Abstract
Systemic treatment for cutaneous T-cell lymphoma (CTCL) involves the use of less aggressive, well-tolerated therapies. Pralatrexate is a novel antifolate with high affinity for reduced folate carrier-1. A dose de-escalation strategy identified recommended pralatrexate dosing for patients with CTCL that demonstrated high activity, good rates of disease control, and an acceptable toxicity profile for continuous long-term dosing. Eligibility included mycosis fungoides, Sézary syndrome, or primary cutaneous anaplastic large cell lymphoma, with disease progression after ≥ 1 prior systemic therapy. The starting dose and schedule was 30 mg/m2/wk intravenously for 3 of 4 (3/4) weeks. Subsequent starting doses were 20, 15, and 10 mg/m2/wk for 3/4 or 2 of 3 (2/3) weeks. Response was evaluated by the modified severity-weighted adjustment tool. Fifty-four patients were treated. The recommended regimen was identified as 15 mg/m2/wk for 3/4 weeks and was explored in the expansion cohort. In 29 patients treated overall with the recommended dosing regimen, the median number of prior systemic therapies was 4. Pralatrexate was administered for a median of 4 cycles; response rate was 45%. The most common grade 3 adverse event (AE) was mucositis (17%); the only grade 4 AE was leukopenia (3%). Pralatrexate 15 mg/m2/wk for 3/4 weeks shows high activity with acceptable toxicity in patients with relapsed/refractory CTCL.
Introduction
Cutaneous T-cell lymphomas (CTCLs) are typically indolent T-cell lymphomas that present primarily or exclusively in the skin. The term CTCL comprises several distinct clinical entities including mycosis fungoides (MF), the Sézary syndrome (SS), and primary cutaneous anaplastic large cell lymphoma (ALCL), which are all characterized by infiltration of the skin by malignant T cells.1
Therapeutic approaches for patients with CTCL depend on the disease stage. For early stage disease, skin-directed therapies are generally used, with systemic treatments reserved for patients with relapsed or more extensive disease.2,3 Durable remissions off-therapy are uncommon in MF/SS, and, historically, more aggressive first-line approaches have not resulted in improved outcomes, but are associated with increased toxicity.4 In pivotal studies of approved systemic therapies for advanced relapsed/refractory CTCLs, response rates were reported for vorinostat (30%), denileukin diftitox (30%), oral bexarotene (45%-54%), and romidepsin (34%-35%), with median response durations of 6 to 15 months.5-10 Recently, denileukin diftitox demonstrated a 44% response rate versus placebo (15.9% response rate) in a randomized study, with a median progression-free survival (PFS) of > 2 years compared with 124 days in the placebo group.11 Several other agents are used in CTCL treatment, although their use is generally derived from smaller studies.12-15 Traditional cytotoxic chemotherapy agents are used later in the disease course because of risks of myelosuppression associated with poor skin integrity and the underlying immunosuppression associated with MF/SS.14,16-19 The moderate response rates and frequent lack of durable responses to current therapies for CTCL underline the need for additional effective and tolerable treatments.
Pralatrexate (FOLOTYN; Allos Therapeutics Inc), an antineoplastic folate analog, has high affinity for the reduced folate carrier type-1 oncoprotein and is an efficient substrate for polyglutamylation by the enzyme folylpolyglutamyl synthetase, resulting in extensive internalization and accumulation within tumor cells. Pralatrexate inhibits dihydrofolate reductase, resulting in disruption of DNA synthesis and subsequent tumor cell death. This agent has been extensively studied in preclinical and clinical settings, alone and in combination,20 and has been shown to have superior activity compared with methotrexate against human non-Hodgkin lymphoma in both in vitro and in vivo models.21,22 The pharmacokinetics and toxicology of pralatrexate, as well as its efficacy and safety in peripheral T-cell lymphoma (PTCL), are previously well described.23-31 Pralatrexate received accelerated approval from the US Food and Drug Administration for patients with relapsed or refractory PTCL at a dose of 30 mg/m2 weekly by IV push for 6 of 7 (6/7) weeks, based on the results of the PROPEL study.29 To reduce the risk of mucositis, the most common toxicity encountered in the PROPEL study, vitamin B12 and folate supplementation, are used with treatment.23,26,28,32
CTCL is approached as a more indolent disease compared with PTCL, and combination chemotherapies are rarely used. Because of the activity in several patients with CTCL in prior phase 1-2 pralatrexate studies at various doses, this dose de-escalation study (PDX-010) was designed to try to identify an effective dose with acceptable toxicity for patients with CTCL.29,32
Methods
This study was approved by the institutional review boards of all participating institutions and was conducted in accordance with the principles set forth in the Declaration of Helsinki.
Patients
Eligible patients included those with histologically confirmed CTCL subtypes of MF (stage ≥ IB), SS, or primary cutaneous ALCL with measurable disease at the time of enrollment. Patients must have progressed after ≥ 1 prior systemic therapy, had an Eastern Cooperative Oncology Group performance status of 0-2, adequate hematologic, hepatic, and renal function, and provided written informed consent before study entry.
Study design
This was a dose-finding study to determine an effective and well-tolerated pralatrexate dosing regimen with vitamin B12 and folic acid supplementation in patients with relapsed/refractory CTCLs. Patients in the dose-finding cohorts were treated at sequentially decreasing pralatrexate dose intensities to identify an optimal dosing regimen, and an expansion cohort further explored that identified regimen. The optimal regimen was defined as the dose and schedule with ≥ 1 response in up to 9 patients, an incidence of dose-limiting toxicities (DLTs) of < 33%, and no patients with grade 4 hematologic toxicity, grade 3-4 infection, or febrile neutropenia.
The pralatrexate starting dose and schedule was 30 mg/m2/wk by IV push for 3 consecutive weeks followed by 1 rest week in a 4-week cycle. This regimen was based on a previous phase 1-2 dose-escalation study in patients with relapsed/refractory lymphoid malignancies and was the lowest dose explored in that study.32 On occurrence of predefined DLTs, which included many grade 2 toxicities in an effort to find a minimally toxic dose, each subsequent cohort was treated at the next lower pralatrexate dose of 20, 15, or 10 mg/m2, or the next less frequent dosing schedule of 3 of 4 (3/4) or 2 of 3 (2/3) weeks.
Protocol-defined DLTs that would trigger dose and/or schedule de-escalation included grade 3-4 neutropenia, grade 2-4 thrombocytopenia or any thrombocytopenia with clinically significant bleeding (excluding epistaxis), any febrile neutropenia, grade 2-4 nonhematologic adverse event (AE), dose reduction/omission in cycle 1 for treatment-related AE, or initiation of cycle 2 delayed more than 1 week for treatment-related AE.
Pralatrexate was continued until progressive disease (PD) or 1 of the following criteria for treatment discontinuation was met: initiation of subsequent lymphoma therapy; intolerance of pralatrexate treatment; lapse of 2 weeks between pralatrexate doses within a cycle or 3 weeks between cycles; withdrawal of consent; or investigator/sponsor decision. Patients received treatment for a maximum of 12 months; however, on clinical or radiologic evidence of benefit in the investigator's opinion, patients could continue to receive pralatrexate. All patients received supplementation with vitamin B12 (1 mg intramuscularly every 8-10 weeks) and folic acid (1 mg orally once daily) before study treatment initiation (at least 10 days prior for folic acid and within 10 weeks prior for vitamin B12) through 30 days after study treatment discontinuation. Patients were not permitted to initiate treatment with systemic or topical corticosteroids while on study; however, patients could continue to receive topical corticosteroids or a dose of no more than 10 mg/d of prednisone (or equivalent) provided the corticosteroids were ongoing for at least 1 month before study entry.
Pralatrexate dose modifications for mucositis were: (1) for grade 2, treatment was held until recovery to grade 0-1, with the following dose administered without reduction; and (2) for recurrent grade 2 or grade 3-4, treatment was held until recovery to grade 0-1, followed by reduction to the next lower dose level, with discontinuation for recurrence. For other nonhematologic toxicities, absolute neutrophil counts, and platelet counts, doses were omitted for grade 3-4 until recovery to grade 0-2, followed by reduction to the next lower dose, with discontinuation for recurrence.
Assessments
Disease staging was performed using the tumor, node, metastasis (TNM) classification system.33 During the course of the study, the TNM system was modified to account for blood involvement (TNMB).34 Disease staging was conducted using the TNMB for patients enrolled in this study after the modified criteria were published; however, no retrospective staging was performed for previously enrolled patients.
Response to treatment was evaluated by the investigator every 2 cycles for 6 months and every 4 cycles thereafter using a standard response assessment for CTCL studies: the modified severity-weighted adjustment tool.5 For patients with lymph node involvement, computed tomography scans were obtained at baseline and on clinical response or end of treatment, whichever occurred first. Clinical photographs were not required for response assessment. Response rate, PFS, and response duration were determined. Patients were evaluable for response if they received ≥ 1 pralatrexate dose.
Safety was evaluated by physical examinations, clinical laboratory evaluations, and treatment-emergent AEs. The incidence and severity of AEs were graded using the National Cancer Institute Common Terminology Criteria for AEs Version 3.0, and were coded using the Medical Dictionary for Regulatory Activities Version 11.0.
Statistical analysis
Safety and efficacy data, baseline values, and demographics were analyzed for all patients who received ≥ 1 pralatrexate dose. Data were collected during treatment and for 35 (± 5) days after the last pralatrexate dose. Comparative statistical testing was not performed in this single-arm study.
Investigator assessment of response was defined as either complete response (CR) or partial response (PR). Duration of response was calculated from the day of first response (per investigator) until PD or death. Responding patients who had not progressed were censored at the last response assessment before study end. Progression was defined as either PD or death, and PFS was calculated from the first pralatrexate dose until PD or death. Patients who came off therapy without PD or death were censored for PFS at the last response assessment before study end.35 Kaplan-Meier estimates of the median duration of response and PFS were generated.
Results
Overall patient characteristics
Between August 2007 and October 2010, 54 patients were treated at 8 US sites (31 in the dose-finding cohorts and 23 in the expansion cohort). Patient characteristics are shown in Table 1. This was a heavily pretreated population, with a median of 6.5 prior regimens (range, 1-25) and 4 prior systemic regimens (range, 1-11), given either sequentially or in combination. Overall, 69% of patients had received prior oral bexarotene, 83% had received other prior systemic chemotherapy, and 70% had received prior immunotherapy (mostly interferon and denileukin diftitox); most had received both systemic chemotherapy and immunotherapy.
Dose-finding cohorts
In the dose-finding cohorts, 31 patients were enrolled sequentially and treated at de-escalating doses and schedules until an optimal dosing regimen was determined. The regimens evaluated, number of patients treated, and observed DLTs and responses are summarized in Table 2. The optimal dosing regimen was originally defined as the dose and schedule with ≥ 1 response in up to 9 patients and a DLT incidence rate of < 33%. The only dosing regimen to demonstrate this DLT rate was pralatrexate 10 mg/m2 weekly for 3/4 weeks (cohort 6: 3/10 patients [30%] with DLTs), but this cohort did not demonstrate sufficient activity, with only one response in 10 patients. As no dosing regimen met the predefined criteria for expansion, a review of toxicity and efficacy data was performed across the overall pralatrexate safety profile to assess the risk:benefit profiles of each cohort.
Table 2 presents responses observed in the dose-finding cohorts. High response rates (≥ 50%) were seen at 30 mg/m2 for 3/4 weeks, 20 mg/m2 for 3/4 and 2/3 weeks, and 15 mg/m2 for 3/4 weeks, with a combined response rate at these doses (cohorts 1-4) of 11/18 (61%), and an apparent threshold for activity was observed with a response rate of 1/13 (8%) in patients treated at lower dose intensities (cohorts 5-6). While an active and essentially nontoxic dose was not identified, cohorts 3 (20 mg/m2/wk for 2/3 weeks) and 4 (15 mg/m2/wk for 3/4 weeks) had the greatest risk:benefit profiles and both were considered for the recommended dose. After review among the investigators, DLTs in cohort 4 were considered to be less clinically significant with an absence of grade 3 toxicities compared with those in cohort 3. Therefore, the dose and schedule evaluated in cohort 4 (15 mg/m2/wk for 3/4 weeks) was chosen as the recommended dosing regimen to be explored in the expansion cohort, and the protocol was amended to reflect this change.
Expansion cohort
The cohort that received 15 mg/m2 weekly for 3/4 weeks (n = 6) was further expanded to an additional 23 patients, for a total of 29 patients treated with this recommended dosing regimen. At the time of this analysis, 6 of these patients remained on pralatrexate therapy and 23 patients had discontinued therapy. Reasons for discontinuation were: 6 patients (21%) experienced PD; 7 patients (24%) per investigator decision because of lack of response (n = 5), intolerance of therapy (n = 1), and surgery for preexisting condition (n = 1); 3 patients (10%) for AEs; 3 patients (10%) for receiving subsequent therapy (includes one patient who went on to a transplantation); and 4 patients (14%) per patient decision (because of withdrawal of consent [n = 2] and patient relocation [n = 2]).
All 29 patients in the expansion cohort were evaluable. Pralatrexate was administered for a median of 4 cycles (range, 1-23 cycles), and the median treatment duration was 99 days. The response rate among patients treated with a dose of 15 mg/m2 weekly for 3/4 weeks was 45% (13/29; 1 CR, 12 PRs; 95% confidence interval [CI]: 26.4%-64.3%), as displayed in Table 3 by CTCL stage and subtype shown. The median time to best response was 57 days (range, 44-168 days). The median response duration was not reached because of censoring (range, 1*-372* days; * = censored for subsequent therapy, study termination, in response at time of analysis, or 1 day if no further response assessments were performed after response was observed), although the Kaplan-Meier estimate shows 73% of responses continuing at 6 months. Among the 13 patients who responded at the pralatrexate dose of 15 mg/m2 weekly for 3/4 weeks, the median number of cycles administered was 7 (range, 4-23 cycles) and the median duration of treatment was 159 days.
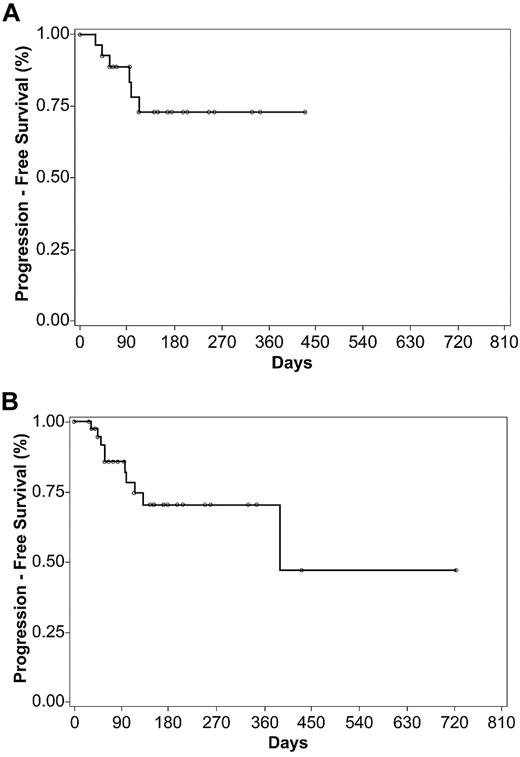
PFS was calculated for the 29 patients treated with 15 mg/m2 weekly for 3/4 weeks (Figure 1A) and for all patients treated at a dose intensity of ≥ 15 mg/m2 weekly for 3/4 weeks (Figure 1B). Median PFS was not reached for the 15 mg/m2 weekly for 3/4 weeks regimen (range, 1*-429* days; * = censored for subsequent therapy, study termination, continuation of follow-up, or censored to treatment day 1). Median PFS for the 41 patients who received ≥ 15 mg/m2 weekly for 3/4 weeks was 388 days (range, 1*-722* days).
Kaplan-Meier estimates of progression-free survival based on investigator assessment of response. (A) PFS for 29 patients treated at the optimal dosing regimen of pralatrexate 15 mg/m2 weekly for 3 of 4 (3/4) weeks and (B) for 41 patients treated at praltrexate ≥ 15 mg/m2 weekly for 3/4 weeks.
Kaplan-Meier estimates of progression-free survival based on investigator assessment of response. (A) PFS for 29 patients treated at the optimal dosing regimen of pralatrexate 15 mg/m2 weekly for 3 of 4 (3/4) weeks and (B) for 41 patients treated at praltrexate ≥ 15 mg/m2 weekly for 3/4 weeks.
Two patients in the 20 mg/m2 for 2/3 weeks dose-finding cohort stopped therapy per protocol while still in response to pralatrexate (1 PR [response duration at study end = 218 days] and 1 CR [response duration at study end = 94 days]). On disease recurrence or progression, both patients were reentered into the study as permitted by the protocol. The patient with a PR on pralatrexate was retreated at 20 mg/m2 weekly for 2/3 weeks and again achieved a PR that was continuing as of the data collection end point. The patient with a CR on pralatrexate was retreated at a lower dose (15 mg/m2/wk for 2/3 weeks) and again achieved a CR on pralatrexate that lasted 154 days; this patient was still in response when he discontinued pralatrexate treatment because of a nontreatment-related AE.
Dose escalation to 20 mg/m2 was permitted per investigator discretion for patients in the expansion cohort who did not experience a CR and tolerated treatment for 2 complete cycles. Two of the 23 patients treated in the expansion cohort met the criteria for dose escalation to 20 mg/m2 weekly for 3/4 weeks. One of these patients achieved a PR at the assessment directly following escalation, and received a total of 5 doses at 20 mg/m2 weekly for 3/4 weeks. The other patient maintained stable disease and received 13 doses at 20 mg/m2 weekly for 3/4 weeks.
The most common AEs, regardless of causality, reported in patients treated with pralatrexate 15 mg/m2 weekly for 3/4 weeks included mucositis (48%), fatigue (41%), nausea (31%), edema (28%), epistaxis (24%), pyrexia (21%), anorexia (21%), and skin toxicity (21%). The only grade 3 AEs that occurred in > 1 patient in this dosing regimen were mucositis (17%) and skin toxicity (7%), and the only grade 4 AE was leukopenia, which was reported in 1 patient ∼ 2 weeks after discontinuing pralatrexate treatment. Treatment-related AEs in ≥ 5% of patients are detailed in Table 4. At a dose of 15 mg/m2 weekly for 3/4 weeks, no treatment-related AEs of neutropenia were reported, the only treatment-related AE of anemia was grade 2, and no grade 4 treatment-related AEs were reported.
All patients
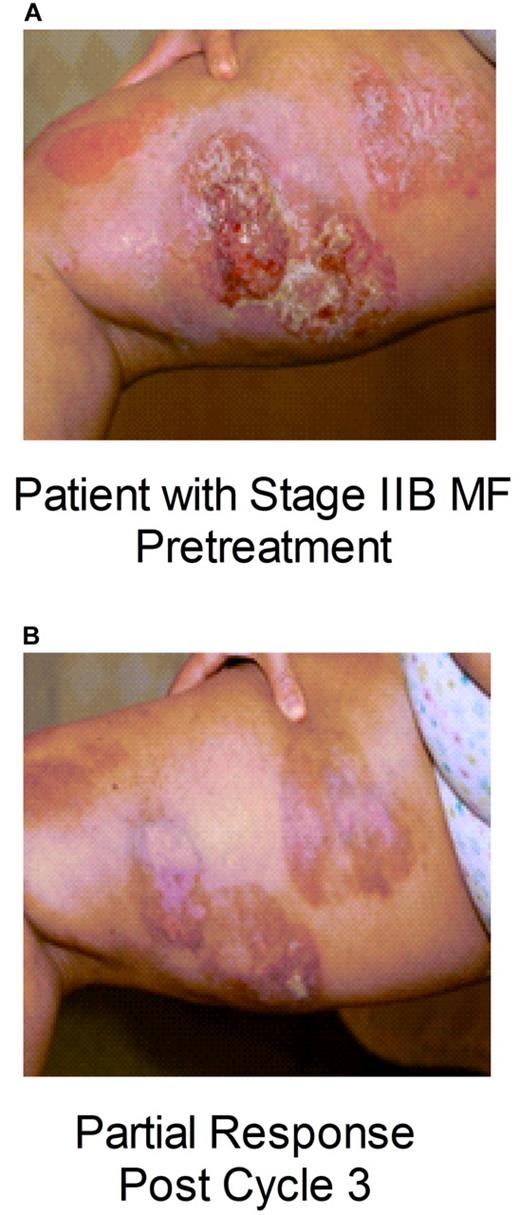
For the entire study, the overall response rate was 41% (22/54; 95% CI: 27.6%-55.0%), including 3 CRs and 19 PRs. Response is presented by CTCL subtype and disease stage in Table 3 for the overall patient population (N = 54), for patients treated with 15 mg/m2 weekly for 3/4 weeks (n = 29), for patients treated with ≥ 15 mg/m2 weekly for 3/4 weeks (n = 41), and for patients treated with < 15 mg/m2 weekly for 3/4 weeks (n = 13). Patients with both advanced MF and SS achieved responses with pralatrexate; Figure 2 shows a patient with MF stage II B who experienced a PR. There was only one patient with ALCL in this study, and this patient achieved a CR on pralatrexate, as illustrated in Figure 3. Logistic regression analyses revealed no correlations between response and disease stage or number of prior therapies (data not shown).
Response to pralatrexate in patients with cutaneous T-cell lymphoma. Skin photographs documenting disease in a patient with mycocis fungoides (MF) stage IIB (A) before the start of treatment with pralatrexate and (B) after 4 cycles of treatment with pralatrexate.
Response to pralatrexate in patients with cutaneous T-cell lymphoma. Skin photographs documenting disease in a patient with mycocis fungoides (MF) stage IIB (A) before the start of treatment with pralatrexate and (B) after 4 cycles of treatment with pralatrexate.
Response to pralatrexate in patients with cutaneous T-cell lymphoma. Skin photographs documenting disease in a patient with anaplastic large cell lymphoma before the start of treatment with pralatrexate and after 4 cycles of treatment.
Response to pralatrexate in patients with cutaneous T-cell lymphoma. Skin photographs documenting disease in a patient with anaplastic large cell lymphoma before the start of treatment with pralatrexate and after 4 cycles of treatment.
The type of prior therapy received did not predict responses to pralatrexate. Response rates in patients who were previously treated with and progressed following bexarotene (n = 37), histone deacetylase (HDAC) inhibitors (n = 27; mostly vorinostat), interferon (n = 28), and methotrexate (n = 13) were 46%, 41%, 36%, and 46%, respectively.
Among the entire population, 50 patients (93%) experienced an AE on study or within 30 days of the last dose. The most frequently reported AEs regardless of causality were mucositis (54%), fatigue (43%), nausea (39%), skin toxicity (28%), edema (26%), anemia (22%), and pyrexia (22%). Grade 3 treatment-related AEs reported in > 1 patient included mucositis (n = 8 [15%]), skin toxicity (n = 3 [6%]), and anemia, lymphopenia, and thrombocytopenia (each in 2 patients [4%]). Only one grade 4 treatment-related AE was reported (pulmonary embolism in a patient treated in the 15 mg/m2/wk for 2/3 weeks cohort). The only serious AE reported in > 2 patients was skin toxicity (n = 3 [6%]); serious AEs reported in 2 patients (4%) included failure to thrive, hypotension, mucositis, acute renal failure, and sepsis. When the incidence of ≥ grade 2 mucositis was compared with baseline methylmalonic acid levels (above and below 200 nmol/L), no definitive correlation was observed.
Discussion
None of the current therapeutic options available for patients with CTCLs are curative. Many patients are treated sequentially and suffer from frequent morbidity because of the burden of their disease and the cumulative toxicity of therapy.
The present study identified an effective CTCL dosing regimen for pralatrexate with an AE profile that is acceptable for continuous long-term use. For patients treated with 15 mg/m2 weekly for 3/4 weeks and ≥ 15 mg/m2 weekly for 3/4 weeks, the response rates were 45% and 51%, respectively. These results were obtained in heavily pretreated patients with relapsed/refractory CTCLs with a median of 4 prior systemic therapies. These results compare with the single agents commonly used for the treatment of CTCLs (vorinostat, denileukin diftitox, oral bexarotene, and romidepsin) for which these patients had been previously treated and have reported response rates between 30% and 54% with response durations of 6 to 15 months.5-10 The median time to response for patients in the expansion cohort (57 days) is also comparable with or shorter than those reported for these other agents, which range from 42 to 180 days.5-10 While most of the responses observed in the expansion cohort were PRs (12 of 13; 92%), this proportion falls within the range of other published data, where 67% to 96% of all responses were PRs.5-10 The most frequent AEs experienced with this effective pralatrexate regimen were stomatitis (48%; 14 of 29) and fatigue (38%; 11 of 29). Similar incidence and grades of AEs have been reported for these other agents.5-10
In patients receiving pralatrexate 15 mg/m2 weekly for 3/4 weeks, the median response duration was not reached (1*-372* days [* = censored]), and the Kaplan-Meier estimate for duration of response at 6 months was 73%. Many of the single agents used for CTCL treatment are biologic agents or non-traditional cytotoxic chemotherapies such as retinoids, interferons, and HDAC inhibitors. These agents are preferred because they can be administered long-term without cumulative toxicities, particularly immunosuppression. At the pralatrexate dosing regimens explored in this study, patients were treated for up to ∼ 2 years without cumulative myelosuppression or an increased rate of infection. Among the 54 treated patients, there was only one patient (2%) each with treatment-related grade 3 neutropenia and grade 3-4 staphylococcal infection, which is one of the more significant and dangerous toxicities commonly associated with chemotherapy for patients with CTCLs. Furthermore, the rate of AEs reported across all doses was significantly lower than that seen in the pivotal study in PTCL, where the administered dose intensity was 30 mg/m2 weekly for 6/7 weeks.
Interestingly, pralatrexate had a 46% response rate in patients who progressed following prior methotrexate, reinforcing that pralatrexate may exhibit potentially non–cross-resistant mechanisms of action compared with methotrexate.
In conclusion, the starting dose of pralatrexate 15 mg/m2 weekly for 3/4 weeks demonstrated high activity with acceptable toxicity in a relapsed/refractory CTCL population. While this 54-patient study is reasonably sized for this uncommon illness, a confirmatory study is warranted. Pralatrexate represents a viable treatment option with the potential to provide durable benefit for patients with advanced-stage CTCLs who have been previously treated with many of the currently available agents. Moreover, the relatively low toxicity profile coupled with the described activity suggests that pralatrexate could form part of future combination studies with some of the active and nonmyelosuppressive agents used in CTCLs. Currently, phase 1 studies exploring pralatrexate in combination with HDAC inhibitors and retinoids are under way.
Presented in poster form (Poster 2800) at the American Society of Hematology 52nd Annual Meeting, Orlando, FL, December 4, 2010.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
Monica Ramchandani, PhD, and Lindsey Lozano of Allos Therapeutics Inc provided medical writing support with direction and guidance from authors. Adrienne Schreiber of inScience Communications provided editing support, which was funded by Allos Therapeutics Inc.
This study was sponsored by Allos Therapeutics Inc.
The opinions expressed are those of the authors, who received no honoraria for manuscript development.
Authorship
Contribution: S.M.H. contributed to conceiving and designing the study, collecting, analyzing, and interpreting the data, and writing and approving the manuscript; Y.H.K. contributed to designing, collecting, and analyzing the data, interpreting the data, writing/commenting and approving the manuscript, and providing study materials or patients; F.F. contributed to writing/commenting and approving the manuscript; J.M.Z. contributed to collecting data, providing study material or patients, writing/commenting, and final approval of the manuscript; P.L.M. contributed to collecting and assembling data, writing/commenting, and approving the manuscript; M.J.L. contributed to analyzing and interpreting data, writing/commenting, and approving the manuscript; D.C.F. contributed to collecting, assembling, and interpreting data, writing/commenting on the manuscript, and providing final approval; A.R.S. contributed to collecting, analyzing, and interpreting the data, writing/commenting and approving the manuscript, and providing study material and patients; N.L.B. contributed to performing research, collecting and analyzing the data, writing/commenting, and approving the manuscript; M.L.D. contributed to collecting data and writing/commenting and approving the manuscript; T.K. contributed to concept, design, and analysis of the data, interpreting data and performing statistical analysis, writing/commenting, and approving the manuscript; M.E.S. contributed to the conception and design of the study, the collection and assembly of data, analysis and interpretation of the data, writing/commenting, and approving the final manuscript; O.A.O. contributed to designing research, analyzing and interpreting data, and writing/commenting on the manuscript; and M.D. contributed to the concept and design of the study, the collection, analysis, and interpretation of the data, writing/commenting, and approving the manuscript.
Conflict-of-interest disclosure: S.M.H. has served as a consultant for and received research funding from Allos Therapeutics Inc, Celgene, and Seattle Genetics, and has also received honorarium from Merck. Y.H.K. has served as a compensated consultant or advisor for Allos Therapeutics Inc, Gloucester, Celegene, Kyowa Kirin, Seattle Genetics, Millennium, Therakos, Merck, and Eisai Co Ltd, and has received research funding from Kyowa Kirin, Merck, Allos Therapeutics Inc, Yaupon, and Seattle Genetics. F.F. has served as a consultant for Celgene, Eisai Co Ltd, and Merck, and received honoraria from Celgene, Cephalon, Eisai Co Ltd, and Merck. J.M.Z. has received honoraria for a speaking engagement for Allos Therapeutic Inc. M.J.L. has consulted with Allos Therapeutics Inc and was compensated. D.C.F. has served on an advisory board for Allos Therapeutics Inc for which he received honoraria. A.R.S. has received research funding and honorarium from Allos Therapeutics Inc. N.L.B. has received research funding from Allos Therapeutics Inc. M.L.D. has served as a compensated consultant and received honoraria for a speaking engagement for Allos Therapeutics Inc. T.K. is the Vice President of Biometrics for Allos Therapeutics Inc. M.E.S. is the Vice President of Clinical Drug Safety and Pharamcovigilance for Allos Therapeutics Inc, and has stock or other ownership interest in Allos Therapeutics Inc. O.A.O. has received grant funding from the US Food and Drug Administration (1 RO1 FD0003498-01), and has received funding for clinical trial support from Allos Therapeutics Inc. M.D. has received funding for clinical trial support from Allos Therapeutics Inc, and has served as a compensated advisor for Allos. P.L.M. declares no competing financial interests.
Correspondence: Steven M. Horwitz, MD, Dept of Medicine, Memorial Sloan-Kettering Cancer Center, 1275 York Ave, New York, NY 10065; e-mail: horwitzs@mskcc.org.