Abstract
In classical Hodgkin lymphoma, circulating clonotypic malignant cells express CD20, which potentially explains the observed activity of rituximab. This multicenter phase 2 study investigated the combination of rituximab-ABVD (doxorubicin, bleomycin, vinblastine, dacarbazine) for stage II-IV untreated classical Hodgkin lymphoma. A goal was to assess the behavior of circulating clonotypic B cells clinically. Of 49 evaluable patients, 69% had stage IIB-IV disease; 8% had CD20+ Hodgkin and Reed-Sternberg cells. Rituximab-ABVD was generally well tolerated. Delivered relative dose intensity was 94% for AVD and 79% for bleomycin. After 6 cycles, 81% of patients were in complete remission. Only 8% received radiation therapy. The actuarial 3-year event-free and overall survival rates were 83% and 98%, respectively. EBV copy number in plasma fell dramatically during cycle 1 in patients with EBV+ tumors. Persistence of detectable circulating clonotypic B cells was associated with a greater relapse frequency (P < .05). Rituximab-ABVD and clonotypic B cells warrant additional study in classical Hodgkin lymphoma. This trial was registered at www.clinicaltrials.gov as NCT00369681.
Introduction
In classical Hodgkin lymphoma (cHL), responses to rituximab can occur in patients whose Hodgkin and Reed-Sternberg (HRS) cells lack CD20 expression.1,2 Interestingly, morphologically distinct subpopulations were described within a human cHL cell line more than 20 years ago.3 Clonogenic potential appeared to be largely limited to rare CD19+ mononuclear cells that were phenotypically distinct from HRS cells.3 The Johns Hopkins group recently reported that within cHL cell lines, this small population has a memory B-cell phenotype, is enriched for the stem cell marker aldehyde dehydrogenase, and appears to generate and sustain the growth of HRS cells.4 Furthermore, such rare clonotypic B cells circulated in most patients with newly diagnosed cHL, including those with limited-stage disease, and had the same immunoglobulin gene rearrangements as lymph node–derived HRS cells.4 These findings potentially explain the observed activity of rituximab in cHL and raise the possibility that anti–B-cell therapies represent new approaches for this disease.
A phase 2 trial of rituximab combined with ABVD (doxorubicin, bleomycin, vinblastine, dacarbazine) for cHL was conducted to explore its biologic effects in correlative laboratory studies and secondarily to evaluate clinical outcomes.
Methods
This single-arm, institutional review board–approved study was conducted through an inter-SPORE (Lymphoma Specialized Program of Research Excellence) collaboration. All participants gave written consent. Eligibility criteria included untreated stage II-IV cHL; age ≥ 18 years; negative for HIV and hepatitis B surface antigen; creatinine ≤ 2 mg/dL; and bilirubin ≤ 5 mg/dL.
Specified treatment was 6-8 cycles of standard ABVD with rituximab 375 mg/m2 1 week before ABVD initiation; on days 1, 8, 15, and 22 of cycle 1 ABVD; and on day 1 of cycles 2, 4, and 6. Once-weekly rituximab was given to maximize B-cell depletion early in the course.1
Sample size was based on correlative laboratory end points. Some pretreatment clonotypic B-cell data were included in a previous report.4 Blood specimens were collected at baseline, during chemotherapy, 2-4 weeks afterward, and then every 2-6 months.
Results and discussion
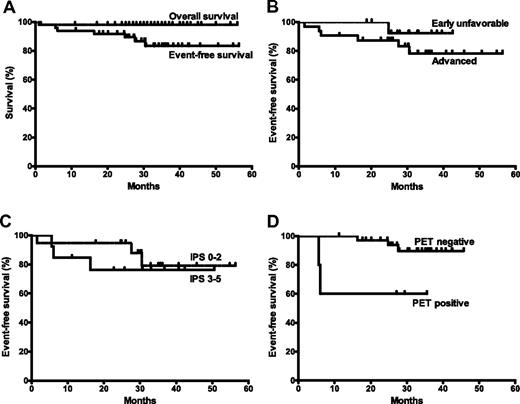
Table 1 summarizes the 49 evaluable patients. After 6 cycles, 39 (81%) of 48 patients had achieved complete remission, 7 (15%) had achieved partial remission, 1 had stable disease, and 2 had progressive disease.5 With a median follow-up of 33 months (range 11-56 months) in patients without events, 42 were alive and in continued remission. At 3 years, the estimated event-free survival (EFS) was 83% (95% confidence interval [CI] 68%-92%) and overall survival was 98% (95% CI 86%-100%; Figure 1A). EFS and failure-free survival were the same. In advanced disease (Figure 1B,C), the 3-year EFS was 79% (95% CI 47%-93%), with an International Prognostic Score ≤ 2 and 76% (95% CI 43%-92%) with an International Prognostic Score ≥ 3.
Survival outcomes with rituximab-ABVD in classical Hodgkin lymphoma. (A) EFS and overall survival after treatment initiation, estimated by the Kaplan-Meier method. An event is defined as relapse, progression, or death. (B) EFS in patients with early unfavorable or advanced disease. (C) EFS according to the International Prognostic Score (IPS) in patients with advanced disease. (D) EFS according to the interim PET result.
Survival outcomes with rituximab-ABVD in classical Hodgkin lymphoma. (A) EFS and overall survival after treatment initiation, estimated by the Kaplan-Meier method. An event is defined as relapse, progression, or death. (B) EFS in patients with early unfavorable or advanced disease. (C) EFS according to the International Prognostic Score (IPS) in patients with advanced disease. (D) EFS according to the interim PET result.
On centralized review of cycle 2 positron emission tomography (PET)/computed tomography, 36 (88%) of 41 scans were PET-negative by modified London criteria.6 The estimated 3-year EFS was 90% in the interim PET-negative group and 60% in the PET-positive group (P = .02; Figure 1D).
Frequencies of circulating clonotypic B cells were prospectively followed in 25 patients by use of previously described methods.4 Of 24 having a baseline sample, 21 (88%) had detectable clonal CD27+ALDH+ B cells before rituximab or rituximab-ABVD. These represented a median of 0.3% (range 0.2%-2%) of total circulating CD19+ cells, expressed κ-light chain in 6 cases, and expressed λ-light chain in 15 cases. After rituximab-ABVD, they became undetectable in 19 patients, of whom 18 had > 1 measurement after treatment completion. Three of these 19 patients have had documented reemergence of the clone but remain in remission. The clone persisted in 2, both of whom relapsed. With 11- to 41-month follow-up, 0 of 16 patients whose clone disappeared have relapsed versus 2 of 5 patients who had persistence or documented reemergence of the clone (P < .05, Fisher exact test).
Serial blood specimens were available for viral studies in 34 patients: 4 with EBV+ tumors, 29 EBV−, and 1 not characterized. At baseline, EBV copy number assessed by real-time PCR7 distinguished EBV+ from EBV− tumors. Among patients with EBV+ tumors, EBV copy number was reduced dramatically during cycle 1, disappearing in 3 patients and showing > 50-fold reduction in 1 patient. None of these patients have relapsed at 25- to 56-month follow-up.
Before ABVD cycles beyond cycle 1, the median absolute neutrophil count ranged from 600-1043 cells/mm3 (minimum 27-168 cells/mm3). The mean relative dose intensity8 was 94% for doxorubicin, vinblastine, and dacarbazine and 79% for bleomycin. The mean and median percentages of projected dose intensity delivered for the ABVD combination were 90% and 96%, respectively.
Rituximab-ABVD typically was associated with the expected toxicities; however, 27 hospitalizations occurred among 16 patients during or within 2 months after chemotherapy because of febrile neutropenia (7 cases), nonneutropenic infection (9), thrombosis (4), pulmonary toxicity (3), and other causes (4). No increase in viral infections was seen. Infections were grade 3 or less9 with 2 exceptions: a 42-year-old patient developed fatal, nonneutropenic sepsis, and a 28-year-old patient developed grade 4 neutropenic infection but recovered. Rituximab possibly increased the infection risk.10 A high delivered-dose intensity, coupled with avoidance of growth factors, possibly contributed, although others have reported low infection incidences with similar strategies.11
Rituximab has been increasingly evaluated in cHL,2,12-16 although its role and mechanism in cHL are not fully understood. Rituximab may potentiate the effects of cytotoxic chemotherapy, perhaps in part by depleting benign supporting B cells from the tumor microenvironment.1,2 Alternatively, rituximab might target neoplastic HRS stem cells or a B-cell population that is clonally related to these stem cells.4
The clinical outcomes are consistent with the possibility that rituximab improves long-term remission rates in cHL17,18 ; however, its contribution cannot be ascertained from the present small single-arm study. The outcome reporting is largely descriptive. Longer follow-up and randomized trials are needed to define the role of anti-CD20 therapy, with attention to both toxicity and tumor control end points.
Frontline regimens incorporating brentuximab vedotin are also under investigation.19 Although the ultimate role of these agents in cHL requires further study, the present clonotypic B-cell data suggest that brentuximab and rituximab are likely to target different cellular compartments in cHL.
Rare, biologically distinct cell populations with stem cell properties have now been reported in many cancers. Although their clinical significance remains hotly debated,20 recent data suggest they may be responsible for relapse in myelodysplastic syndrome21 and acute myeloid leukemia.22 Whether such circulating B cells in cHL represent “cancer stem cells” or a molecularly related population not critical for tumor maintenance or progression is unknown.4 These data suggest that further definition of their clinical significance in cHL and of the utility of their monitoring is warranted.
In cHL, EBV copy number in plasma is tumor-derived viral DNA rather than virion-packaged DNA. Its presence reflects tumor cell turnover rather than viremia.23,24 Consistent with previous reports,23,24 we found that EBV copy number in plasma rapidly disappeared in patients with EBV+ tumors, compatible with the possibility that EBV DNA is useful in monitoring tumor response in this setting. However, the small number of patients with EBV+ cHL and their uniformly good outcomes in the present series preclude definitive evaluation of the approach.
In conclusion, rituximab-ABVD was generally well tolerated. This study was not powered to demonstrate a clinical benefit. The study intended to begin to explore a new platform of B-cell targeting and therapeutic monitoring in cHL. We believe this is the first investigation of clonotypic B cells as prognostic markers in a clinical trial in cHL.
There is an Inside Blood commentary on this article in this issue.
Presented in poster form at the American Society of Clinical Oncology annual meeting, Chicago, IL, June 6, 2011.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Steven Piantadosi (Cedars-Sinai Medical Center, Los Angeles, CA) for input on study design.
This work was supported by the National Institutes of Health (K23 CA124465 to Y.L.K., and P01 CA015396 to R.J.J.), the National Cancer Institute Lymphoma SPORE (P50 CA09688) (R.F.A.), and Genentech Inc.
National Institutes of Health
Authorship
Contribution: Y.L.K. designed and performed research, collected data, analyzed and interpreted data, and wrote the manuscript; H.A.J. designed research and collected and analyzed imaging data; C.D.G. and W.H.M. contributed vital analytical tools; L.J.S., D.E.G., B.K.L., and T.M.H. performed research, collected and interpreted data, and revised the manuscript; B.P. performed research and analyzed data; L.L.P. performed research and collected and interpreted data; J.M.H. designed research; R.J.J. and R.F.A. designed and performed research, contributed vital analytical tools, collected data, analyzed and interpreted data, and wrote the manuscript; and all authors approved the final manuscript.
Conflict-of-interest disclosure: Y.L.K. has received research funding from Genentech, Syndax, and Seattle Genetics and has a compensated advisory role with Seattle Genetics; H.A.J. has received research funding from GlaxoSmithKline; L.J.S. has received research funding and honoraria from Genentech. B.K.L. has a compensated advisory or consultant role with Genentech, GlaxoSmithKline, and Allos Therapeutics; and R.J.J. holds the patent for Aldefluor and, under a licensing agreement between Aldagen and the Johns Hopkins University, is entitled to a share of royalties received by the University (the terms of this arrangement are being managed by the Johns Hopkins University in accordance with its conflict-of-interest policies). The remaining authors declare no competing financial interests.
Correspondence: Yvette L. Kasamon, MD, CRB I, Rm 388, 1650 Orleans St, Baltimore, MD 21287; e-mail: ykasamon@jhmi.edu.