Abstract
Rising BCR-ABL1 transcripts indicate potential loss of imatinib response in CML. We determined whether the BCR-ABL1 doubling time could distinguish nonadherence from resistance as the cause of lost response. Distinct groups were examined: (1) acquired clinical resistance because of blast crisis and/or BCR-ABL1 mutations; and (2) documented imatinib discontinuation/interruption. Short doubling times occurred with blast crisis (median, 9.0 days; range, 6.1-17.6 days; n = 12 patients), relapse after imatinib discontinuation in complete molecular response (median, 9.0 days; range, 6.9-26.5 days; n = 17), and imatinib interruption during an entire measurement interval (median, 9.4 days; range, 4.2-17.6 days; n = 12; P = .72). Whereas these doubling times were consistently short and indicated rapid leukemic expansion, fold rises were highly variable: 71-, 9.5-, and 10.5-fold, respectively. The fold rise depended on the measurement interval, whereas the doubling time was independent of the interval. Longer doubling times occurred for patients with mutations who maintained chronic phase (CP: median, 48 days; range, 17.3-143 days; n = 29; P < .0001). Predicted short and long doubling times were validated on an independent cohort monitored elsewhere. The doubling time revealed major differences in kinetics according to clinical context. Long doubling times observed with mutations in CP allow time for intervention. A short doubling time for a patient in CP should raise the suspicion of nonadherence.
Introduction
The primary genetic abnormality of chronic myeloid leukemia (CML) is the BCR-ABL1 gene, and transcripts are measured to assess response to the kinase inhibitor imatinib.1 An appropriate frequency of molecular monitoring potentially provides early warning of pending relapse for timely therapeutic intervention before overt relapse. The recommended monitoring frequency is every 3 months until a major molecular response (MMR) is confirmed, and then at least every 6 months.1
The molecular warning for potential resistance or nonadherence is a BCR-ABL1 transcript rise.2-6 However, there is no consensus or clarity on the lower limit of a fold rise that should prompt BCR-ABL1 kinase domain mutation analysis or an evaluation of compliance.1,7 A 10-fold rise is considered clinically significant by the National Comprehensive Cancer Network,7 whereas Press et al found that a 2.6-fold rise was optimal for predicting mutations and concluded that a 10-fold rise is set too high and lacks diagnostic sensitivity.8 A rise is undoubtedly a useful trigger to screen for causes of treatment resistance, but the fold rise is itself a product of 2 factors: the velocity of the rise (a property of the leukemic clone) and the measurement interval (which is arbitrary). Both factors will influence the fold rise; however, they are not considered in current monitoring guidelines.
The velocity of a BCR-ABL1 rise has significance for disease phase at relapse.2,9 A more rapid rise, assessed by the number of days over which BCR-ABL1 doubled (doubling time), occurred with relapse into accelerated phase (AP) or blast crisis (BC) after transplantation,9 and with loss of imatinib response,2 compared with chronic phase (CP) relapse. The doubling time provides an evaluation of tumor growth kinetics and has been used to assess clinical response, determine optimal therapy, and predict survival for various solid tumors.10-13
Rapid BCR-ABL1 increases have occurred when imatinib was interrupted,14-16 and poor adherence to the prescribed dose was associated with suboptimal response or imatinib failure.6,17,18 Distinguishing between loss of response resulting from resistance or nonadherence is important because intervention may differ. However, distinction is not straightforward and is particularly difficult when evidence of biologic resistance is lacking, such as BCR-ABL1 mutations and/or progression to AP/BC. We identified patients with documented imatinib interruption or discontinuation and those who acquired defined mechanisms of imatinib resistance (mutations or BC relapse). We determined whether interruption or discontinuation was associated with a BCR-ABL1 rise and loss of response. The majority of these patients did indeed have a rise and loss of response. The kinetics of the rise, assessed by the BCR-ABL1 doubling time, was compared with that of patients with a known mechanism of resistance. The aim was to determine whether (1) the doubling time provided information on the kinetics of the rise that was not evident from the fold rise, (2) imatinib interruption or discontinuation was associated with characteristic kinetics, and (3) whether the doubling time could help to differentiate nonadherence from drug resistance.
Methods
Patients
Patients included for this retrospective analysis were in CP at imatinib start and monitored at our institution since July 2000 (n = 584, median molecular follow-up, 33 months; range, 3-132 months).19-23 See supplemental Methods for trial descriptions (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The trials were conducted according to the Helsinki Declaration and approved by national/international ethics committees. An assessment of BCR-ABL1 dynamics when values are more than or equal to 10% on the international scale (IS) may be inaccurate.24 Therefore, only patients with reduction less than 10% IS were considered further (n = 539). Of these 539, all patients who met the following criteria were identified: (1) relapsed directly into BC, (2) detection of a BCR-ABL1 mutation, (3) discontinued imatinib in the Australian discontinuation study (TWISTER),22 and (4) had one or more documented imatinib interruptions for any reason (dose was documented and available for 296 of 539 patients from the start of imatinib). An interruption was defined as consecutive days of zero imatinib amounting to at least 10% of the total days of a BCR-ABL1 measurement interval. A measurement interval was the number of days between measurements. The molecular data of all patients who met one or more of these criteria were examined to determine which patients, if any, had a BCR-ABL1 rise. Figure 1 shows the grouping of patients according to the clinical context and dose information.
The clinical and molecular data were examined to identify patients with known mechanisms of resistance and/or documented imatinib interruption or discontinuation. Of the 584 patients available for investigation, 539 achieved a substantial reduction of BCR-ABL1 during imatinib therapy (< 10% IS) and were further assessed. Among these 539 patients, those who relapsed directly into BC and/or had an emergent BCR-ABL1 mutation, discontinued imatinib after sustained CMR or had a documented dose interruption were identified. Among these patients, doubling times were calculated for those with a BCR-ABL1 rise. The number of patients with a rise is indicated for each group.
The clinical and molecular data were examined to identify patients with known mechanisms of resistance and/or documented imatinib interruption or discontinuation. Of the 584 patients available for investigation, 539 achieved a substantial reduction of BCR-ABL1 during imatinib therapy (< 10% IS) and were further assessed. Among these 539 patients, those who relapsed directly into BC and/or had an emergent BCR-ABL1 mutation, discontinued imatinib after sustained CMR or had a documented dose interruption were identified. Among these patients, doubling times were calculated for those with a BCR-ABL1 rise. The number of patients with a rise is indicated for each group.
Molecular analysis
The BCR-ABL1 quantitative RT-PCR and mutation detection techniques were described previously.25,26 Criteria for mutation analysis varied between studies: every 6 months for the first 2 years of imatinib20 ; failure to achieve a major cytogenetic response (MCyR) by 6 months or MMR by 12 months23 ; or a BCR-ABL1 rise according to our previously determined coefficient of variation and assay variability.2 A rise was defined as more than 2-fold for values more than 0.01% IS, and more than 5-fold when less than or equal to 0.01% IS. We did not consider a rise within the inherent variability of the assay as a true rise. For patients where BCR-ABL1 became detectable after imatinib discontinuation in CMR, a rise was assessed from the first positive value. The schedule of quantitative RT-PCR analysis for the discontinuation study was monthly for the first 12 months, which was more frequent than other studies (every 3 months after the first 3 months), although samples were collected outside of the scheduled time points, or occasional collections were missed. BCR-ABL1 less than 10% IS was considered representative of MCyR and less than or equal to 1% IS, a complete cytogenetic response (CCyR) in the absence of cytogenetic analysis.27
Statistical and result analysis
The following formulas were used: doubling time = ln2/k, where (k) is the fold BCR-ABL1 rise divided by the number of days over which the rise occurred [k = (ln(b) − ln(a))/d], where (a) is the value before the rise, (b) the value at the rise, and (d) days.2 The supplemental Excel file contains the formulas for automatic calculation of the measurement interval, fold rise, and doubling time. The constant gradient of the exponential rise was confirmed by graphing BCR-ABL1 values of patients with more than 2 consecutive measurements without therapeutic intervention. The number of days over which the rise occurred was plotted against log10BCR-ABL1 for the period over which the rise occurred for each patient. The equation of the correlation coefficient was calculated using least squares analysis. The paired t test was used to compare the first and second doubling times of individual patients. Groups were compared using the unpaired t test, the Mann-Whitney Rank Sum Test, or the Kruskal-Wallis test. The Fisher Exact test and χ2 test were used to compare frequencies.
Results
Patients with biologic evidence of imatinib resistance
Kinetics were assessed in patients who relapsed directly into BC from CP and/or had BCR-ABL1 mutations. We did not consider other patients with loss of response because a prior study found loss of response in 50% of patients was closely related to dose interruption.20 None of these patients progressed or had mutations. Therefore, it is difficult to determine whether loss of response in such patients should truly be classified as resistance.20 Progression to AP occurred in few patients and was therefore not included in our analysis.
Short doubling times with BC relapse.
Twelve of the 539 patients relapsed into BC, and all had a BCR-ABL1 rise. The median doubling time was 9.0 days (range, 6.1-17.6 days; Table 1). Before BC, 7 patients were in CCyR (none had an MMR). Fourteen mutations were detected in 8 patients (67%). Their median doubling time was similar to 4 patients without mutations (9.0 vs 8.1 days, respectively).
Long doubling times with BCR-ABL1 mutations and maintenance of CP.
Mutations (n = 35) were detected in 30 patients who maintained CP at the time of mutation detection. A rise occurred in 29. The mutation was detectable in the sample collected before the rise in 16 of 27 evaluable patients. The median doubling time was 48 days (range, 17-140 days; Table 1).
Before mutation detection, 25 patients were in CCyR (14 in MMR). Twenty patients lost response: loss of CCyR (n = 11), MCyR (n = 5), complete hematologic response (n = 3) and lymphoid BC (1 patient, 8 months after mutation detection: D276G). This patient initially lost MMR and maintained CCyR on a higher dose. At the time of BC, the doubling time had shortened from 29 to 9.6 days. The other 10 patients met the criterion of imatinib failure for mutations poorly sensitive to imatinib1 (6 with the highly resistant Y253H). Six of the 10 patients lost MMR before therapeutic intervention. Four patients had therapeutic intervention by 4 months after mutation detection and did not lose their best response.
Short doubling times with imatinib discontinuation after sustained CMR
Thirty-six patients were enrolled in the Australian discontinuation trial22 and had at least 6 months of follow-up. Twenty-two of the 36 patients met a criterion for restarting imatinib: BCR-ABL1 less than or equal to 0.10% IS followed by another positive result at any level. Seventeen of the 22 had a rise on consecutive measurements before imatinib restart. The other 5 patients did not have a rise, a pattern of “relapse” that was also observed in some patients in the Stop Imatinib study.28 There is currently no conclusive explanation for the altered kinetics observed in these patients. The doubling time was calculated for the 17 patients with a rise: median 9.0 days (range, 6.9-26.5 days; Table 1).
Imatinib interruption was associated with a BCR-ABL1 rise and loss of response
The dosing schedule was available for 296 patients for a median follow-up of 15 months (range, 3-97 months). The dose was examined of each patient to determine whether documented interruption led to a rise and loss of response and whether the kinetics differed between patients with complete or partial interruption. Interruptions were documented for 45 of 296 patients (55 interruptions). A rise occurred in 43 of the 55 interruptions and loss of response in 31, as detailed in “Consistently short doubling times after complete interruption” and “Variable doubling times after partial interruption.”
The 45 patients with an interruption were divided into 2 groups: interruption for 100% of measurement interval days (complete interruption, 12 patients); and interruption for 10% to 99% (partial interruption, 34 patients). One patient had 3 interruptions and overlapped both groups.
Consistently short doubling times after complete interruption.
Twelve responding patients had a complete interruption for various reasons: intolerance/adverse event (n = 8), treatment for a second malignancy (n = 1), out of medication (n = 1), and nonadherence (n = 2). At the time of interruption, 11 patients had a CCyR (MMR in 6). A rise occurred for all 12 patients during the interruption. The patient with the smallest rise of 2.5-fold had the shortest measurement interval of 7 days, and the patient with the largest rise of 2400-fold had the longest interval of 93 days. The median doubling time was 9.4 days (range, 4.2-17.6 days; Table 1).
The complete interruption led to loss of response in 11 of 12 patients (92%): loss of complete hematologic response (n = 1), MCyR (n = 8), CCyR (n = 1), and MMR (n = 2). The remaining patient commenced nilotinib after only 7 days, without losing MMR. None of the patients progressed to AP/BC during the interruption, and none had a mutation.
Variable doubling times after partial interruption.
Thirty-four patients had one or more partial interruptions (median, 28% days of zero dose; range, 10%-79%). Seven patients had 2 partial interruptions and one patient had 3. Therefore, there were 43 partial interruptions for intolerance/adverse event (n = 31), nonadherence, (n = 6) other medical reasons (n = 2), interruption during a stem cell collection (n = 1), or the reason was not documented (n = 3). At the time of interruption, 30 patients were in CCyR (10 in MMR). Loss of response occurred for 20 of the 43 interruptions (47%): loss of MCyR (n = 8), CCyR (n = 7), and MMR (n = 5).
A rise occurred for 31 of the 43 interruptions (72%). The median doubling time was 37 days (range, 11.6-104 days; Table 1). There was a nonlinear relationship between the doubling time and the duration of the interruption (supplemental Figure 1). When the days of zero dose were doubled, the doubling time was approximately halved.
For 12 interruptions, a rise did not occur. BCR-ABL1 remained stable in 8 (≤ 2-fold change in consecutive measurements) and declined in 4. Stable or declining BCR-ABL1 may be related to the timing of the interruption relative to imatinib start. The median month of imatinib therapy after the interruption for patients without a rise was 6 months (range, 3-42 months), which was significantly earlier than for patients with a rise (20 months; range, 5-107 months; P = .02). The most substantial reductions of leukemic cells occur within 3 to 12 months of starting imatinib because of rapid clearance of differentiated cells.16 Therefore, a rise related to interruption in the early months of therapy could potentially be masked by a rapid reduction when imatinib is restarted.
Imatinib interruption was not associated with BC or BCR-ABL1 mutations.
Of all 55 measurement intervals with an interruption, a rise occurred for 43 (78%) and loss of response for 31 (56%), including 6 of 8 interruptions for nonadherence. No patient progressed to AP/BC during the interruption. However, 2 patients progressed to BC at 3 and 7 months after the interruption. No patient had a mutation detected during the interruption. Mutations were subsequently detected in 5 patients at 3 to 22 months. In total, 296 patients had dose documented, including 14 with mutations. Five of 45 patients (11%) with an interruption subsequently had a mutation, and 9 of 251 (3.6%) without an interruption had a mutation (P = .045).
Significant differences between the BCR-ABL1 kinetics according to the clinical context
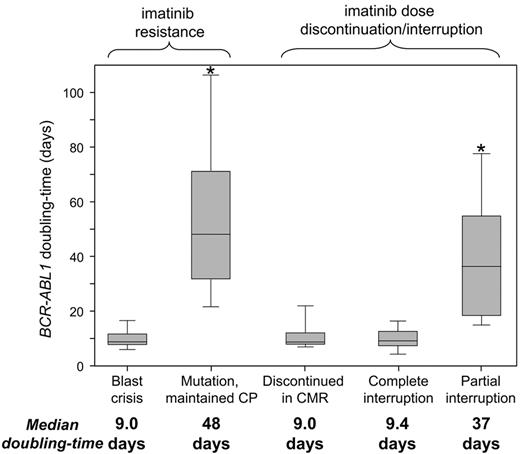
The consistently short doubling times of patients with BC relapse and those who discontinued imatinib or had a complete interruption suggest complete lack of kinase inhibition. There was no difference in the doubling times (P = .72). In contrast, the doubling times of patients with mutations who maintained CP were significantly longer (P < .0001; Figure 2). The longer doubling times suggest that partial kinase inhibition was maintained.
BCR-ABL1 doubling times according to the clinical context. The doubling times suggest a complete lack of kinase inhibition for patients with BC and those who discontinued imatinib or had a complete interruption, compared with partial kinase inhibition for patients with mutations who maintained CP. *Significant differences between the doubling times (P < .0001), compared with the BC, discontinued in CMR and complete interruption groups.
BCR-ABL1 doubling times according to the clinical context. The doubling times suggest a complete lack of kinase inhibition for patients with BC and those who discontinued imatinib or had a complete interruption, compared with partial kinase inhibition for patients with mutations who maintained CP. *Significant differences between the doubling times (P < .0001), compared with the BC, discontinued in CMR and complete interruption groups.
The BCR-ABL1 rise was exponential and the doubling time remained constant
The rise represented a constant logarithmic increase when the value before the rise was within the IS dynamic range (< 10%). Twenty-nine patients had more than 2 consecutive measurements over the course of the rise and did not have a change of therapy (patients who discontinued/complete interruption did not restart). The correlation coefficient (r) was calculated for each rise, which was close to 1 in each case (median, 0.997; range, 0.976-1.0). There was no difference between the correlation coefficients of 15 of 29 patients with a rise related to discontinuation/complete interruption (median, 0.996; range, 0.976-1.0) compared with 14 patients who maintained CP with a mutation-associated rise (median, 0.998; range, 0.983-1.0; P = .21).
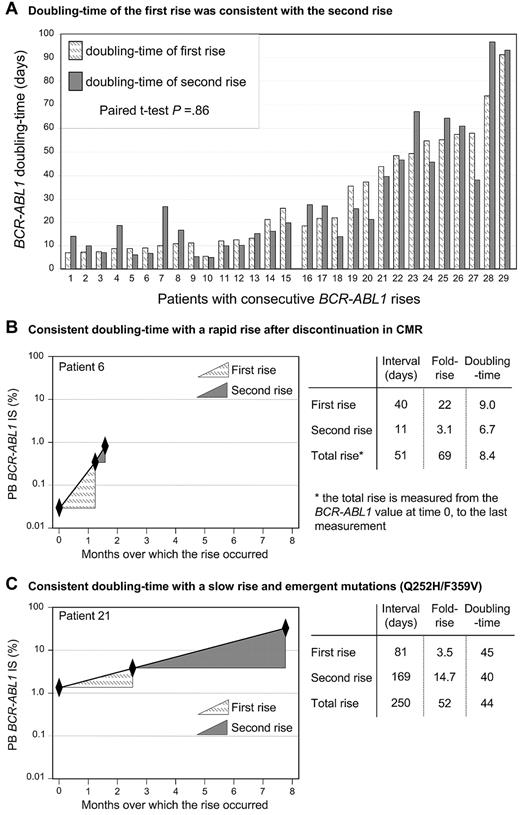
The doubling time remained constant for individual patients over time (Figure 3). The doubling time of the first rise for each of the 29 patients with consecutive increases was compared with the doubling time of the second rise. There was no difference between the first and second doubling times (P = .86, paired t test; mean doubling time 29.2 days for the first rise and 29.5 days for the second rise). Furthermore, the transcript dynamics were analyzed in the context of the accuracy of quantitative RT-PCR at different BCR-ABL1 levels, and there was no difference in the dynamics (supplemental Tables 1 and 2).
The BCR-ABL1 rise was consistent with an exponential growth model; therefore, the doubling time for individual patients remained constant over time. (A) Twenty-nine patients had more than 2 consecutive BCR-ABL1 measurements during the time of the rise and did not have a change of therapy. Patients 1 to 15 discontinued imatinib in CMR or had a complete interruption during the measurement intervals, whereas patients 16 to 29 had a mutation and maintained CP. The exponential nature of the rise meant that the doubling time of the first rise was consistent with the rise that occurred in the second measurement interval. Only 1 patient had a doubling time that differed by greater than 2.5 times at the second rise (patient 7). (B-C) Representative plots of the BCR-ABL1 rise of 2 patients to demonstrate that the doubling time remained constant wherever the rise was measured: over the first rise, the second rise, or over the duration of the total rise. In contrast, the fold rise was highly variable for each of these measurements. A shorter measurement interval led to a smaller rise (B, second rise; and C, first rise), whereas a longer measurement interval led to a greater rise (B, first rise; and C, second rise). The total fold rise was similar for both patients, although the kinetics were markedly different.
The BCR-ABL1 rise was consistent with an exponential growth model; therefore, the doubling time for individual patients remained constant over time. (A) Twenty-nine patients had more than 2 consecutive BCR-ABL1 measurements during the time of the rise and did not have a change of therapy. Patients 1 to 15 discontinued imatinib in CMR or had a complete interruption during the measurement intervals, whereas patients 16 to 29 had a mutation and maintained CP. The exponential nature of the rise meant that the doubling time of the first rise was consistent with the rise that occurred in the second measurement interval. Only 1 patient had a doubling time that differed by greater than 2.5 times at the second rise (patient 7). (B-C) Representative plots of the BCR-ABL1 rise of 2 patients to demonstrate that the doubling time remained constant wherever the rise was measured: over the first rise, the second rise, or over the duration of the total rise. In contrast, the fold rise was highly variable for each of these measurements. A shorter measurement interval led to a smaller rise (B, second rise; and C, first rise), whereas a longer measurement interval led to a greater rise (B, first rise; and C, second rise). The total fold rise was similar for both patients, although the kinetics were markedly different.
The doubling time was not altered by the interval of analysis, unlike the fold rise
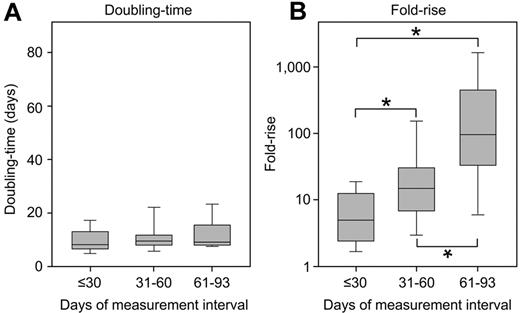
There were significant differences in the measurement interval between patients. Patients with BC relapse had a longer interval than those with a rise associated with imatinib discontinuation or complete interruption: median 68 days versus 28 days, respectively (P < .0001). However, these differences did not influence the doubling time. When patients were grouped according to the days over which BCR-ABL1 was measured (≤ 30 days, 31-60 days, and 61-93 days), there was no significant difference between the doubling times (P = .32; Table 2; Figure 4). Among the 12 patients with intervals of 61 to 93 days, 8 had BC. There was no difference between the doubling times of those with BC (median, 10.1 days; range, 7.7-17.6 days) and the other patients (median, 9.3 days; range, 8.3-26.9 days; P = .36). In contrast, there were significant differences in the fold rise. The longer the measurement interval, the greater the fold rise (P < .0001; Table 2; Figure 4).
The doubling time was not influenced by the interval of analysis. Among the patients with a rapid BCR-ABL1 rise, there was a significant difference in the measurement interval. However, when divided into groups according to the days over which BCR-ABL1 was measured, there was no difference between the doubling times (A). In contrast, there were significant differences in the fold rise (B), where a longer measurement interval led to a greater fold rise. *Significant differences between the groups (P ≤ .005, comparison between each group).
The doubling time was not influenced by the interval of analysis. Among the patients with a rapid BCR-ABL1 rise, there was a significant difference in the measurement interval. However, when divided into groups according to the days over which BCR-ABL1 was measured, there was no difference between the doubling times (A). In contrast, there were significant differences in the fold rise (B), where a longer measurement interval led to a greater fold rise. *Significant differences between the groups (P ≤ .005, comparison between each group).
Similar doubling times for other patient cohorts using a different quantitative RT-PCR method
Two patient cohorts were available at the Catholic University of Korea for analysis of doubling times (supplemental Methods). Three of 20 patients enrolled in an imatinib discontinuation study after sustained CMR29 showed loss of CMR and a BCR-ABL1 rise. The median doubling time (7.9 days; range, 5.8-9.9 days) was similar to our discontinuation cohort (median, 9.0 days, P = .51). Nine patients had a mutation and met the same criteria for assessment as our cohort. All patients maintained CP at mutation detection, and 8 of the 9 had a rise (median doubling time, 47 days; range, 38-128 days). This was similar to our cohort (median, 48 days, P = .65). Of the patients analyzed in Korea, the doubling time of those with imatinib discontinuation after CMR was significantly shorter than those with mutations who maintained CP (P = .012), as in our cohort.
Discussion
Our goal was to examine the link between BCR-ABL1 kinetics and (1) acquired clinical resistance to imatinib and (2) nonadherence, to determine whether nonadherence could be distinguished. For this purpose, we needed to define each group clearly. The kinetics of acquired resistance were assessed in patients with BCR-ABL1 mutations and/or BC relapse, and compared with that of responding patients with documented imatinib discontinuation or interruption for any reason, including nonadherence. The kinetics were evaluated by calculating the BCR-ABL1 doubling time for patients in these groups with a BCR-ABL1 rise. Imatinib interruption or discontinuation was highly associated with a rise and loss of response. The doubling time provided evidence for dose interruption or nonadherence and revealed differences in kinetics for patients with mutations.
Consistently short doubling times (median, 9.0-9.4 days) occurred with BC relapse and in responding patients with a rise related to imatinib discontinuation after sustained CMR or complete interruption during a measurement interval. The short doubling times with discontinuation were consistent with the kinetics of molecular relapse in the Stop Imatinib study, where the BCR-ABL1 rise was 10-fold per month28 (approximate doubling time, 9 days) and the Korean discontinuation study29 (median, 7.9 days). The kinetics are consistent with the calculated rate at which terminally differentiated leukemic cells arise from leukemic progenitors in the absence of imatinib (doubling time, 8 days).16 This suggests complete lack of kinase inhibition with imatinib discontinuation or complete interruption, and BC relapse.
Doubling times associated with mutations and maintenance of CP were significantly longer (median, 48 days) than those associated with complete lack of kinase inhibition. This was confirmed in a cohort tested in Korea (median, 47 days). The long doubling times could be the result of maintenance of partial kinase inhibition if there were mixed populations of imatinib-sensitive and -resistant clones, although we found no association between the proportion of mutant and the doubling time. The longer doubling times could be the result of differences in the growth kinetics of the clones containing the mutations. Marked differences in relapse kinetics, assessed by doubling times of molecular markers of relapsed patients with acute myeloid leukemia, were observed according to the underlying gene mutation.30 In our study, there were no consistent differences in the relapse kinetics for specific mutations or when mutations were grouped according to their in vitro resistance profile.1 This contrasts with previous reports of poorer outcome or more rapid relapse with higher IC50 mutations.31-36 However, unlike our cohort, these studies included patients treated with imatinib in advanced phases and those without MCyR before relapse. The dynamics of relapse may be different in these cases. T315I had the highest growth rate of mutants in an in vitro study.37 However, among our patients, T315I was associated with variable kinetics, ranging from a short doubling time with BC relapse (10.6 days) to a long doubling time with maintenance of CP (77 days). A long doubling time is consistent with an indolent course for many CP patients with T315I.38 Kinase domain mutations may lead to resistance, but other factors, such as acquired genomic mutations, could determine the rate of leukemic cell expansion.39 A recent study of the dynamics of low-level BCR-ABL1 mutations explained discrepancies between in vitro and in vivo resistance profiles as related to competition between drug-resistant clones.40
A study of kinetics in patients with acquired clinical resistance of unknown mechanism was not performed. Many studies have demonstrated that acquired resistance is highly associated with emergent mutations, which occur in more than 50% of patients.8,20,41-46 Loss of response in the absence of a known mechanism of resistance could be related to dose interruption. In the Australian TIDEL study, loss of response in 50% of patients was linked to dose interruption or discontinuation.20 Therefore, we could not develop a greater understanding of disease kinetics if patients were included in the clinical resistance group who did not have an identified mechanism of resistance because loss of response could potentially be related to undocumented, poor drug adherence.
Most of the interruptions in our cohort were for intolerance or adverse events. We cannot exclude that other patients had interruptions that were not documented. Nevertheless, our study was inclusive of all patients with a documented interruption (55 interruptions), and we observed a strong association between an interruption of more than or equal to 10% of measurement interval days and a rise/loss of response, irrespective of the reason for the interruption. Marin et al found nonadherence of more than or equal to 10% of a 3-month measurement interval was associated with unexplained BCR-ABL1 increases at any time during follow-up.6 A rise in the absence of clinical evidence of resistance or known imatinib discontinuation should raise the suspicion of nonadherence. A clinical benefit of the doubling time calculation for patients with short doubling times in the absence of BC is for the indication of complete interruption. Our data suggest that, unlike plasma drug levels that are very dependent on the dose over the 24-hour period before assessment, the doubling time provides an indicator of kinase inhibition during a substantially longer interval and argues for frequent molecular monitoring for adherence assessment. This is analogous to the measurement of HBA1C that provides an indicator of blood glucose control over the previous 60 to 90 days.47
There are some situations where the kinetics may not be informative. Frequent, intermittent nonadherence, which could be the most common form of nonadherence, may not lead to a rise. However, shorter measurement intervals for a patient with compliance issues might provide a clearer indication of nonadherence. The exponential nature of a rise suggests the practical relevance of the doubling time should remain valid with longer measurement intervals, providing therapy is not modified. The timing of measurements relative to the timing of an event, such as an emergent mutation, could potentially influence the doubling time. In our cohort, mutations were detectable in most patients in the sample before the rise using a monitoring frequency of every 3 months, which supports regular quantitative RT-PCR analysis. The reliability of the doubling time could vary according to the laboratory performing the analysis. The international effort to standardize methods has demonstrated that desirable performance is not achieved in all laboratories.24,48 Nevertheless, our findings provide further evidence for the clinical value of frequent, good-quality molecular monitoring, which we have demonstrated is available at many, but not all, molecular laboratories.
In cases of drug resistance, imatinib discontinuation, or complete interruption, doubling times were not altered by the duration of measurement, unlike the fold rise, which was highly variable. Therefore, using a defined value as a trigger for resistance screening or compliance evaluation, such as the 10-fold rise of the National Comprehensive Cancer Network,7 is problematic. We and others suggested that individual laboratories determine the fold rise indicating a true biologic change that is distinguishable from assay variation.8,49 A small, true rise occurring over a short time frame may be of immediate consequence by indicating a rapidly proliferating clone, whereas a greater fold rise occurring over a longer time frame could indicate a slowly proliferating clone. Mutation analysis is warranted in cases of a true biologic rise that is not related to interruption/discontinuation. The doubling time calculation should only be applied in cases of a true rise, rather than fluctuations related to inherent assay variability.
In conclusion, poor drug adherence is one of the major challenges confronting clinicians, and a marker of nonadherence would be of clinical benefit.6,17,18,50 The doubling time can identify patients potentially not adhering to their drug therapy. We found clear differences in the velocity of leukemic growth in various clinical situations, which was not evident by the BCR-ABL1 fold rise. Dose interruption was associated with variable kinetics. A short doubling time in a patient still in CP is strong evidence in favor of complete interruption, whereas a longer doubling time in the absence of a mutation may indicate partial interruption. The slow kinetics for most patients with mutations could be reassuring for clinicians in that a CML clone with a resistant mutation may proliferate slowly, allowing time for consideration of therapeutic options. A shorter doubling time for patients with mutations may be a warning that prompt intervention is required to avoid overt relapse. We suggest the adoption of the doubling time calculation into clinical practice: (1) to function as a marker of potential nonadherence; and (2) to assess the rate of leukemic cell expansion in cases of kinase domain mutation emergence.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the many patients, clinicians, and study coordinators who contributed samples and follow-up data to this study; the staff of the Leukemia Unit, Genetics and Molecular Pathology, SA Pathology for their excellent technical support; Dr Wendy Parker for critical review of the manuscript; and the Australasian Leukaemia and Lymphoma group for their support.
This study was supported in part by Novartis Pharmaceuticals and Bristol-Myers Squibb (S.B. and T.P.H.).
Authorship
Contribution: S.B. designed and performed the research, analyzed data, and wrote the manuscript; D.M.R. and T.P.H. contributed to the experimental design and contributed significantly to manuscript preparation; J.A.P. performed research and contributed to manuscript preparation; D.T.Y. contributed to the experimental design, performed research, and contributed to manuscript preparation; S-Y.C., J.-h.B., and J.E.P. performed research in Korea; and D.-W.K. contributed to manuscript preparation.
Conflict-of-interest disclosure: S.B., T.P.H., and D.-W.K. received research funding and honoraria from Novartis Pharmaceuticals, Bristol-Myers Squibb, and Ariad Pharmaceuticals. D.M.R. received research funding from Novartis Pharmaceuticals and honoraria from Novartis Pharmaceuticals and Bristol-Myers Squibb. D.T.Y. received research funding from Novartis Pharmaceuticals and Bristol-Myers Squibb. The remaining authors declare no competing financial interests.
Correspondence: Susan Branford, Department of Genetics and Molecular Pathology, SA Pathology, PO Box 14, Rundle Mall, Adelaide, South Australia, 5000, Australia; e-mail: susan.branford@health.sa.gov.au.