Abstract
Salidroside is a phenylpropanoid glycoside isolated from the medicinal plant Rhodiola rosea, which has potent antioxidant properties. Here we show that salidroside prevented the loss of hematopoietic stem cells (HSCs) in mice under oxidative stress. Quiescent HSCs were recruited into cell cycling on in vivo challenge with oxidative stress, which was blocked by salidroside. Surprisingly, salidroside does not prevent the production of reactive oxygen species but reduces hydrogen peroxide–induced DNA-strand breaks in bone marrow cells enriched for HSCs. We tested whether salidroside enhances oxidative DNA damage repair in mice deficient for 5 DNA repair pathways known to be involved in oxidative DNA damage repair; we found that salidroside activated poly(ADP-ribose)polymerase-1 (PARP-1), a component of the base excision repair pathway, in mouse bone marrow HSCs as well as primary fibroblasts and human lymphoblasts. PARP-1 activation by salidroside protects quiescent HSCs from oxidative stress–induced cycling in native animals and self-renewal defect in transplanted recipients, which was abrogated by genetic ablation or pharmacologic inhibition of PARP-1. Together, these findings suggest that activation of PARP-1 by salidroside could affect the homeostasis and function of HSCs and contribute to the antioxidant effects of salidroside.
Introduction
Hematopoietic stem cells (HSCs) are a rare population of pluripotent cells that can self-renew and differentiate into various types of cells of the blood lineage.1 Under steady physiologic conditions, the most primitive HSCs are in a quiescent state and reside in the bone marrow (BM) niche where they preserve the capacity to self-renew and to continue to produce all types of blood cells throughout a prolonged life span without depleting the regenerative cell pool.2,3 In response to stress or stimulation, the HSCs can move out of the BM niche, entering cell cycle and undergoing division. In addition, the cycling HSCs may return to the BM niche and regain their quiescent state.4 Disruption of HSC quiescence prematurely exhausts the stem cell pool and causes hematologic failure under various stresses, such as oxidative stress, cell cycling, and aging.5,6
Oxidative stress, defined as an imbalance between the production of reactive oxygen species (ROS) and antioxidant defense, is most evident in states of aging and diseases such as BM failure and cancer.7,8 Even in a healthy state, HSCs are exposed to various ROS, which are routinely generated during metabolic or inflammatory process.9 ROS induce a variety of responses in HSCs, including cellular proliferation and apoptosis.10,11 ROS can also cause DNA damage and drive HSCs into cell division, which is essential for DNA repair processes.12,13 Similar to stem cells from other tissues, HSCs have developed several mechanisms to prevent the damage induced by oxidative stress. Antioxidant enzymes, including superoxide dismutases, catalase, glutathione peroxidases, and peroxiredoxins, can directly eliminate ROS.10 Other cellular enzymes can function to repair DNA damage induced by ROS in hematopoietic tissues.8,14 There is strong evidence that HSCs are activated and thus functionally exhausted by oxidative stress. Mice with mutations in the ATM or FOXO genes, as well as various DNA repair genes, exhibit premature exhaustion of HSCs because of accumulation of ROS or DNA damage, indicating that cellular balance between ROS and antioxidant defense, as well as DNA repair, is crucial for the maintenance of HSC self-renewal and hematopoietic function.15,16
Salidroside is a phenylpropanoid glycoside isolated from the medicinal plant Rhodiola rosea that grows in high altitude or cold regions of the world and was used as a folk medicine in France, Germany, and many European countries to fight fatigue in the 19th century.17 In present days, extract of R rosea has been used to enhance both the physical and mental performance.18,19 Salidroside (2-[4-hydroxyphenyl]ethyl β-D-glucopyranoside) has been reported to have anti-aging, anti-cancer, anti-inflammatory, and antioxidative functions.20-23 A recent study shows that salidroside promotes erythropoiesis and up-regulates the level of antioxidative enzymes glutathione peroxidase-1 and thioredoxin-1 to counteract oxidative stress.24 In this study, we show that salidroside prevented quiescent HSCs and progenitor cells from being recruited into cell cycling on in vivo challenge with oxidative stress in mice. Using several mouse models deficient for DNA repair pathways known to be involved in oxidative DNA damage repair (ODDR), we demonstrate that salidroside protects quiescent hematopoietic stem progenitor cells (HSPCs) from oxidative stress–induced cycling through stimulation activity of poly(ADP-ribose)polymerase-1 (PARP-1), a component of the base excision repair pathway.
Methods
Mice and treatments
Parp1−/− or Xpc−/− mice were generated by backcrossing 129S-Parp1tm1zqw/J or B6;129-Xpctm1Ecf/J (The Jackson laboratory), respectively, with wild-type (WT) C57BL/6 mice. Fancd2−/− mice (provided by Dr Markus Grompe, Oregon Health & Science University) or DNA-PKcs3A/3A mice (a gift from Benjamin P. C. Chen, University of Texas Southwestern Medical Center at Dallas)25 were generated by interbreeding heterozygous Fancd2± or DNA-PKcs+/3A mice, respectively. Brca2−/− mice were generated by Cre-mediated deletion of floxed alleles by crossing Brca2tm1Brn (NCI)26 with a Cre-ERT2 strain.27
For H2O2 treatment, mice were first screened with increasing doses of H2O2 (Sigma-Aldrich; 0, 0.05, 0.15, 0.25, 0.35, and 0.50 μmol/g body weight), and that the optimal dose (0.25 μmol/g body weight) was chosen for further experiments. For antioxidant treatment, mice were injected with salidroside (PhytoLab; 75μg/g body weight), manganese (III) tetrakis (4-benzoic acid) porphyrin (MnTBAP, Sigma-Aldrich; 10 μg/g body weight), N-acetyl-L-cysteine (NAC; Sigma-Aldrich; 50 μg/g body weight) or saline vehicle, intraperitoneally followed by H2O2 (0.25 μmol/g body weight). For NU1025 treatment, mice were injected with NU1025 (25 mg/kg body weight; Sigma-Aldrich) followed by H2O2 treatment. All experimental procedures conducted in this study were approved by the Institutional Animal Care and Use Committee of Cincinnati Children's Hospital Medical Center.
Parp1 activity
Parp1 activity was detected by flow cytometry as previously described.28 Briefly, cells were centrifuged and resuspended in 100% ethanol and left at −20°C for at least 20 minutes. Cells were then resuspended in ∼10 mL buffer A (10mM Tris-HCl pH 7.8, 1mM EDTA [ethylenediaminetetraacetic acid], 4mM MgCl2, and 30mM 2-mercaptoethanol). Then cells were centrifuged again and resuspended in buffer A again and transferred to a V-shaped 96-well plate on ice for at least 5 minutes. Then 20 μL of 3X reaction buffer (with or with NAD+) plus 13 μL of 15mM NaCl incorporating were added to the reaction mix followed by 37°C incubation for 10 minutes. Then second fixation was done by adding 60 μL of 4% formaldehyde/phosphate-buffered saline (PBS) for 20 minutes at room temperature. PBS was then added to quench the reaction. Cells were then centrifuged and resuspended in 100 μL primary antibody diluted in fluorescence-activated cell sorter (FACS) buffer and incubated at 37°C for 1 hour or overnight at 4°C. Then the cells were washed and resuspended in 100 μL of diluted secondary antibody (Alexa 488–conjugated goat anti–mouse; Invitrogen) followed by 37°C incubation for 30 minutes. Cells were washed and resuspended for flow cytometry analysis.
For flow cytometry and cell cycle analysis, BrdU incorporation, determination of ROS production, comet assay, and BM transplantation, see supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Results
Salidroside prevents oxidative stress–induced HSC loss in mice
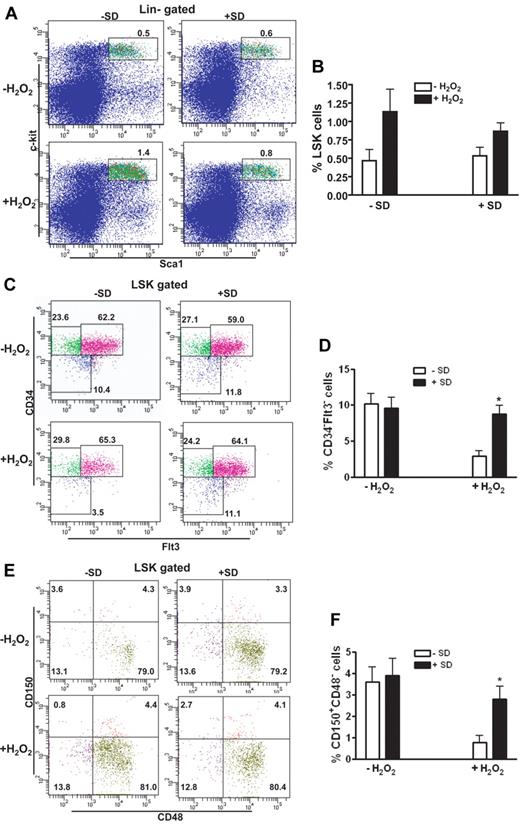
In an attempt to search for new chemopreventive and antioxidant agents that are effective and less toxic in hematopoietic improvement for patients with BM failure syndromes, such as Fanconi anemia (FA), in which oxidative stress is identified as a physiologic mediator of HSC loss,7 we investigated the antioxidant effect of salidroside on HSC maintenance. Salidroside (2-[4-hydroxyphenyl]ethyl β-D-glucopyranoside; supplemental Figure 1A), a phenylpropanoid glycoside found in the medicinal plant R rosea, has a wide range of biologic activities, such as antiaging, anticancer, anti-inflammatory, and antioxidative functions.20-23 To test the effect of salidroside on oxidative stress in HSC in mice, we first determined the optimal dose of hydrogen peroxide (H2O2) and found that 0.25 μmol/g body weight was the most effective dose that effectively induced DNA damage without causing death (supplemental Figure 1B). In addition, this range of H2O2 doses gave an inverse correlation between DNA damage and HSC repopulation (supplemental Figure 1C). We then used this dose of H2O2 to treat the mice and found that salidroside effectively antagonized H2O2-induced effect on WT HSCs in vivo (Figure 1). Although both salidroside and H2O2 treatments did not change absolute number of total BM cells (supplemental Figure 3), H2O2-treated mice had a much higher frequency of Lin-c-kit+Sca-1+ (LSK) cells in the BM than untreated mice, and salidroside partially limited this expansion (Figure 1A-B). Further analysis of the LSK compartment indicated that H2O2-treated mice had much lower LSK CD34−Flt3− cells, a population enriched for long-term (LT)–HSCs,29,30 than untreated control mice (Figure 1C-D). Significantly, salidroside almost completely abrogated H2O2-induced loss of LT-HSCs. We also determined the effect of salidroside on the BM HSC compartment using the CD150 and CD48-based immunophenotyping.31,32 Similarly, we observed that salidroside effectively rescued LT-HSCs (CD150+CD48− LSK) in H2O2-treated mice (Figure 1E-F). Moreover, salidroside protected H2O2-induced HSC loss in mice deficient for the FA gene, Fanca (Fanca−/−) or Fancc (Fancc−/−; supplemental Figure 2). Together, these data suggest that salidroside plays a selective role in the maintenance of LT-HSCs.
Salidroside prevents oxidative stress–induced HSC loss. (A) salidroside partially limits H2O2-induced LSK expansion. BM cells from WT C57BL/6 mice pretreated with or without salidroside (75μg/g body weight) followed by H2O2 (0.25 μmol/g body weight) injection were harvested for LSK (Lin-Sca1+c-kit+) cell frequency assessment using flow cytometry. (B) Quantification of LSK frequency in mice described in panel A. (C) SD prevents H2O2-induced LSK CD34-Flt3- cell loss. BM cells described in panel A were subjected to clow cytometry analysis for LSK CD34−Flt3− frequency. (D). Quantification of LSK CD34−Flt3− frequency in mice described in panel A. (E) SD prevents H2O2-induced LSK CD150+CD48− cell loss. BM cells described in panel A were subjected to Flow Cytometry analysis for LSK CD150+CD48− frequency. (F) Quantification of CD150+CD48− frequency in mice described in panel A. Results are means ± standard deviation (SD) of 3 independent experiments (n = 9 per group).
Salidroside prevents oxidative stress–induced HSC loss. (A) salidroside partially limits H2O2-induced LSK expansion. BM cells from WT C57BL/6 mice pretreated with or without salidroside (75μg/g body weight) followed by H2O2 (0.25 μmol/g body weight) injection were harvested for LSK (Lin-Sca1+c-kit+) cell frequency assessment using flow cytometry. (B) Quantification of LSK frequency in mice described in panel A. (C) SD prevents H2O2-induced LSK CD34-Flt3- cell loss. BM cells described in panel A were subjected to clow cytometry analysis for LSK CD34−Flt3− frequency. (D). Quantification of LSK CD34−Flt3− frequency in mice described in panel A. (E) SD prevents H2O2-induced LSK CD150+CD48− cell loss. BM cells described in panel A were subjected to Flow Cytometry analysis for LSK CD150+CD48− frequency. (F) Quantification of CD150+CD48− frequency in mice described in panel A. Results are means ± standard deviation (SD) of 3 independent experiments (n = 9 per group).
Salidroside enhances the ability of stressed HSCs to repopulate mouse hematopoietic system
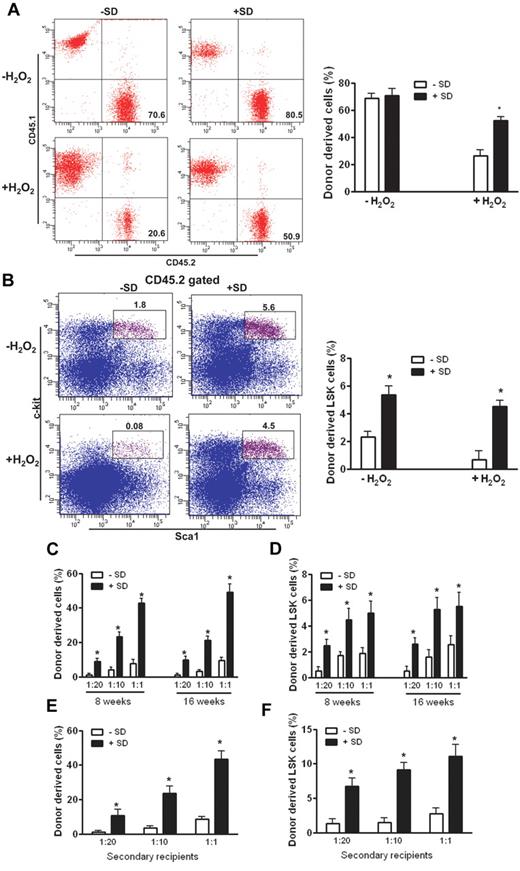
We next assessed whether salidroside improved the repopulating ability of oxidative stressed HSCs. We transplanted LSK cells from H2O2-treated WT C57BL/6 mice (CD45.2+) pretreated with or without salidroside, along with BM cells (depleted of c-Kit+ cells to provide short-term hematopoiesis after irradiation) obtained from congenic WT Boy J mice (CD45.1+), into lethally irradiated congenic recipients (CD45.1+). We observed a significant increase in both repopulated hematopoiesis in the peripheral blood (Figure 2A) and HSC-enriched LSK cells in the BM of recipients that received LSK cells from salidroside-treated mice (Figure 2B) at 4 months after transplantation. We also performed BM transplantation using total BM from stressed WT mice, which shows similar results (supplemental Figure 4). Further, we performed competitive reconstitution using limiting dilutions of LSK cells from stressed mice with defined ratios of WT LSK competitor cells, and analyzed hematopoietic reconstitution in recipient mice by donor cells at 8 and 16 weeks after transplantation. The results show that donor-repopulated hematopoiesis was greater in the recipients that received all doses of LSK cells from salidroside-treated mice than in those that received LSK cells from untreated mice (Figure 2C). Furthermore, the recipients transplanted with cells from salidroside-treated mice showed greater abundance of donor-derived LSK cells in the BM (Figure 2D). Analysis of the frequency of CD45.2+ lymphoid and myeloid as well as erythroid lineage cells in the peripheral blood showed that both groups of donor LSK cells had a similar multilineage-reconstitution capacity (supplemental Figure 5), indicating that salidroside does not affect cell fate or differentiation potential of HSCs. To investigate the long-term-repopulation abilities of salidroside-conditioned HSCs, we performed serial transplantation experiments by transplanting primary recipients of salidroside-treated or untreated mouse BM at 16 weeks after transplantation into lethally irradiated secondary recipients. Sixteen weeks after transplantation, we analyzed hematopoiesis derived from donor (CD45.2+) cells. The results show that donor-derived hematopoiesis from primary recipients of salidroside-treated BM was significantly greater in the secondary recipients than that from primary recipients of untreated BM (Figure 2E). Notably, this was correlated with an increased number of LSK cells in the bone marrow (Figure 2F). Together, these results demonstrate that salidroside enhances the ability of stressed HSCs to repopulate the hematopoietic system.
Salidroside enhances HSC repopulation. (A) Salidroside enhances the repopulating ability in recipient mice. LSK (Lin-Sca1+c-kit+) cells from WT C57BL/6 mice (CD45.2+) pretreated with or without salidroside (75μg/g body weight) followed by H2O2 (0.25 μmol/g body weight) treatment were isolated by cell sorting using FlowAria II. One thousand sorted LSK cells plus 1 million c-kit–depleted competitors (CD45.1+) were injected to lethally irradiated recipients. Donor chimerism was examined at 4 months after transplantation using peripheral blood from recipients. Representative images (left) and quantifications (right) were shown. Results are means ± SD of 3 independent experiments (n = 15 per group). (B) Salidroside increases HSC-enriched LSK cells in recipient mice. One thousand sorted LSK cells from WT C57BL/6 mice (CD45.2+) pretreated with or without salidroside followed by H2O2 treatment were injected along with 1 million c-kit–depleted competitors (CD45.1+) to lethally irradiated recipients. BM cells from recipient mice were harvested and subjected to flow cytometry analysis for LSK frequency. (C) Salidroside enhances competitive reconstitution of stressed HSCs. Various numbers of donor (CD45.2+) LSK cells (50, 100, or 1000) from stressed WT C57BL/6 mice were mixed with 1000 competitor LSK (CD45.1+) cells and the mixtures were transplanted intravenously into lethally irradiated congenic (CD45.1+) recipients. Donor-derived hematopoietic reconstitution was analyzed by flow cytometry 8 and 16 weeks after transplantation using peripheral blood from recipients. (D) Salidroside increases HSC-enriched LSK in recipient mice. BM cells from recipient mice described in panel C were subjected to flow cytometry analysis for LSK frequency. (E-F) Salidroside enhances LT-HSC repopulation. BM cells from primary recipient mice described in panel B were used for secondary transplantation by injecting 5 × 106 BM cells to lethally irradiated recipients. Donor-derived chimerism (E) and LSK (F) were analyzed by flow cytometry 16 weeks after BMT. Results are means ± SD of 2 independent experiments (n = 10 per group).
Salidroside enhances HSC repopulation. (A) Salidroside enhances the repopulating ability in recipient mice. LSK (Lin-Sca1+c-kit+) cells from WT C57BL/6 mice (CD45.2+) pretreated with or without salidroside (75μg/g body weight) followed by H2O2 (0.25 μmol/g body weight) treatment were isolated by cell sorting using FlowAria II. One thousand sorted LSK cells plus 1 million c-kit–depleted competitors (CD45.1+) were injected to lethally irradiated recipients. Donor chimerism was examined at 4 months after transplantation using peripheral blood from recipients. Representative images (left) and quantifications (right) were shown. Results are means ± SD of 3 independent experiments (n = 15 per group). (B) Salidroside increases HSC-enriched LSK cells in recipient mice. One thousand sorted LSK cells from WT C57BL/6 mice (CD45.2+) pretreated with or without salidroside followed by H2O2 treatment were injected along with 1 million c-kit–depleted competitors (CD45.1+) to lethally irradiated recipients. BM cells from recipient mice were harvested and subjected to flow cytometry analysis for LSK frequency. (C) Salidroside enhances competitive reconstitution of stressed HSCs. Various numbers of donor (CD45.2+) LSK cells (50, 100, or 1000) from stressed WT C57BL/6 mice were mixed with 1000 competitor LSK (CD45.1+) cells and the mixtures were transplanted intravenously into lethally irradiated congenic (CD45.1+) recipients. Donor-derived hematopoietic reconstitution was analyzed by flow cytometry 8 and 16 weeks after transplantation using peripheral blood from recipients. (D) Salidroside increases HSC-enriched LSK in recipient mice. BM cells from recipient mice described in panel C were subjected to flow cytometry analysis for LSK frequency. (E-F) Salidroside enhances LT-HSC repopulation. BM cells from primary recipient mice described in panel B were used for secondary transplantation by injecting 5 × 106 BM cells to lethally irradiated recipients. Donor-derived chimerism (E) and LSK (F) were analyzed by flow cytometry 16 weeks after BMT. Results are means ± SD of 2 independent experiments (n = 10 per group).
Salidroside prevents oxidative stress–induced HSC cycling in vivo
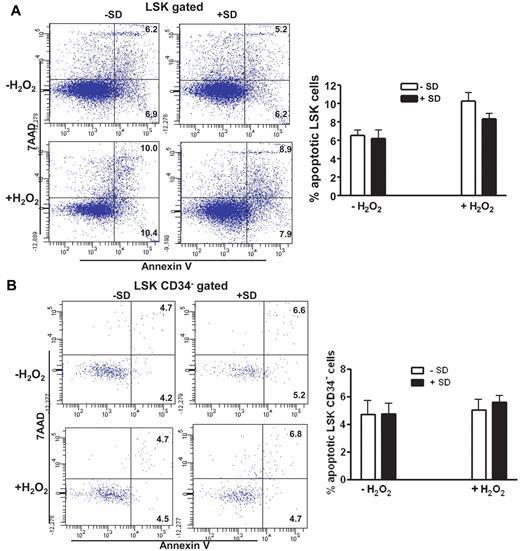
To identify the mechanism of salidroside in HSC maintenance, we asked whether salidroside prevented oxidative stress–induced apoptosis of HSCs in stressed animals. We observed increased apoptosis of LSK cells in H2O2-treated WT C57BL/6 mice compared with untreated mice, and salidroside partially reduced the stress-induced apoptosis (Figure 3A). However, H2O2 treatment did not induce significant apoptosis in the more primitive CD34− LSK cell compartment29 and salidroside had no effect on the apoptosis in the cell population (Figure 3B). This finding suggests that the loss of HSCs in stressed mice may be because of the recruitment of quiescent HSCs into cell cycle on challenge with oxidative stress. To test whether salidroside played a role in preventing quiescent HSCs from entering cell cycling or proliferation, we first analyzed the cell cycle profile of CD34− LSK cells by staining RNA and DNA with pyronin Y and Hoechst 33342, respectively. Quiescent cell populations can be identified by negative to low pyronin Y staining; whereas actively cycling cells are positive for pyronin Y.33 There was a marked decrease in the number of quiescent (pyronin Y-negative; G0) and an increase in the number of cycling (pyronin Y-positive; G1+S) CD34− LSK cells in H2O2-treated mice compared with the unstressed animals (Figure 3C). Salidroside treatment significantly decreased cycling, and at the same time, increased quiescent (G0) CD34− LSK cells in the stressed mice. We also performed in vivo BrdU labeling in mice to determine the proliferative status of HSCs in the BM. In line with the cell-cycle data, the percentage of CD34− LSK cells that incorporated BrdU was elevated in H2O2-treated mice compared with the unstressed animals, which was largely abrogated by salidroside (Figure 3D). These data suggest that salidroside prevents quiescent HSCs from oxidative stress–induced cycling.
Salidroside prevents HSC cycling in vivo. (A) Salidroside partially reduces H2O2-induced LSK cell apoptosis. BM cells were harvested from WT C57BL/6 mice pretreated with or without salidroside (75μg/g body weight) followed by H2O2 (0.25 μmol/g body weight), and gated for LSK population. Apoptotic LSK cells were determined by annexin-V and 7AAD staining. (B) Salidroside and H2O2 have no effect on apoptosis of CD34-LSK cells. CD34 negative LSK cells were gated for apoptosis analysis. (C-D) Salidroside prevents HSC cycling in vivo. Cells described in panel B were subjected to Hochest and pyronin Y (C) or BrdU (D) staining. Representative images (top) and quantifications (bottom) were shown. Results are means ± SD of 3 independent experiments (n = 9 per group).
Salidroside prevents HSC cycling in vivo. (A) Salidroside partially reduces H2O2-induced LSK cell apoptosis. BM cells were harvested from WT C57BL/6 mice pretreated with or without salidroside (75μg/g body weight) followed by H2O2 (0.25 μmol/g body weight), and gated for LSK population. Apoptotic LSK cells were determined by annexin-V and 7AAD staining. (B) Salidroside and H2O2 have no effect on apoptosis of CD34-LSK cells. CD34 negative LSK cells were gated for apoptosis analysis. (C-D) Salidroside prevents HSC cycling in vivo. Cells described in panel B were subjected to Hochest and pyronin Y (C) or BrdU (D) staining. Representative images (top) and quantifications (bottom) were shown. Results are means ± SD of 3 independent experiments (n = 9 per group).
Salidroside reduces H2O2-induced DNA-strand breaks in HSPCs
Because salidroside has antioxidative activity,20,21,24 we tested whether salidroside could function as a free radical scavenger in BM HSCs of mice stressed with H2O2, which is a potent producer of reactive oxygen species (ROS). We measured ROS by staining BM CD34− LSK cells from H2O2-injected mice with CM-H2 DCFDA, a cell-permeable fluorescent dye that reacts to a broad spectrum of ROS. To accurately evaluate the effect of salidroside on ROS, we used 2 small antioxidant molecules, NAC, and manganese (III) tetrakis (4-benzoic acid) porphyrin (MnTBAP). NAC stimulates the formation of the endogenous reducing agent glutathione, which cells use to scavenge H2O2.10 MnTBAP has catalytic activities similar to the ROS-scavenging enzymes superoxide dismutase (SOD) and catalase.34 We pretreated the mice with salidroside, NAC, or MnTBAP, and then concomitantly with H2O2. Compared with NAC and MnTBAP, both eliminated most of the ROS generated in the BM LSK cells of H2O2-injected mice, salidroside did not prevent the production of ROS in these cells (Figure 4A). This finding suggests that salidroside antagonizes oxidative stress through a distinct mechanism that does not involve ROS-scavenging.
Salidroside reduces DNA strand breaks but not ROS. (A) Salidroside does not reduce H2O2-induced ROS. WT C57BL/6 mice were pretreated with one dose of salidroside (75μg/g body weight), NAC (50μg/g body weight), or MnTBAP (10μg/g body weight), followed by H2O2 (0.25 μmol/g body weight). BM cells were then harvested and labeled with CM-H2DCFDA for flow cytometry analysis of ROS in the LSK-gated cells. Representative images (left) and quantifications (right) were shown. (B) Salidroside reduces DNA strand breaks. BM cells from mice described in panel A were isolated by magnetic beads depletion, and then subjected to analysis for DNA strand breaks by the comet assay. The mean tail moment of untreated vehicle sample is expressed as 100%. Larger tail moment represents higher levels of DNA damage. For each treatment, 50 cells were scored from random sampling. (C) Salidroside reduces 8-oxodG. Protein extractions were prepared using low density BM cells from mice described in panel A. Whole cell lysates were then subjected to SDS-PAGE and immunoblotted with Western Blot antibodies for 8-oxodG and β-actin. (D) Repair kinetics. Low-density BM cells were pretreated with salidroside (250μM), NAC (500μM), or MnTBAP (250μM) for 2 hours, followed by H2O2 for additional 2 hours, and then released for indicated time intervals. Whole cell lysates were prepared and subjected to SDS-PAGE and immunoblotted with antibodies for 8-oxodG and β-actin.
Salidroside reduces DNA strand breaks but not ROS. (A) Salidroside does not reduce H2O2-induced ROS. WT C57BL/6 mice were pretreated with one dose of salidroside (75μg/g body weight), NAC (50μg/g body weight), or MnTBAP (10μg/g body weight), followed by H2O2 (0.25 μmol/g body weight). BM cells were then harvested and labeled with CM-H2DCFDA for flow cytometry analysis of ROS in the LSK-gated cells. Representative images (left) and quantifications (right) were shown. (B) Salidroside reduces DNA strand breaks. BM cells from mice described in panel A were isolated by magnetic beads depletion, and then subjected to analysis for DNA strand breaks by the comet assay. The mean tail moment of untreated vehicle sample is expressed as 100%. Larger tail moment represents higher levels of DNA damage. For each treatment, 50 cells were scored from random sampling. (C) Salidroside reduces 8-oxodG. Protein extractions were prepared using low density BM cells from mice described in panel A. Whole cell lysates were then subjected to SDS-PAGE and immunoblotted with Western Blot antibodies for 8-oxodG and β-actin. (D) Repair kinetics. Low-density BM cells were pretreated with salidroside (250μM), NAC (500μM), or MnTBAP (250μM) for 2 hours, followed by H2O2 for additional 2 hours, and then released for indicated time intervals. Whole cell lysates were prepared and subjected to SDS-PAGE and immunoblotted with antibodies for 8-oxodG and β-actin.
To further elucidate the action of mechanism by salidroside, we determined whether salidroside played a role in preventing oxidative DNA damage in HSPCs of the H2O2-treated mice. We used the comet assay35 using a Fpg-FLARE (fragment length analysis using repair enzymes) assay kit, which measures specifically oxidative DNA damage including single and double-strand DNA breaks.36 There was significant accumulation of DNA damage in BM Lin- cells freshly isolated from H2O2-treated mice, which was largely eliminated by salidroside, as well as by NAC or MnTBAP (Figure 4B). Consistent with this, H2O2 induced expression of γ-H2AX, a robust indicator of DNA strand breaks,37 and all 3 antioxidants suppressed γ-H2AX expression in BM cells from H2O2-treated mice (supplemental Figure 6). A similar increase in 8-oxo-deoxyguanosin (8-oxodG), an established marker of oxidative DNA damage,9 was also demonstrated in BM cells from H2O2-injected mice (Figure 4C). Cotreatment of H2O2-injected mice with salidroside, or the ROS scavenger NAC or MnTBAP reduced the accumulation of 8-oxodG (Figure 4C).
To distinguish the antioxidant property of salidroside from that of NAC or MnTBAP in the context of oxidative DNA damage repair, we pretreated fresh-isolated low-density BM cells with salidroside, NAC, or MnTBAP for 2 hours, followed by H2O2 for additional 2 hours, and then measured the remaining amounts of 8-oxodG and DNA strand breaks for a period of 12 hours after H2O2 treatment. There was significant difference between the effect of salidroside and that of NAC or MnTBAP in terms of the kinetics of 8-oxodG removal. Specifically, the level of oxidative DNA damage was not decreased until 4 hours after salidroside treatment with almost no 8-oxodG remaining at 12 hours (Figure 4D). However, NAC and MnTBAP reduced the level of 8-oxodG at each time point after H2O2 incubation. This result indicates that the ROS scavengers NAC and MnTBAP have no effect on the repair of 8-oxodG, which is consistent with their function in reducing ROS formation and preventing DNA damage. We observed similar repair kinetics of DNA strand breaks in a comet assay, in which DNA strand breaks remained high in salidroside-treated cells during 8 hours after H2O2 incubation (supplemental Figure 7). Together, these results suggest that salidroside may use different mechanism from that of the ROS scavenger NAC or MnTBAP in repair of oxidative DNA damage.
Salidroside stimulates PARP-1 activity
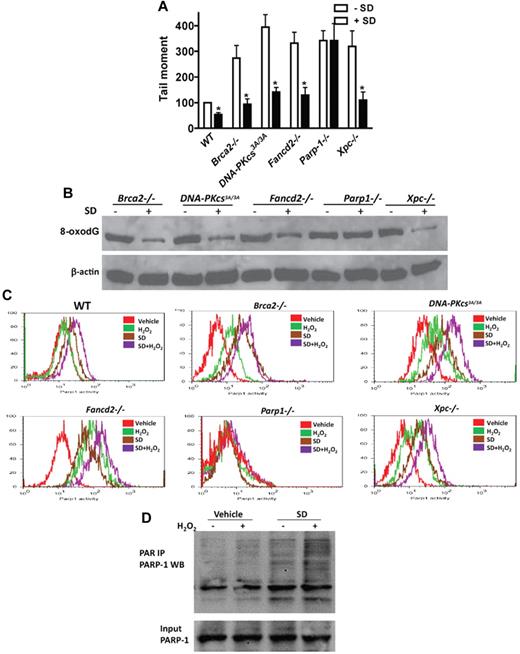
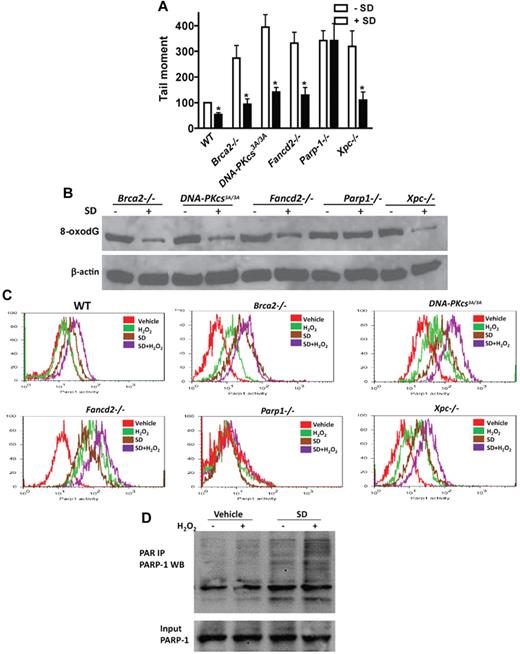
To identify the mechanism by which salidroside antagonists the effect of oxidative stress on HSC maintenance, we next tested whether salidroside enhanced ODDR in mice deficient for 5 different DNA repair pathways known to be involved in ODDR, including homologous recombination (HR), nonhomologous end-joining (NHEJ), base excision repair (BER), nucleotide excision repair (NER), and FA pathways.7,38-40 We observed reduction of H2O2-induced DNA strand breaks by salidroside in BM Lin- cells from mice deficient for genes functioning in HR (Brca2−/−),41 NHEJ (DNA-PKcs3A/3A),42 NER (Xpc−/−),43 and FA (Fancd2−/−)44 pathways (Figure 5A). However, salidroside failed to reduce H2O2-induced DNA strand breaks in HSPCs from mice deleted for the gene encoding poly(ADP-ribose)polymerase-1 (PARP-1), a component of the BER pathway (Figure 5A). Analysis of H2O2-induced 8-oxodG in these gene-knockout BM cells showed similar results, with salidroside accelerating clearance of the oxidative DNA adducts in Brca2−/−, DNA-PKc3A/3A, Fancd2−/−, and Xpc−/− cells but having no effect in Parp-1−/− cells (Figure 5B). These results suggest that salidroside may specifically target PARP-1 in response to oxidative DNA damage. To test this notion, we used a flow-cytometric assay to determine the effect of salidroside on the enzyme activity of PARP-1 in BM LSK cells from WT or knockout mice deficient for each of the 5 repair pathways. We found that salidroside stimulated PARP-1 activity in cells from all except Parp-1 knockout mice treated with or without H2O2 (Figure 5C). We next determined whether salidroside stimulated PARP-1 activity in other cell types. We isolate mouse embryonic fibroblasts (MEFs) from WT mice and cotreated the cells with salidroside and H2O2. We observed similar stimulation of PARP-1 activity by salidroside in these MEFs (supplemental Figure 8A). We also found that salidroside enhanced PARP-1 activity in human lymphoblasts (supplemental Figure 8B). To substantiate these observations, we performed immunoprecipitation to determine the stimulatory effect of salidroside on PARP1 activity in vivo. We cotreated fresh-isolated BM cells with H2O2 and salidroside, and subjected the cell lysates to immunoprecipitation with PAR antibodies followed by Western blot with PARP1 antibodies. Consistent with the results obtained from the flow-cytometric assay, we observed enhanced PARP1 poly-ADP-ribosylation by salidroside in both stressed and unstressed cells (Figure 5D). Together, these results indicate that salidroside reduces oxidative DNA damage through stimulation of PARP-1 activity.
Salidroside stimulates PARP-1 activity. (A) Salidroside fails to reduce H2O2-induced DNA strand breaks in Parp-1−/− cells. Lin− cells were isolated from Brca2−/−, DNA-PKcs3A/3A, Fancd2−/−, Parp-1−/−, or Xpc−/− mice. Cells were treated with or without salidroside, NAC, or MnTBAP in the presence of H2O2, and subjected to Comet Assay. The mean tail moment of H2O2-treated WT sample is expressed as 100%. For each sample, 50 cells were scored from random sampling. (B) Salidroside fails to reduce H2O2-induced 8-oxodG in Parp-1−/− cells. Protein lysates were prepared from low-density BM cells treated as described in panel A. Whole cell lysates were subjected to SDS-PAGE and immunoblotted with antibodies against 8-oxodG and β-actin. (C) Salidroside stimulates Parp-1 activity. Low-density BM cells isolated from mice described in panel A were treated with or without H2O2 and salidroside, and BM cells were isolated and subjected to flow cytometry analysis for Parp-1 activity in the LSK population using antibody against PARP-1. (D) Salidroside activates Parp-1 in vivo. Low-density BM cells were isolated from WT mice, and treated with or without SD and H2O2 for 2 hours. Whole cell lysates were subjected to immunoprecipitation using PAR antibody. Precipitated samples were resolved by SDS-PAGE and blotted with antibody specific for PARP-1.
Salidroside stimulates PARP-1 activity. (A) Salidroside fails to reduce H2O2-induced DNA strand breaks in Parp-1−/− cells. Lin− cells were isolated from Brca2−/−, DNA-PKcs3A/3A, Fancd2−/−, Parp-1−/−, or Xpc−/− mice. Cells were treated with or without salidroside, NAC, or MnTBAP in the presence of H2O2, and subjected to Comet Assay. The mean tail moment of H2O2-treated WT sample is expressed as 100%. For each sample, 50 cells were scored from random sampling. (B) Salidroside fails to reduce H2O2-induced 8-oxodG in Parp-1−/− cells. Protein lysates were prepared from low-density BM cells treated as described in panel A. Whole cell lysates were subjected to SDS-PAGE and immunoblotted with antibodies against 8-oxodG and β-actin. (C) Salidroside stimulates Parp-1 activity. Low-density BM cells isolated from mice described in panel A were treated with or without H2O2 and salidroside, and BM cells were isolated and subjected to flow cytometry analysis for Parp-1 activity in the LSK population using antibody against PARP-1. (D) Salidroside activates Parp-1 in vivo. Low-density BM cells were isolated from WT mice, and treated with or without SD and H2O2 for 2 hours. Whole cell lysates were subjected to immunoprecipitation using PAR antibody. Precipitated samples were resolved by SDS-PAGE and blotted with antibody specific for PARP-1.
Inhibition of PARP-1 abrogates the effect of salidroside on HSC maintenance
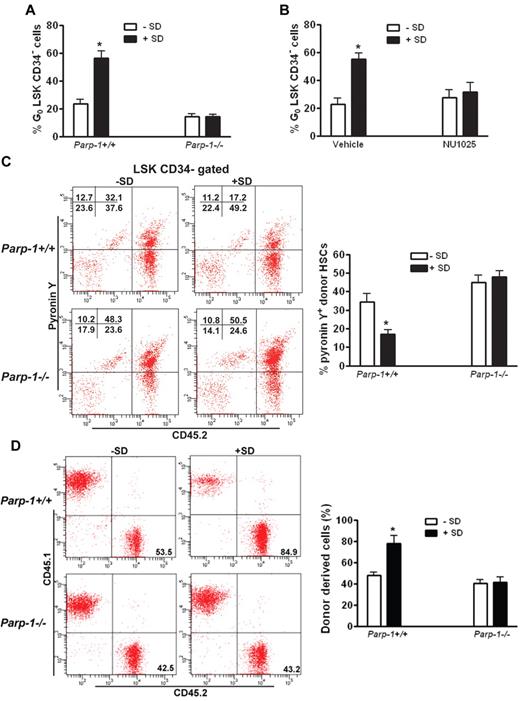
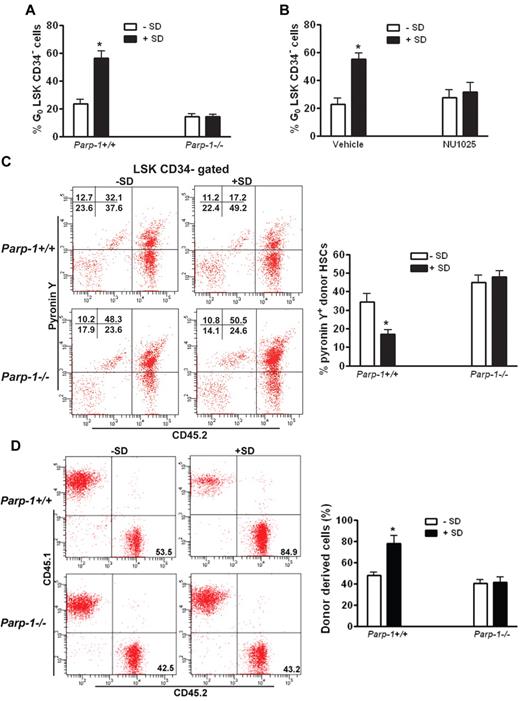
We next sought to test whether PARP-1 activation by salidroside protected quiescent HSCs from oxidative stress–induced cycling. We took 2 approaches: genetic ablation of the Parp-1 gene and pharmacologic inhibition of PARP-1 enzymatic activity in oxidative stressed mice to further elucidate the specific action of salidroside in PARP-1 stimulation. Compared with WT mice in which salidroside increased quiescent HSC pool significantly, salidroside had no discernible effect on oxidative stress–induced HSC cycling in Parp-1−/− mice (Figure 6A). To substantiate this finding, we made use of a specific small molecule inhibitor of PARP-1, 8-hydroxy-2-methylquinazolinone (NU1025).45 Consistent with PARP-1 knockout, NU1025 treatment abolished salidroside-mediated increase in quiescent HSC frequency in stressed mice (Figure 6B). As expected, both salidroside and NU1025 had no effect on oxidative stress–induced HSC cycling in Parp-1−/− mice (supplemental Figure 9). Collectively, these results provided convincing evidence to show that protection of quiescent HSCs by salidroside from oxidative stress–induced cycling requires PARP-1.
Inhibition of PARP-1 abrogates the effect of salidroside on HSC maintenance. (A) Deletion of Parp-1 abolishes salidroside-mediated increase in quiescent HSC frequency in stressed mice. Parp1−/− mice as well as their WT littermates were pretreated with or without salidroside (75 μg/g body weight) followed by H2O2 (0.25 μmol/g body weight). BM cells were subjected to flow cytometry analysis for quiescent HSC (G0 phase). Results are means ± SD of 3 independent experiments (n = 9 per group). (B) NU1025 treatment abolishes salidroside-mediated increase in quiescent HSC frequency in stressed mice. H2O2-treated (0.25 μmol/g body weight) WT mice were treated with or without salidroside (SD; 75μg/g body weight) and NU1025 (25 mg/kg body weight). BM cells were subjected to flow cytometry analysis for quiescent HSC. Results are means ± SD of 3 independent experiments (n = 9 per group). (C) The maintenance of HSC quiescence by salidroside requires Parp-1. 1000 LSK cells from WT or Parp1−/− mice were injected to lethally irradiated WT recipients. Recipient mice were then subjected to salidroside and H2O2 treatments. Cycling donor-derived (CD45.2+) cells were assessed by Flow Cytometry analysis by pyronin Y staining. Representative images (top) and quantifications (bottom) were shown. Results are means ± SD of 3 independent experiments (n = 9 per group). (D) The enhancing effect of salidroside on the long-term repopulation abilities of stressed HSCs requires Parp1. Cells from primary recipients described in panel C were used for second transplantation by injecting 10 million whole BM cells to lethally irradiated WT recipients. Donor-derived cells were determined by flow cytometry analysis. Representative images (top) and quantifications (bottom) were shown. Results are means ± SD of 3 independent experiments (n = 9 per group).
Inhibition of PARP-1 abrogates the effect of salidroside on HSC maintenance. (A) Deletion of Parp-1 abolishes salidroside-mediated increase in quiescent HSC frequency in stressed mice. Parp1−/− mice as well as their WT littermates were pretreated with or without salidroside (75 μg/g body weight) followed by H2O2 (0.25 μmol/g body weight). BM cells were subjected to flow cytometry analysis for quiescent HSC (G0 phase). Results are means ± SD of 3 independent experiments (n = 9 per group). (B) NU1025 treatment abolishes salidroside-mediated increase in quiescent HSC frequency in stressed mice. H2O2-treated (0.25 μmol/g body weight) WT mice were treated with or without salidroside (SD; 75μg/g body weight) and NU1025 (25 mg/kg body weight). BM cells were subjected to flow cytometry analysis for quiescent HSC. Results are means ± SD of 3 independent experiments (n = 9 per group). (C) The maintenance of HSC quiescence by salidroside requires Parp-1. 1000 LSK cells from WT or Parp1−/− mice were injected to lethally irradiated WT recipients. Recipient mice were then subjected to salidroside and H2O2 treatments. Cycling donor-derived (CD45.2+) cells were assessed by Flow Cytometry analysis by pyronin Y staining. Representative images (top) and quantifications (bottom) were shown. Results are means ± SD of 3 independent experiments (n = 9 per group). (D) The enhancing effect of salidroside on the long-term repopulation abilities of stressed HSCs requires Parp1. Cells from primary recipients described in panel C were used for second transplantation by injecting 10 million whole BM cells to lethally irradiated WT recipients. Donor-derived cells were determined by flow cytometry analysis. Representative images (top) and quantifications (bottom) were shown. Results are means ± SD of 3 independent experiments (n = 9 per group).
To provide functional evidence that HSC maintenance by salidroside under oxidative stress requires PARP-1, we assessed the ability of salidroside to preserve the self-renewal and hematopoietic reconstitution capacity of HSCs in the context of PARP-1 function. We transplanted BM LSK cells from WT or Parp-1−/− mice into lethally irradiated WT recipient mice, which were treated with salidroside and H2O2 at 4 months after transplantation. On injection with H2O2, we observed that cell cycle progression induced by oxidative stress was not prevented by salidroside in donor-derived HSCs deficient for Parp-1 (Figure 6C), which suggests that the action of salidroside in maintaining quiescent HSCs requires PARP-1. To determine whether the effect of salidroside on the long-term repopulation abilities of HSCs also required PARP-1, we did serial transplantation experiments by transplanting BM cells from the salidroside and H2O2-treated primary recipients of Parp-1+/+ or Parp-1−/− BM cells into lethally irradiated WT secondary recipients. Four months after transplantation, we obtained peripheral blood from the secondary recipient mice and analyzed hematopoietic reconstitution derived from donor (CD45.2+) cells. Our analysis indicated that salidroside failed to enhance donor-derived hematopoiesis in recipients transplanted with Parp-1−/− HSCs (Figure 6D). These findings suggest that the effect of salidroside on the maintenance of quiescent HSCs under oxidative stress requires PARP-1.
Discussion
Oxidative stress has been linked to aging and cancer as well as other major human health problems. A promising strategy for preventing oxidative damage to the cell is to use readily available natural compounds isolated from vegetables, fruits, and herbs. Many of the phytochemicals have already been identified to have chemopreventive potential, capable of intervening in tumorigenesis. We investigated the potential of the natural antioxidant salidroside as a new chemopreventive and antioxidant agent that has therapeutic value for patients with BM failure or leukemia. Salidroside has been reported to have antiaging, anticancer, anti-inflammatory, and antioxidative functions.20-23 In this study, we demonstrate that salidroside promotes the maintenance of mouse HSCs under oxidative stress. There are several findings that highlight the significance of our study: first, salidroside prevents quiescent HSCs from oxidative stress–induced activation; second, salidroside does not function as a ROS scavenger but enhances oxidative DNA damage repair through a mechanism involving stimulation of repair enzyme PARP-1 activity; and third, PARP-1 activation by salidroside protects quiescent HSCs from oxidative stress–induced cycling and repopulating defect. Thus, salidroside plays a significant role in inhibiting oxidative DNA damage; and thereby regulates the homeostasis of HSCs.
Quiescence has been postulated to prevent HSC exhaustion.3 In the BM, HSCs are kept in a low proliferative, relatively quiescent state within the BM microenvironment termed niches. Whereas numerous molecular factors that contribute to quiescence exist in the HSCs and the BM niche, HSCs are inevitably exposed to stress, such as accumulation of ROS and DNA damage, which can drive HSCs into uncontrolled cell-cycle entry and excessive proliferation. Indeed, we found that on challenge with oxidative stress, quiescent HSCs were recruited into cell cycle, evidenced by a marked decrease in the number of quiescent and an increase in the number of cycling CD34− LSK cells in H2O2-treated mice compared with the unstressed animals. We confirmed this finding by showing elevated BrdU-positive CD34− LSK cells in H2O2-treated mice. Functionally, we demonstrate that oxidative stress impaired long-term repopulation abilities of HSCs. These results are consistent with the recent reports describing functional exhaustion of HSCs, because of uncontrolled accumulation of ROS within the HSC population, in mice deficient in several oxidative damage response and repair pathways.15,46,47 It is in this context that we identified salidroside as a potent small molecule that prevents exhaustion of HSCs from oxidative stress–induced uncontrolled cell-cycle entry and excessive proliferation.
Another interesting finding of our study is that unlike other antioxidants, such as NAC, MnTBAP, and quercetin that function as free radical scavengers, salidroside does not prevent the production of ROS but acts to eliminate oxidative DNA damage in BM cells enriched for HSCs, as demonstrated by a robust reduction of DNA-stranded breaks and expression of γ-H2AX and 8-oxodG. More importantly, we present evidence of distinct kinetics of 8-oxodG clearance by salidroside compared with those by the ROS scavenger NAC or MnTBAP. That is, the effect of salidroside on repair kinetics consistently lags those of NAC and MnTBAP. We propose that salidroside acts directly in the process of oxidative DNA damage repair. Oxidative DNA damage by increased ROS accumulation in HSCs has been documented in several recent studies using age-related murine HSCs15,16 and more recently human HSCs.13 Although these studies suggest a correlation between oxidative DNA damage and impaired HSC maintenance, little is known about the mechanism by which oxidative DNA damage influences the function of HSCs in disease states, such as BM failure and leukemia, which are commonly accompanied by defective DNA repair. Our results suggest that oxidative DNA damage drives HSCs into cell cycling, which may be a prerequisite for DNA repair to proceed. Inefficient repair of oxidative DNA damage in these disease conditions may prolong cell cycling, which leads to HSC exhaustion. In addition, patients with BM failure and leukemia often have an extremely low number of HSCs, which critically limits the success of stem cell therapy. Therefore, one of the key issues in these disease states is to identify conditions to increase the number of HSCs under stressful conditions, either in vivo or during ex vivo growth cultures. We show here that salidroside promotes HSC self-renewal and preservation under oxidative stress and thus may be valuable for HSC expansion.
Although further studies are required to reveal the underlying molecular mechanisms, it is intriguing that salidroside promotes HSC maintenance by reducing oxidative DNA damage through stimulation of PARP-1 activity. Studies conducted using mutant mice have underscored the importance of DNA repair pathways in maintaining the functionality of HSCs. A number of studies demonstrate hematopoietic defects in mice deficient for HR, NHEJ, NER, and telomere maintenance pathways.15,16 In the context of oxidative DNA damage response/repair in HSC maintenance, recent studies in mice deficient for the ATM kinase or the Foxo transcription factors showed impaired HSC function as a result of increased accumulation of ROS in HSCs.47-50 Cumulatively, these studies suggest that genomic DNA integrity is a limiting factor in the maintenance of functional HSCs. Indeed we observed defective long-term hematopoietic repopulation by oxidative stressed HSCs from mice lacking the BER enzyme Parp-1. Furthermore, our results indicate that salidroside reduces oxidative DNA damage through stimulation of PARP-1 activity, and that inhibition of PARP-1 abrogates the beneficial effect of salidroside on HSC maintenance. These data suggest that stimulation of PARP-1 activity by salidroside could account for the accelerated repair of the oxidative DNA damage and enhanced repopulating capacity of the stressed HSCs.
Another interesting finding of this study is the observation that salidroside was able to maintain the oxidative stressed HSCs from WT but not Parp-1−/− mice in quiescent state in transplanted mice. Cell-cycle analysis shows that on challenge with oxidative stress, donor-derived HSCs exited quiescence and underwent cycling. However, these proliferating HSCs were able to regain quiescence in recipient mice conditioned with salidroside. This phenomenon was probably because of Parp-1 stimulation by salidroside, because donor-derived HSCs deficient for Parp-1 failed to regain quiescent in salidroside-conditioned recipient mice. These results might suggest that cell-cycle status of HSCs could reversibly change from oxidative stress–induced activation back to quiescence after repair of the oxidative DNA damage. This finding raises important questions as to whether activation is required for efficient repair of oxidative DNA damage in the stressed HSCs and whether elimination of the DNA damage is sufficient for the activated HSCs to return to quiescent state. Although further evidence needs to be provided, it is tempting to speculate that reversibility between quiescence and activation may be a physiologic function of activated HSCs at the interface between damage repair and reestablishment of homeostasis. In this context, it is noteworthy that recent studies in both mice and human show that HSCs can reversibly switch between dormancy and self-renewal at the interface between homeostasis and repair.12,13
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Markus Grompe (Oregon Health & Science University) for Fancd2± mice, Benjamin Chen (University of Texas Southwestern Medical Center at Dallas) for DNA-PKcs+/3A mice, Dr Liang Li for technical assistance, and the Comprehensive Mouse and Cancer Core of the Cincinnati Children's Research Foundation (Cincinnati Children's Hospital Medical Center) for BM transplantation service.
This work was supported by a Visiting Scholarship from South China Normal University (X.L.) and partially by National Institutes of Health (NIH) grants R01 HL076712 and R01 CA157537. Q.P. is supported by a Leukemia & Lymphoma Scholar award. W.D. is supported by an NIH T32 training grant.
National Institutes of Health
Authorship
Contribution: X.L. designed research and performed research and analyzed data; J.S. performed research; Q.P. designed research and analyzed data; and W.D. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Wei Du, Division of Experimental Hematology and Cancer Biology, Cincinnati Children's Hospital Medical Center, 3333 Burnet Ave, Cincinnati, OH 45229; e-mail: wei.du@cchmc.org.