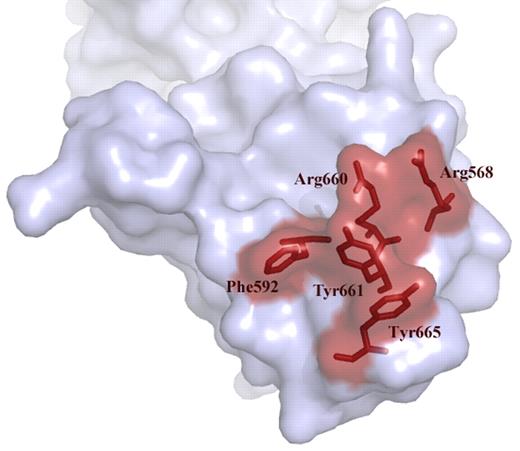
Over the past years we have learned that a major binding site for antibodies resides in the spacer domain (reviewed in Pos et al4 ). Detailed mutagenesis studies pointed at an exposed surface area in the spacer domain composed of Arg660, Tyr661, and Tyr665 as being a crucial part of the epitope.6 Examination of a large panel of plasma from patients with acquired TTP revealed that Arg568 and Phe592 also contribute to the binding of anti-ADAMTS13 antibodies.7 Progressive replacement of residues Arg568, Phe592, Arg660, Tyr661, and Tyr665 for Ala reduced antibody binding to the spacer domain (see figure).7 Building on these results, Jian and colleagues made conservative changes within these 5 residues. Unexpectedly, the resulting “M5-variant” exhibited a 10- to 12-fold increase in activity.1 In excellent agreement with earlier results from Pos and coworkers, the M5-variant was resistant to inhibition of a panel of autoantibodies from acquired idiopathic TTP patients.1,7 The gain-of-function and autoantibody-resistant ADAMTS13 variant provides perspective of a novel therapeutic avenue for treatment of patients with acquired TTP.
Exposed VWF binding surface in the spacer domain comprising residues Arg568, Phe592 Arg661, Tyr660, Tyr661, and Tyr665. These residues are targeted by autoantibodies that develop in patients with acquired TTP. Conservative changes in these 5 residues result in a gain-of-function ADAMTS13 variant.
Exposed VWF binding surface in the spacer domain comprising residues Arg568, Phe592 Arg661, Tyr660, Tyr661, and Tyr665. These residues are targeted by autoantibodies that develop in patients with acquired TTP. Conservative changes in these 5 residues result in a gain-of-function ADAMTS13 variant.
The mainstay of treatment of acquired idiopathic TTP is daily plasma exchange with replacement of plasma until normalization of platelet count and lactate dehydrogenase levels as well as stabilization of clinical symptoms is achieved. Suppression of anti-ADAMTS13 autoantibody formation is attempted by the addition of corticosteroids and more and more by the administration of the monoclonal anti-CD20 antibody rituximab. During the past 20 years the mortality rate of acute TTP bouts has remained in the range of approximately 20%8 and morbidity of treatment complications is considerable. The gain-of-function and autoantibody-resistant ADAMTS13 variant thus provides perspective of therapeutic potential in acquired (and hereditary) TTP. Using recombinant ADAMTS13, Plaimauer et al recently demonstrated the existence of a linear relationship between inhibitor titer and recombinant ADAMTS13 necessary to restore 50% of VWF-cleaving activity in plasma of acquired TTP patients in vitro and that the required amount (∼ 0.5 U recombinant ADAMTS13 per mL of plasma and inhibitor titer) is within technical reach.9 The gain-of-function variant M5 of Jian et al should be even better off, as the primary step of neutralizing inhibitory antibodies before restoring proteolytic activity wouldn't be required. In 20% to 30% of patients with acquired TTP, anti–TSP2-8 and anti–CUB1-2 antibodies are present,7,10 which would neutralize or clear any recombinant or plasma-derived ADAMTS13 variant similarly. Although immune complexes are generally cleared rapidly, their continuous formation may eventually exceed the body's clearance capacity, upholding a proinflammatory state with an activated endothelium and release of the ADAMTS13 substrate, large VWF multimers, and possibly involvement of other organs. This may hamper the use of recombinant ADAMTS13 (wild-type or gain-of-function mutant) as a single agent in acquired TTP.
Finally, the phenomenon of “inhibitor boosting” that occurs in about half of patients treated with plasma exchange and whose underlying mechanism has not been elucidated yet needs to be considered. After an initial improvement of clinical symptoms and laboratory parameters, platelets fall again despite continued daily plasma exchange, sometimes in association with new neurologic symptoms and/or signs of cardiac ischemia. In a number of cases a significant rise in ADAMTS13 inhibitor titers is observed at the same time. The administration of exogenous ADAMTS13 may very well provoke the differentiation of ADAMTS13-specific memory B cells into antibody-producing plasma cells, although the experience of patients diagnosed with severe congenital ADAMTS13 deficiency on regular prophylactic plasma infusions—worldwide about 150 affected patients (at least one-third on regular plasma treatment) and so far no report of a single case with the formation of inhibitory allo-antibodies—suggests that plasma-derived ADAMTS13 per se is not very immunogenic, at least not in hereditary TTP.
Jian and coworkers clearly show that modification of the VWF A2 binding surface in the spacer domain results in an impressive enhancement of VWF cleavage. Therefore, their elegant report provides an excellent basis for future studies aimed at improving the catalytic activity of ADAMTS13 toward VWF. This may not only be relevant for treatment of congenital and acquired TTP; gain-of-function variants of ADAMTS13 might also be potentially useful as a novel treatment regimen for other thrombotic disorders such as stroke.
Conflict-of-interest-disclosure: J.A.K.H. is a consultant for Baxter Healthcare for the development of rADAMTS13 and receives a grant for the investigator-driven study on hereditary TTP (www.ttpregistry.net; registered at www.clinicaltrials.gov as NCT01257269) from the same company. J.V. declares no competing financial interests. ■