Abstract
Thrombotic thrombocytopenic purpura (TTP) is primarily caused by immunoglobulin G (IgG) autoantibodies against A Disintegrin And Metalloprotease with ThromboSpondin type 1 repeats, 13 (ADAMTS13). Nearly all adult idiopathic TTP patients harbor IgGs, which bind the spacer domain of ADAMTS13, a region critical for recognition and proteolysis of von Willebrand factor (VWF). We hypothesize that a modification of an exosite in the spacer domain may generate ADAMTS13 variants with reduced autoantibody binding while preserving or enhancing specific activity. Site-directed mutagenesis was used to generate a series of ADAMTS13 variants, and their functional properties were assessed. Of 24 novel ADAMTS13 variants, 2 (ie, M4, R660K/F592Y/R568K/Y661F and M5, R660K/F592Y/R568K/Y661F/Y665F) exhibited increased specific activity approximately 4- to 5-fold and approximately 10- to 12-fold cleaving a peptide VWF73 substrate and multimeric VWF, respectively. More interestingly, the gain-of-function ADAMTS13 variants were more resistant to inhibition by anti-ADAMTS13 autoantibodies from patients with acquired idiopathic TTP because of reduced binding by anti-ADAMTS13 IgGs. These results shed more light on the critical role of the exosite in the spacer domain in substrate recognition. Our findings also help understand the pathogenesis of acquired autoimmune TTP. The autoantibody-resistant ADAMTS13 variants may be further developed as a novel therapeutic for acquired TTP with inhibitors.
Introduction
ADAMTS13 (A Disintegrin And Metalloprotease with ThromboSpondin type 1 repeats, 13) cleaves ultra large (UL) von Willebrand factor (VWF) on endothelial cells,1 soluble VWF in the flowing blood,2,3 and VWF adhering to sites of injury where VWF-rich platelet thrombi are formed.4-6 This cleavage by ADAMTS13 is highly specific, occurring at the Tyr1605-Met1606 bond in the A2 domain.7 In vivo, fluid shear stress accelerates the cleavage of cell bound ULVWF1,8 and soluble VWF multimers in circulation.2,3 In vitro, addition of a denaturant, such as urea9 or guanidine,7 also markedly accelerates the proteolytic cleavage of soluble VWF by ADAMTS13. These findings greatly facilitate the development of various biochemical assays for assessing ADAMTS13 activity.
The importance of VWF proteolysis by ADAMTS13 is highlighted by the development of a potentially fatal syndrome, thrombotic thrombocytopenic purpura (TTP), when plasma ADAMTS13 activity is severely deficient. This can result from either hereditary mutations of ADAMTS13 gene10 or acquired formation of autoantibodies that inhibit plasma ADAMTS13 activity.11-13 Nearly all adult idiopathic TTP patients with severely deficient plasma ADAMTS13 activity harbor polyclonal immunoglobulin Gs (IgGs) that bind the Cys-rich and spacer domains, particularly the spacer domain of ADAMTS13.13-17 Recent studies have shown that exosite 3 (ie, Y659-Y665) and several other adjacent amino acid residues (ie, R568 and F592) in the spacer domain compose a major antigenic epitope for IgG autoantibodies in idiopathic TTP.18,19 This region is also found to play an essential role in proteolytic cleavage of VWF under various conditions6,20-24 and modulation of arterial thrombus formation in vivo.6
In the present study, we test a hypothesis that a modification of the exosite 3 in the spacer domain might create an ADAMTS13 variant with reduced binding and inhibition by autoantibodies from patients with acquired idiopathic TTP while preserving or enhancing its specific activity. To this aim, a series of recombinant ADAMTS13 variants were engineered by replacing several surface charged/hydrophobic residues in the exosite 3 with those having similar chemical structures. Proteolytic activity and sensitivity of the novel variants to patient anti-ADAMTS13 autoantibodies were assessed. Of 24 novel ADAMTS13 variants, 2 exhibit dramatically enhanced specific activity but are more resistant to inhibition by a panel of autoantibodies from acquired idiopathic TTP patients. These results indicate that the novel gain-of-function and autoantibody-resistant ADAMTS13 variants may be further developed for therapy of acquired idiopathic TTP patients with inhibitors.
Methods
Constructs
QuickChange site-directed mutagenesis regents from Stratagene were used to replace one or a clustered of surface charged amino acid residues (R660, F592, R568, Y661, and Y665) in the β9-β10 variable region of the spacer domain. A pcDNA3.1 vector containing wild-type (WT) ADAMTS13-V5-His, as described previously,23 was used as a template. The resulting variants with a desired mutation or mutations were sequenced to confirm the accuracy at the Nucleic Acid Core Facility, Children's Hospital of Philadelphia.
Preparations of recombinant WT ADAMTS13 and variants
COS-7 cells were transfected with plasmid and polyethylenimine (PEI) according to the manufacturer's instruction (Advanced Cell System). Serum-free conditioned medium was collected 4 days after transfection and concentrated 50 to 100 times using a filtration column (Millipore) in the presence of 1% protease inhibitor cocktail (Sigma-Aldrich).
ELISA
The concentrations of WT ADAMTS13 and variants in the concentrated conditioned medium were determined by an in-house ELISA. Briefly, a high-binding microtiter plate was coated with 100 μL of monoclonal anti-disintegrin IgG (40 μg/mL; custom-made in Green Mountain Antibody) overnight. The remaining binding sites were blocked for 30 minutes with 150 μL/well of 2.5% BSA in PBS. WT and variants diluted with PBS were added and incubated for 2 hours. After being washed with PBS, monoclonal anti–V5-HRP IgG (Invitrogen; 1:1000) was added for detection. Purified ADAMTS13 was used as a calibration. Each quantitation was repeated 3 times for consistency.
Western blot
The integrity of WT and variants in the concentrated conditioned medium were assessed by Western blotting after fractionation on 8% SDS-polyacrylamide gel under reduced conditions as described previously.23 After being transferred to a nitrocellulose membrane, recombinant WT and variants were blotted by anti-V5 IgG (Invitrogen; 1:5000) and IRdye800CW-labeled goat anti–mouse IgG (1:20 000; LI-COR) in 20mM Tris-HCl, 150mM NaCl containing 0.05% Tween-20 and 1% casein (TBSTc). The fluorescent signals obtained with an Odyssey imaging system (LI-COR) were converted to gray images.
Proteolytic cleavage of a VWF73 peptide
A 5′-maleimide fluorescein-labeled recombinant VWF73 peptide (rF-VWF73; 2μM) previously described25,26 was incubated with WT and variants (0.2nM) in 5mM Bis-Tris, pH 6.0, containing 25mM CaCl2 and 0.005% Tween-20 in a 96-well white plate (Corning). The rate of fluorescence generation was monitored at 37°C with a SpectroMax fluorescent microtiter plate reader (Molecular Devices; Ex/Em 485/535 nm) every minute for 30 minutes. Normal human plasma from George Kings Biotech was used as a reference.
Proteolytic cleavage of multimeric VWF under denaturing conditions
Purified plasma VWF (37.5 μg/mL or 150nM) was incubated with WT and variants in the conditioned medium (0.2nM and 0.04nM) at 37°C for 4 hours on a dialysis membrane (0.25 μm, pore size) floating over 50 mL dialysis buffer (10mM Tris-HCl, pH 8.0, containing 1.5M urea in a conical tube). The digested materials were withdrawn and denatured with a multimer sample buffer (70mM Tris-HCl, pH 6.5, 2.4% SDS, 0.67M urea, and 4mM EDTA) at 60°C for 20 minutes. The denatured VWF was fractionated with 1% (weight/volume) SeaKem HGT agarose gel (Cambrex). The proteins were then transferred onto a nitrocellulose membrane, blocked with TBSTc, and incubated with anti-VWF IgG (Dako North America; 1:5000) in TBSTc. After being washed with TBST 3 times, the bound primary antibody was detected by an IRDye 800CW-labeled goat anti–rabbit IgG (LI-COR; 1:10 000) in TBSTc, followed by Odyssey imaging system (LI-COR) as described previously.6,27
TTP patient plasma
This study was approved by the Internal Review Boards at the Children's Hospital of Philadelphia and the University of Pennsylvania, as well as Northwestern University. Informed consent was obtained from each participant before blood was drawn in accordance with the Declaration of Helsinki. Blood (5 mL) was collected into a tube containing 3.8% sodium citrate and centrifuged at 1500g for 15 minutes at 25°C. Plasma was collected and stored at −80°C. All patients (n = 12) included in the study had acquired idiopathic TTP. Patients did not have other concurrent conditions, such as hematopoietic progenitor cell transplantation, cancer, drugs, and pregnancy, which were known to cause secondary TTP. The median age of TTP patients was 43 years, with a female-to-male ratio of 3:1. The median platelet count, hematocrit, and serum levels of lactate dehydrogenase were 15.5 × 109/L, 23.5%, and 1242 units/L, respectively. Central nervous system symptoms were present in 10 of 12 patients. Renal insufficiency (creatinine > 1.4 mg/dL) was only present in 3 of 12 patients. All patients exhibited severely deficient plasma ADAMTS13 activity (< 5% of normal), and positive inhibitors detected by a 50:50 mixing study with peptide-based and multimer assays. An anti-ADAMTS13 IgG ELISA from American Diagnostica was used to confirm the presence of IgG autoantibodies in all patients (Table 1).
Clinical characteristics, plasma ADAMTS13 activity, inhibitors of TTP patients, and sensitivity of WT ADAMTS13 and novel variants to the inhibition by patient plasma
| Patient no. . | Age, y . | Sex . | Platelet count, × 109/L . | Hemato-crit, % . | LDH, U/L . | Creatinine, mg/dL . | CNS, signs and symptoms . | ADAMTS13 activity by rF-vWF73, % . | Plasma anti-ADAMTS13 inhibitors . | Plasma anti-ADAMTS13IgG, U/mL . | Sensitivity to patient plasma inhibition . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WT . | M1 . | M2 . | M3 . | M4 . | M5 . | |||||||||||
| 1 | 44 | Female | 15 | 23 | 695 | 1.1 | Yes | < 5 | + | 84.2 | ++ | ++ | ++ | + | − | − |
| 2 | 56 | Female | 19 | 29 | 654 | 1.1 | Yes | < 5 | + | 35.4 | ++ | ++ | ++ | + | + | + |
| 3 | 45 | Female | 88 | 28 | 995 | 0.7 | Yes | < 5 | + | 81.9 | ++ | ++ | ++ | − | − | − |
| 4 | 52 | Female | 52 | 20 | 866 | 1.1 | Yes | < 5 | + | 132.0 | ++ | ++ | ++ | − | − | − |
| 5 | 79 | Female | 7 | 27 | 1660 | 1.4 | Yes | < 5 | + | 118.0 | ++ | ++ | ++ | + | − | − |
| 6 | 34 | Male | 15 | 23 | 2703 | 0.9 | Yes | < 5 | + | 127.5 | ++ | ++ | ++ | + | − | − |
| 7 | 34 | Male | 0 | 33 | 859 | 1.9 | No | < 5 | + | 162.0 | ++ | ++ | ++ | − | − | − |
| 8 | 23 | Female | 11 | 18 | 3412 | 0.8 | Yes | < 5 | + | 132.0 | ++ | ++ | ++ | − | − | − |
| 9 | 42 | Female | 23 | 17 | 3259 | 0.8 | Yes | < 5 | + | 168.0 | ++ | ++ | ++ | − | − | − |
| 10 | 21 | Female | 9 | 13 | 1489 | 1.5 | Yes | < 5 | + | 72.0 | ++ | ++ | ++ | + | + | + |
| 11 | 61 | Female | 33 | 28 | 511 | 0.8 | Yes | < 5 | + | 221.0 | ++ | ++ | ++ | − | − | − |
| 12 | 42 | Male | 16 | 24 | 6517 | 1.2 | No | < 5 | + | 147.2 | ++ | ++ | ++ | + | − | − |
| N = 12 | 43* | 15.5* | 23.5* | 1242* | 1.1* | (83.3) | (100) | (100) | (100) | (100) | (100) | (100) | (50) | (17) | (17) | |
| Patient no. . | Age, y . | Sex . | Platelet count, × 109/L . | Hemato-crit, % . | LDH, U/L . | Creatinine, mg/dL . | CNS, signs and symptoms . | ADAMTS13 activity by rF-vWF73, % . | Plasma anti-ADAMTS13 inhibitors . | Plasma anti-ADAMTS13IgG, U/mL . | Sensitivity to patient plasma inhibition . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WT . | M1 . | M2 . | M3 . | M4 . | M5 . | |||||||||||
| 1 | 44 | Female | 15 | 23 | 695 | 1.1 | Yes | < 5 | + | 84.2 | ++ | ++ | ++ | + | − | − |
| 2 | 56 | Female | 19 | 29 | 654 | 1.1 | Yes | < 5 | + | 35.4 | ++ | ++ | ++ | + | + | + |
| 3 | 45 | Female | 88 | 28 | 995 | 0.7 | Yes | < 5 | + | 81.9 | ++ | ++ | ++ | − | − | − |
| 4 | 52 | Female | 52 | 20 | 866 | 1.1 | Yes | < 5 | + | 132.0 | ++ | ++ | ++ | − | − | − |
| 5 | 79 | Female | 7 | 27 | 1660 | 1.4 | Yes | < 5 | + | 118.0 | ++ | ++ | ++ | + | − | − |
| 6 | 34 | Male | 15 | 23 | 2703 | 0.9 | Yes | < 5 | + | 127.5 | ++ | ++ | ++ | + | − | − |
| 7 | 34 | Male | 0 | 33 | 859 | 1.9 | No | < 5 | + | 162.0 | ++ | ++ | ++ | − | − | − |
| 8 | 23 | Female | 11 | 18 | 3412 | 0.8 | Yes | < 5 | + | 132.0 | ++ | ++ | ++ | − | − | − |
| 9 | 42 | Female | 23 | 17 | 3259 | 0.8 | Yes | < 5 | + | 168.0 | ++ | ++ | ++ | − | − | − |
| 10 | 21 | Female | 9 | 13 | 1489 | 1.5 | Yes | < 5 | + | 72.0 | ++ | ++ | ++ | + | + | + |
| 11 | 61 | Female | 33 | 28 | 511 | 0.8 | Yes | < 5 | + | 221.0 | ++ | ++ | ++ | − | − | − |
| 12 | 42 | Male | 16 | 24 | 6517 | 1.2 | No | < 5 | + | 147.2 | ++ | ++ | ++ | + | − | − |
| N = 12 | 43* | 15.5* | 23.5* | 1242* | 1.1* | (83.3) | (100) | (100) | (100) | (100) | (100) | (100) | (50) | (17) | (17) | |
Values in parentheses indicate positive rates in percentages.
LDH indicates lactate dehydrogenase; −, negative inhibition (< 10% reduction in activity); +, mild inhibition (10%-30% reduction in activity); ++, moderate inhibition (30%-50% reduction in activity); and +++, strong inhibition (> 50% reduction in activity) after 50:50 mixing with patient plasma.
Median values.
Inhibition of cleavage of VWF by autoantibodies from TTP patients
Recombinant WT and variants (0.2nM) were incubated at 25°C for 30 minutes with 35μM of human monoclonal antibody against the spacer domain of ADAMTS13 previously isolated from a patient with acquired idiopathic TTP (mAb II-1)6,14-16 (kindly provided by Dr Jan Voorberg at Sanquin-AMC Landsteiner Laboratory, Amsterdam, The Netherlands) or with heat-inactivated (56°C for 60 minutes) normal human plasma or patient plasma (2.5-10 μL) in PBS for 30 minutes. The residual proteolytic activity was determined by the cleavage of VWF multimers using agarose gel electrophoresis and Western blotting as described previously.6,27 The percentage of inhibition was determined by comparing the residual activity in WT and variants after incubation with control plasma versus patient plasma.
Binding of patient anti-ADAMTS13 IgGs to WT and variants
A modified immunoprecipitation protocol plus Western blotting was used to detect the antigen-antibody interaction in solution as described previously.13,19 WT and variants (50 ng) were incubated 5 to 10 μL of normal human plasma or patient plasma and 30 μL of protein A/G-Sepharose 4B (Invitrogen) in 50mM Tris-HCl, pH 7.6, containing 0.15M NaCl, 1% BSA, 1% Triton X-100, and 0.1% Tween-20 at 4°C, overnight. After being washed with same buffer, bound recombinant WT and variants were eluted from the beads and determined by Western blotting with anti-V5 IgG (Invitrogen; 1:5000) in TBSTc, followed by IRDye 800CW-labeled goat anti–mouse IgG (1:20 000; LI-COR) as previously described.13
Model of ADAMTS13 and VWF interaction
The interaction between ADAMTS13-spacer domain and VWF-A2 was modeled using the HHPred server plugin in PyMol software (http://www.pymol.org). The supplemental Videos (available on the Blood Web site; see the Supplemental Materials link at the top of the online article) were made with Adobe Photoshop CS 5 software.
Results
Identification of the optimal residue at position of 660 for ADAMTS13 activity
We28 and others18 have previously shown that R660 in the spacer domain of ADAMTS13 plays an essential role for substrate recognition. A substitution of arginine at the position of 660 with alanine (R660A) nearly abolished proteolytic activity toward various substrates.18,28 To determine the optimal residue at this position, we prepared a series of ADAMTS13 variants by replacing the R with 18 other amino acid residues. The resulting constructs were transiently expressed in COS-7 cells, which ran at approximately 195 kDa with little degradation on an SDS-polyacrylamide gel under reduced condition (Figure 1A). The specific activity was assessed by the cleavage of rF-VWF73 and multimeric VWF. A replacement of R660 with any other residues except for K (M1, R660K) resulted in dramatically reduced cleavage of rF-VWF73 (Figure 1B) and multimeric VWF (Figure 1C). These results suggest that a positively charged residue, such as arginine or lysine at position 660 in the spacer domain, is required for ADAMTS13 activity.
Western blot and proteolytic activity of ADAMTS13 and mutants. (A) Western blotting with anti-V5 IgG detects WT and single point mutants at the position 660 in the concentrated condition medium (∼ 50nM per lane). Arrowhead indicates the intact full-length ADAMTS13, ∼ 195 kDa; and double stars, degradation product. (B) Relative proteolytic activity (%) of WT and single point variants assessed by the cleavage of rF-VWF73. Data are mean ± SD (n = 3). All ADAMTS13 mutants except for R660K had relative activity less than 20% of WT. (C) Proteolytic cleavage of multimeric VWF by ADAMTS13 and single point mutants under denaturing conditions. Plasma-derived VWF (37.5 μg/mL or 150nM) was incubated at 37°C with 0.2nM of recombinant WT-ADAMTS13 and point mutants in the presence of 1.5M urea for 4 hours. The proteolytic cleavage of VWF was determined by 1% agarose gel electrophoresis and Western blotting. + indicates the presence of 10mM EDTA in the reaction; −, the absence of EDTA in the reaction; HMW, high molecular weight multimers; and P, cleavage product.
Western blot and proteolytic activity of ADAMTS13 and mutants. (A) Western blotting with anti-V5 IgG detects WT and single point mutants at the position 660 in the concentrated condition medium (∼ 50nM per lane). Arrowhead indicates the intact full-length ADAMTS13, ∼ 195 kDa; and double stars, degradation product. (B) Relative proteolytic activity (%) of WT and single point variants assessed by the cleavage of rF-VWF73. Data are mean ± SD (n = 3). All ADAMTS13 mutants except for R660K had relative activity less than 20% of WT. (C) Proteolytic cleavage of multimeric VWF by ADAMTS13 and single point mutants under denaturing conditions. Plasma-derived VWF (37.5 μg/mL or 150nM) was incubated at 37°C with 0.2nM of recombinant WT-ADAMTS13 and point mutants in the presence of 1.5M urea for 4 hours. The proteolytic cleavage of VWF was determined by 1% agarose gel electrophoresis and Western blotting. + indicates the presence of 10mM EDTA in the reaction; −, the absence of EDTA in the reaction; HMW, high molecular weight multimers; and P, cleavage product.
Identification of gain-of-function ADAMTS13 variants
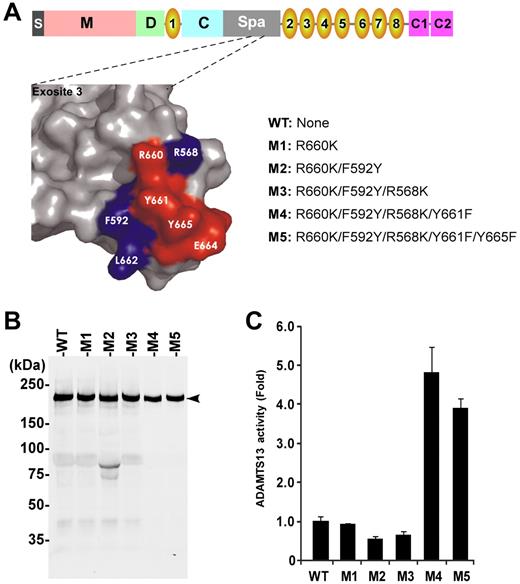
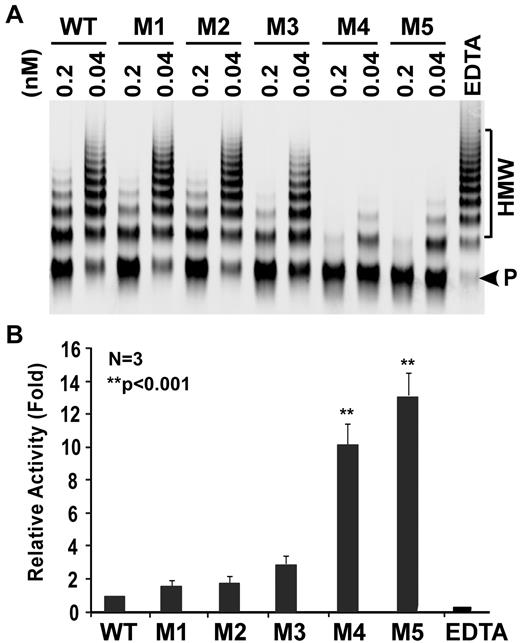
Based on our preliminary results described in the previous section, we performed additional site-directed mutagenesis experiments by sequentially replacing several other charged/hydrophobic residues on the surface loop in the spacer domain (ie, R660, F592, R568, Y661, and Y665) with K, Y, K, F, and F, respectively (Figure 2A). The resulting ADAMTS13 variants (ie, M2, M3, M4, and M5) were expressed in COS-7 cells. All ran at approximately 195 kDa on SDS-polyacrylamide gel under reduced conditions (Figure 2B). The specific activity was assessed by the cleavage of both rF-VWF73 and multimeric VWF. The variants M1, M2, and M3 exhibited similar activity to WT in cleaving VWF73 pep-tide (Figure 2C) and multimeric VWF (Figure 3). However, the variants M4 and M5 exhibited increased specific activity by approximately 4- to 5-fold (P < .001) and approximately 10- to 12-fold (P < .001) in cleaving rF-VWF73 peptide (Figure 2C) and multimeric VWF (Figure 3), respectively. These results demonstrate, for the first time, that gain-of-function ADAMTS13 variants can be engineered and identified through a modification of exosite 3 in the spacer domain.
Characterization of single and compound ADAMTS13 variants. (A) Schematic domain organization of full-length ADAMTS13 showing a signal peptide (S), metalloprotease domain (M), disintegrin domain (D), 8 TSP1 repeats (1-8), Cys-rich domain (C) and spacer domain (Spa), as well as 2 CUB domains (C1 and C2; top), surface representation of exosite 3 and adjacent residues in the spacer domain of ADAMTS13 (left), and names of various ADAMTS13 variants with amino acid substitution (right). (B) Western blotting with anti-V5 detects recombinant WT and variants in the conditioned medium (50 ng per lane). Arrowhead indicates full-length protein of ADAMTS13 WT and variants (∼ 195 kDa) with little degradation. (C) Relative specific activity of ADAMTS13 variants compared with WT. Data are mean ± SD (n = 3). **P < .001, statistically highly significant.
Characterization of single and compound ADAMTS13 variants. (A) Schematic domain organization of full-length ADAMTS13 showing a signal peptide (S), metalloprotease domain (M), disintegrin domain (D), 8 TSP1 repeats (1-8), Cys-rich domain (C) and spacer domain (Spa), as well as 2 CUB domains (C1 and C2; top), surface representation of exosite 3 and adjacent residues in the spacer domain of ADAMTS13 (left), and names of various ADAMTS13 variants with amino acid substitution (right). (B) Western blotting with anti-V5 detects recombinant WT and variants in the conditioned medium (50 ng per lane). Arrowhead indicates full-length protein of ADAMTS13 WT and variants (∼ 195 kDa) with little degradation. (C) Relative specific activity of ADAMTS13 variants compared with WT. Data are mean ± SD (n = 3). **P < .001, statistically highly significant.
Proteolytic cleavage of multimeric VWF by ADAMTS13 and variants under denaturing conditions. (A) Human plasma-derived VWF (37.5 μg/mL or 150nM) predenatured with 1.5M urea was mixed with WT, M1, M2, M3, M4, and M5 (0.04nM and 0.2nM) in the absence or in the presence of EDTA (10mM; last lane) and dialyzed against 10mM Tris-HCl, pH 8.0, containing 1.5M urea at 37° for 4 hours. The cleavage of VWF was determined by agarose (1%) gel electrophoresis and Western blotting with rabbit anti-VWF IgG (1:5000), followed by IRDye 800CW-labeled goat anti–rabbit IgG (1:10 000). (B) The relative activity was determined by ImageJ quantitation of the ratio of cleavage product (P, arrowhead) to high molecular weight (HMW) VWF multimers in each sample. The specific activity was normalized to that of WT (1 arbitrary unit). Data are mean ± SEM from 3 independent experiments (n = 3).
Proteolytic cleavage of multimeric VWF by ADAMTS13 and variants under denaturing conditions. (A) Human plasma-derived VWF (37.5 μg/mL or 150nM) predenatured with 1.5M urea was mixed with WT, M1, M2, M3, M4, and M5 (0.04nM and 0.2nM) in the absence or in the presence of EDTA (10mM; last lane) and dialyzed against 10mM Tris-HCl, pH 8.0, containing 1.5M urea at 37° for 4 hours. The cleavage of VWF was determined by agarose (1%) gel electrophoresis and Western blotting with rabbit anti-VWF IgG (1:5000), followed by IRDye 800CW-labeled goat anti–rabbit IgG (1:10 000). (B) The relative activity was determined by ImageJ quantitation of the ratio of cleavage product (P, arrowhead) to high molecular weight (HMW) VWF multimers in each sample. The specific activity was normalized to that of WT (1 arbitrary unit). Data are mean ± SEM from 3 independent experiments (n = 3).
Identification of ADAMTS13 variants resistant to autoantibodies in TTP patients
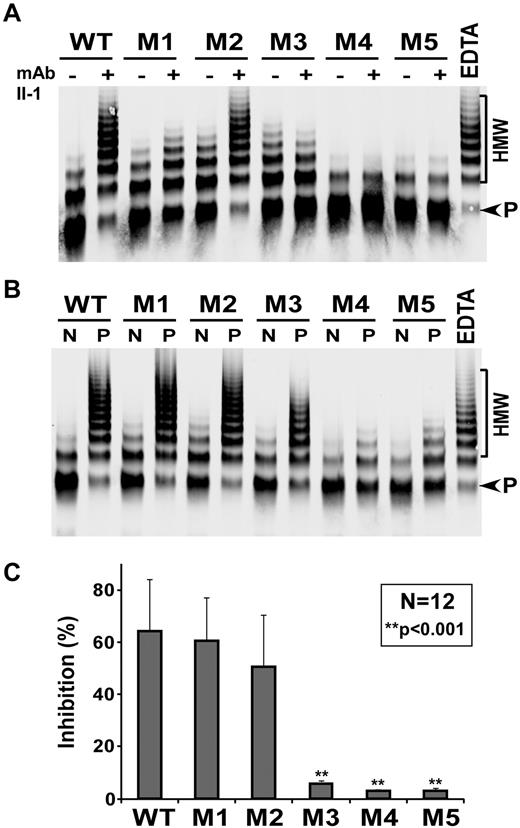
Previous studies have shown that exosite 3 along with several other adjacent residues in the spacer domain contains major binding sites for anti-ADAMTS13 autoantibodies in patients with acquired idiopathic TTP.18,19 We therefore hypothesized that a modification in this region may alter the binding and inhibition of ADAMTS13 variants by patients' anti-ADAMTS13 autoantibodies. First, we assessed the inhibitory effect of mAb II-1 that specifically recognizes the exosite 3 in the spacer domain15,16 on proteolytic cleavage of multimeric VWF by WT and variants. We showed that mAb II-1 dramatically inhibited proteolytic activity of WT and M2, but not M1, M3, M4, and M5 (Figure 4A), suggesting that R660 is critical for autoantibody inhibition. When plasma from TTP patients was used as the source of autoantibodies against ADAMTS13, proteolytic activity of WT, M1, and M2 was almost completely inhibited after 30 minutes of incubation (Figure 4B-C; Table 1), whereas the variant M3 was only variably inhibited, but the variants M4 and M5 were not inhibited by the same amount of patient plasma under the same conditions (Figure 4B-C; Table 1). These results indicate that the novel gain-of-function ADAMTS13 variants, especially M4 and M5, are more resistant to inhibition by both monoclonal and polyclonal anti-ADAMTS13 autoantibodies in patients with acquired idiopathic TTP.
Inhibition of proteolytic activity of ADAMTS13 and variants by autoantibodies. Recombinant WT or variants M1 to M5 (final concentration of 0.2nM) was incubated without (−) or with (+) 35μM of mAb II-1 (A) or 5 to 10 μL of heat-inactivated normal human plasma (N) or plasma from TTP patient 1 (P; B) for 30 minutes. The residual activity was determined by the cleavage of multimeric VWF. EDTA (10mM) was included in the last lane as a negative control. The relative residual activity was determined by the ratio of product (P) to high molecular weight VWF (HMW) multimer using ImageJ Version 1.45m software and normalized to the activity in the presence of normal human plasma. (C) The percentage of inhibition (mean ± SD) by a panel of 12 TTP patient plasmas. **P < .001, statistically highly significant difference between WT and 3 variants (ie, M3, M4, and M5).
Inhibition of proteolytic activity of ADAMTS13 and variants by autoantibodies. Recombinant WT or variants M1 to M5 (final concentration of 0.2nM) was incubated without (−) or with (+) 35μM of mAb II-1 (A) or 5 to 10 μL of heat-inactivated normal human plasma (N) or plasma from TTP patient 1 (P; B) for 30 minutes. The residual activity was determined by the cleavage of multimeric VWF. EDTA (10mM) was included in the last lane as a negative control. The relative residual activity was determined by the ratio of product (P) to high molecular weight VWF (HMW) multimer using ImageJ Version 1.45m software and normalized to the activity in the presence of normal human plasma. (C) The percentage of inhibition (mean ± SD) by a panel of 12 TTP patient plasmas. **P < .001, statistically highly significant difference between WT and 3 variants (ie, M3, M4, and M5).
Binding of patient anti-ADAMTS13 IgGs to recombinant WT and variants
To determine whether the resistance of ADAMTS13 variants to autoantibodies was the result of impaired binding interactions between the variants and autoantibodies, we performed an immunoprecipitation experiment plus Western blotting as described previously.13,19 As shown, mAb II-1 bound to WT and M2 consistently, but not much to the variants M1, M3, M4, and M5 (Figure 5). These results are in agreement with the data from the functional study in Figure 4A, suggesting that the reduction in inhibitory activity is caused by impaired binding of monoclonal antibody to the variants.
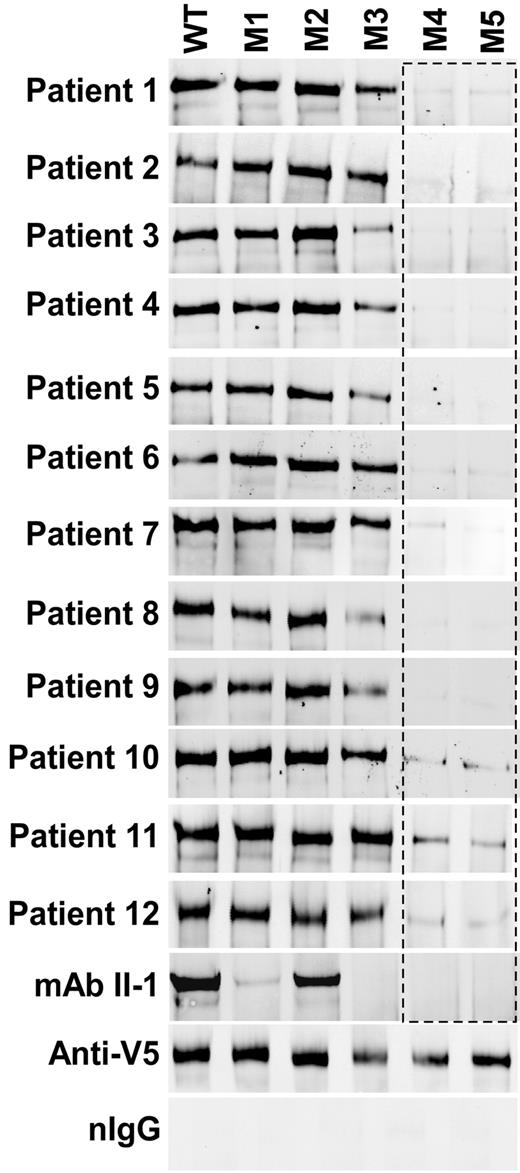
Binding of anti-ADAMTS13 IgGs from TTP patients to ADAMTS13 and variants. WT or variants M1 to M5 (50 ng) were incubated with mAb II-1 (35μM) or TTP patient plasmas (5-10 μL each, depending on plasma IgG concentrations). The immune complexes were pulled down with protein A-Sepharose 4B and detected by Western blotting with anti-V5. Anti-V5 IgG coupled Sepharose 4B beads were used for a positive control. Normal IgG from healthy persons (nIgG) was used for a negative control.
Binding of anti-ADAMTS13 IgGs from TTP patients to ADAMTS13 and variants. WT or variants M1 to M5 (50 ng) were incubated with mAb II-1 (35μM) or TTP patient plasmas (5-10 μL each, depending on plasma IgG concentrations). The immune complexes were pulled down with protein A-Sepharose 4B and detected by Western blotting with anti-V5. Anti-V5 IgG coupled Sepharose 4B beads were used for a positive control. Normal IgG from healthy persons (nIgG) was used for a negative control.
Moreover, plasma anti-ADAMTS13 IgGs from all 12 patients with acquired idiopathic TTP also consistently bound to WT, M1, and M2 but only variably to M3 and rarely to M4 and M5 (Figure 5). Control IgGs from healthy donors did not detectably bind WT and variants, whereas monoclonal anti-V5 IgG bound to all constructs (Figure 5). These results indicate that the inhibition by polyclonal anti-ADAMTS13 IgGs from acquired TTP patients is also primarily mediated by the direct binding of anti-ADAMTS13 IgGs to the exosite 3 in the spacer domain.
Model of interactions between the spacer domain and A2 domain
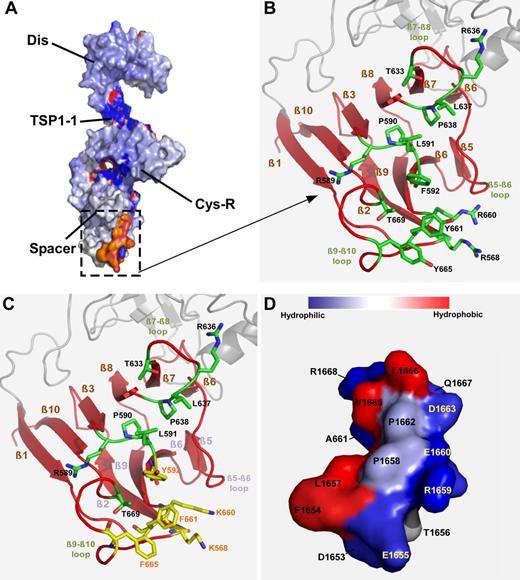
To gain insight into the molecular mechanisms underlying the enhanced specific activity of ADAMTS13 variants, we performed modeling based on the existing crystal structural information of the proximal C-terminal fragment of ADAMTS13 (ie, DTCS29 ; Figure 6A) and the A2 domain of VWF.30 As shown, the spacer domain composes 10 β-sheets (ie, β1-10), a pocket formed by various β-sheets containing a cluster of hydrophobic residues (L591, F592, L637, F638, L668, and T669), and a ring formed by Y661 and Y665 lined by basic residues R568, R589, R660, and R636 (Figure 6B). This pocket appears to directly interact with the α6-helix (residues between D1653 and R1668) in the central A2 domain of VWF (Figure 6D). The hydrophobic residues in the A2 domain presumably face exosite 3 to make strong hydrophobic contacts in conjunction with some hydrogen bonding outside of the pocket (supplemental Video 1). A substitution of R with K residue or Y with F residue or vice versa appears to alter hydrophobicity of the exosite 3 (Figure 6C). The corresponding changes of the hydropathy index were noted as follows: R→K: −4.5 → −3.9 and Y→F: −1.3 → +2.8, thereby enhancing the interaction between VWF and ADAMTS13. Furthermore, a substitution of F592 with Y may open up the pocket even more, thereby better engaging the substrate. There was also a corresponding backbone shift that appears to take place in the β2-, β5-, β6-, and β9-sheets to compensate for the increased hydrophobicity (Figure 6C). These changes allow greater engagement of the exosite 3 with the A2 domain, particularly the amino acid residues between residues D1653 and R1668 (supplemental Video 1). Together, our findings suggest that the modification of an exosite in the spacer domain is a viable approach to improve ADAMTS13 function while reducing autoantibody binding and inhibition.
Modeling of the spacer domain and VWF-A2 interaction. (A) Surface representation of proximal C-terminal DTCS fragment of ADAMTS13. (B) Close-up view of the hydrophobic cluster in the exosite 3 in the spacer domain of ADAMTS13. This pocket contains a cluster of hydrophobic residues (L591, F592, L637, P638, L668, T669, and ring of Y661 and Y665), lined by basic residues (R568, R589, R636, and R660), supported by 8 β-sheets (ie, β1, 2, 3, 6, 7, 8, 9, and 10). (C) A substitution of these surface residues with those in yellow appears to increase hydrophobicity of this pocket. (D) VWF-A2 (1653-1668) forms an amphipathic helix (α6). Hydrophobic residues facing to the top and charged residues to the bottom. This amphipathic helix may govern specificity to the exosite 3 in the spacer domain by inserting its hydrophobic side into the pocket.
Modeling of the spacer domain and VWF-A2 interaction. (A) Surface representation of proximal C-terminal DTCS fragment of ADAMTS13. (B) Close-up view of the hydrophobic cluster in the exosite 3 in the spacer domain of ADAMTS13. This pocket contains a cluster of hydrophobic residues (L591, F592, L637, P638, L668, T669, and ring of Y661 and Y665), lined by basic residues (R568, R589, R636, and R660), supported by 8 β-sheets (ie, β1, 2, 3, 6, 7, 8, 9, and 10). (C) A substitution of these surface residues with those in yellow appears to increase hydrophobicity of this pocket. (D) VWF-A2 (1653-1668) forms an amphipathic helix (α6). Hydrophobic residues facing to the top and charged residues to the bottom. This amphipathic helix may govern specificity to the exosite 3 in the spacer domain by inserting its hydrophobic side into the pocket.
Discussion
In the present study, we have demonstrated that a positively charged residue (ie, arginine or lysine at position 660) is critical for ADAMTS13 enzymatic function (Figure 1). This observation prompts us to test a hypothesis that replacement of the critical residues in the exosite in the spacer domain may generate ADAMTS13 variants that are resistant to binding and inhibition by anti-ADAMTS13 autoantibodies from patients with acquired idiopathic TTP while preserving or enhancing specific proteolytic activity. Of 24 novel ADAMTS13 variants prepared, 2 (ie, M4 and M5) exhibited dramatically enhanced specific activity toward rF-VWF73 peptide (Figure 2) and multimeric VWF (Figure 3). More importantly, these 2 gain-of-function variants are more resistant than WT and other variants to inhibition by either monoclonal or polyclonal autoantibodies against ADAMTS13 in patients with acquired idiopathic TTP (Figure 4; Table 1). As shown, 10 of 12 TTP patient plasmas (83%) do not appear to inhibit proteolytic activity of the variants M4 and M5, whereas the same amount of patient plasma completely inhibits the proteolytic activity of WT, M1, and M2 but only variably inhibits M3 activity under the same conditions. Plasma from 2 patients weakly inhibits the M4 and M5 activity (Table 1). These results further confirm, with a gain-of-function rather than a loss-of-function approach described in the literature,18,28 the critical role of exosite 3 in the spacer domain in substrate recognition.
Molecular modeling of the interaction between the spacer domain and VWF-A2 domain suggests that the α5-helix of VWF-A2 domain appears to directly interact with the residues in the exosite 3 in the spacer domain (Figure 6; supplemental Video 1), primarily through hydrophobic interactions. A substitution of the amino acid residues R, F, R, Y, and Y with K, Y, K, F, and F at the positions of 568, 592, 660, 661, and 665, respectively, appears to increase hydropathy and hydrophobic interactions between the exosite 3 in the spacer domain and the α5-helix in VWF-A2 domain (Figure 6; supplemental Video 1).
Our findings also provide novel insight into the mechanism underlying pathogenesis of acquired idiopathic TTP that is primarily caused by anti-ADAMTS13 autoantibodies. Despite the polyclonal nature of autoantibodies against ADAMTS13 in these patients,13,31 the inhibitory activity of anti-ADAMTS13 autoantibodies appears to be primarily mediated through their binding to the exosite 3 in the spacer domain, as the alteration in this region dramatically reduced binding (Figure 5) and inhibition by patient autoantibodies (Figure 4). These results do not appear to be contradictory to our previous findings in which multiple domains of ADAMTS13 are targeted by anti-ADAMTS13 IgGs in patients with acquired idiopathic TTP.13 The anti-ADAMTS13 IgGs that bind the C-terminal TSP1 2 to 8 repeats and CUB domains are less abundant and exhibit lower affinity than those recognizing the spacer domain.13 In the presence of 1% Triton X-100, the interaction between the middle and distal C-terminal domains and anti-ADAMTS13 IgGs can be disrupted.19 Indeed, our current results are highly consistent with those reported by Pos et al,19 in which a replacement of R568, F592, R660, Y661, and Y665 with A abolishes the binding of anti-ADAMTS13 IgGs from most TTP patients.19 However, alanine substitution in their study results in generation of loss-of-function ADAMTS13 variants, which have little value for therapy.
Our findings may change the way we treat acquired TTP with inhibitors. Plasma exchange remains the main therapy for these patients.32,33 It alone appears to be inadequate to restore severe deficiency of plasma ADAMTS13 activity when high-titer anti-ADAMTS13 IgGs are present.33 Infused ADAMTS13 is rapidly neutralized by anti-ADAMTS13 IgGs in TTP patient plasma. Low ADAMTS13 activity and persistence of anti-ADAMTS13 IgGs in patients correlate with an increased rate of relapse.11,33,34 Other immunosuppressive therapies, such as cyclosporine,35,36 cyclophosphamide,37,38 and rituximab (anti-CD20 antibody),37,38 may reduce the antibody formation, but they take weeks to months to have a clinical effect. Therefore, an autoantibody-resistant ADAMTS13 variant may have a value to instantaneously restore plasma ADAMTS13 activity in patients with inhibitors. The infused ADAMTS13 variants are more likely to survive and work better despite the presence of polyclonal anti-ADAMTS13 IgGs in these patients. The binding affinity of anti-ADAMTS13 IgGs toward other domains besides the spacer domain is relatively weak as previously reported.13-15,31 The clearance of plasma ADAMTS13 as a result of anti-ADAMTS13 IgGs does not appear to be the primary mechanism causing severely low ADAMTS13 activity because plasma ADAMTS13 antigen is relatively normal.39
In conclusion, a subtle alteration of the exosite binding site in the spacer domain represents a viable strategy for re-engineering ADAMTS13 variants with preserved or enhanced specific activity but resistant to inhibition by anti-ADAMTS13 autoantibodies. The gain-of-function variants provide novel tools for understanding the critical role of exosite interactions in proteolytic cleavage of VWF by ADAMTS13. Furthermore, the antibody-resistant ADAMTS13 variants underscore the importance of exosite 3 and adjacent residues in the spacer domain in pathogenesis of acquired idiopathic TTP caused. Finally, we hope that these variants with more desirable properties will be further developed as novel therapeutics for acquired idiopathic TTP.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Jan Voorberg, Sanquin-AMC Landsteiner Laboratory, Amsterdam, The Netherlands, for providing human monoclonal antibody against spacer domain of ADAMTS13 (mAb II-1).
This work was supported in part by the American Heart Association (National Established Investigator Award 0940100N; X.L.Z.) and the National Institutes of Health (grant P01HL074124, Project 3; X.L.Z.).
National Institutes of Health
Authorship
Contribution: C.J. and X.L.Z. designed research, performed experiments, and wrote and revised the manuscript; J.X., L.G., C.G.S., and S.-Y.J. performed experiments and revised the manuscript; and H.C.K. provided patient samples and clinical information and revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: X. Long Zheng, Department of Pathology and Laboratory Medicine, Children's Hospital of Philadelphia, 34th St and Civic Center Blvd, 816G ARC, Philadelphia, PA 19104; e-mail: zheng@email.chop.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal