To the editor:
The 5-year overall survival (OS) rate of patients with acute myeloid leukemia (AML) containing an MLL-AF9 fusion gene is approximately 40%.1,2 We recently showed in 2 independent cohorts that the prognosis among MLL-AF9 positive patients can be refined based on BRE mRNA expression.3 MLL-AF9 positive patients with outlier high BRE expression exhibited a superior outcome (5-year OS of 80% and 64% for the 2 cohorts, respectively) while patients with normal BRE expression exhibited a very poor outcome (5-year OS of 0% and 7%, respectively). Thus, BRE expression may be used for refined risk stratification among MLL-AF9 positive cases. However, the identification of patients with high BRE expression with routinely available techniques such as qPCR is difficult as the fold-difference between normal and high BRE expression is small.3
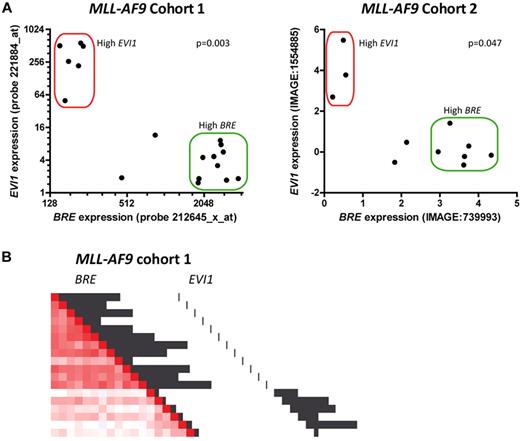
High EVI1 expression occurs in approximately 10% of AML cases and is associated with an inferior outcome. High EVI1 expression has recently been associated with MLL rearrangements including MLL-AF9.4-6 To determine the correlation between BRE and EVI1 expression, we reanalyzed the 2 MLL-AF9 cohorts for which we reported high BRE expression.3,7,8 This showed that high BRE and high EVI1 expression are mutually exclusive (Figure 1A). Hence, the poor prognosis of the patients lacking high BRE expression could be explained by EVI1 expression (eg, see Figure 2C in Noordermeer et al3 ). Of note, in both MLL-AF9 cohorts we identified a few cases without high BRE or EVI1 expression (Figure 1A). As the number of these patients is low, additional studies are required to reliably determine their prognosis.
High BRE and high EVI1 expression are mutually exclusive in MLL-AF9 leukemia. (A) BRE expression was plotted against EVI1 expression for MLL-AF9 positive cases in 2 separate MLL-AF9 cohorts.7,8 In the first cohort (left plot), 33% (6/18) of the samples showed high EVI1 expression, and 55.6% (10/18) showed high BRE expression. In the second cohort (right plot), 27.3% of the samples showed high EVI1 expression (3/11), and 54.5% showed high BRE expression (6/11). Both cohorts contained 2 patients with neither high BRE nor high EVI1 expression. High BRE expression was defined as described before.3 High EVI1 expression was defined as the expression of the upper 10% of the total cohort. P values for negative correlations were calculated using Spearman correlation tests. (B) EVI1-positive patients cluster apart from high BRE expressing patients among MLL-AF9 positive patients in unsupervised clustering analysis. However, EVI1-positive patients show less similar expression profiles among each other compared with high BRE expressing patients (indicated by faint red color compared with bright red color, respectively). Unsupervised clustering was performed on the first cohort as described elsewhere9 and clustering is represented as pairwise correlations between samples with a gradient from red to blue indicating degree of correlation (bright red: high correlation, blue: poor correlation). Black bars represent relative BRE (212645_x_at) and EVI1 (221884_at) expression, as indicated.
High BRE and high EVI1 expression are mutually exclusive in MLL-AF9 leukemia. (A) BRE expression was plotted against EVI1 expression for MLL-AF9 positive cases in 2 separate MLL-AF9 cohorts.7,8 In the first cohort (left plot), 33% (6/18) of the samples showed high EVI1 expression, and 55.6% (10/18) showed high BRE expression. In the second cohort (right plot), 27.3% of the samples showed high EVI1 expression (3/11), and 54.5% showed high BRE expression (6/11). Both cohorts contained 2 patients with neither high BRE nor high EVI1 expression. High BRE expression was defined as described before.3 High EVI1 expression was defined as the expression of the upper 10% of the total cohort. P values for negative correlations were calculated using Spearman correlation tests. (B) EVI1-positive patients cluster apart from high BRE expressing patients among MLL-AF9 positive patients in unsupervised clustering analysis. However, EVI1-positive patients show less similar expression profiles among each other compared with high BRE expressing patients (indicated by faint red color compared with bright red color, respectively). Unsupervised clustering was performed on the first cohort as described elsewhere9 and clustering is represented as pairwise correlations between samples with a gradient from red to blue indicating degree of correlation (bright red: high correlation, blue: poor correlation). Black bars represent relative BRE (212645_x_at) and EVI1 (221884_at) expression, as indicated.
To study whether the mutually exclusive expression of BRE and EVI1 is accompanied by distinct expression profiles, we performed unsupervised genome-wide cluster analysis. The results showed that among MLL-AF9 patients, high BRE expressing patients clustered apart from EVI1-positive patients (Figure 1B). Although the EVI1-positive patients were separated from the BRE-positive patients, they did not share highly similar expression profiles, while BRE-positive patients did. Indeed, in an unsupervised cluster analysis of the total AML cohort, MLL-AF9 positive patients with high EVI1 expression clustered only partially, showing modest similarities in expression profiles (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). This was in contrast to patients with high BRE expression that were almost completely confined to one distinct AML cluster, as described before.3 Thus, MLL-AF9 positive patients with high BRE expression seem to represent a specific AML subclass with highly similar expression profiles, while the MLL-AF9 positive patients with high EVI1 expression do not.
In our previous study, 40% of the MLL-AF9 positive cases were missed by routine cytogenetics.3 In addition, MLL-AF9 positive cases with high EVI1 expression lack chromosomal rearrangements encompassing the EVI1 locus on chromosome 3.4,5 Therefore, risk stratification of these patients could be improved by molecular screening for MLL-AF9–positivity and EVI1 overexpression, in addition to routine cytogenetics.
Authorship
The online version of this article contains a data supplement.
Acknowledgments: This research was financially supported by the Vanderes Foundation.
Contribution: S.N. and B.R. designed the study; S.N., D.M. and M.S. performed the analyses; S.N. and D.M. wrote the manuscript, which was critically revised by M.S., L.B., J.J. and B.R.; and data interpretation was performed by all coauthors.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: B. A. van der Reijden, Geert Grooteplein 8, 6525 GA Nijmegen, The Netherlands; e-mail: b.vanderreijden@labgk.umcn.nl.
References
Author notes
S.M.N. and D.M. contributed equally to this work.